Abstract
The inhibitive behaviour of aloe vera as an eco-friendly inhibitor was studied in the corrosion of mild and stainless steel in 0.5 M H2SO4 medium. The varied aloe vera inhibitor concentrations were studied using weight loss (gravimetric) and linear polarization methods. The methods showed that the inhibition efficiency increased with an increase in the concentration of the inhibitor (up to 10 vol/vol%) for both the mild and stainless steels. Stainless steel was found to exhibit a lower corrosion rate compared to mild steel. The results showed that Langmuir adsorption isotherm was obeyed by the inhibition of mild and stainless steel using aloe vera in 0.5 M H2SO4 with the values of the regression coefficients near unity. The negative values of ∆Gads show the spontaneous adsorption of inhibitor on the mild and stainless steel surfaces and a physisorption adsorption mechanism of the aloe vera inhibitor since the values of ∆Gads obtained are more than − 20 kJ/mol, that is, aloe vera is an efficient corrosion inhibitor with mixed-type inhibition property.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Mild steel, also known as plain-carbon steel, is the most preferred form of steel due to its availability, low price and relatively acceptable physical properties. The problem being faced as regards its use is its susceptibility to corrosion especially in acidic environments. Many manufacturing industries (such as automobile, chemical, food, oil and gas industries) make use of iron and steel vessels in carrying out their operations. In most cases, acidic solutions are used extensively in such environment, thereby causing such materials to be prone to corrosion [1,2,3,4].
Also, stainless steel that is frequently in contact with acidic medium is not exempted from the degradation/deterioration caused by corrosion. Some of the consequences of stainless steel corrosion in industrial operations include reduction in the thickness of the shell or an effective pipe diameter, leakage of hazardous fluids from stainless steel (which acts as a conveyor, container or reservoir), and even plants shutdown resulting from the economy loss [5].
It was reported by [6] that the estimated loss to corrosion of oil pipelines in the Nigerian oil and gas industry is up to $765 million yearly. Directly and indirectly, corrosion has effects on different areas of our lives. For example, sudden failure of an equipment leads to a construction collapse or cause a fire explosion, or some metallic materials when corrode release toxic substances that are harmful to human health, plants and animal in the vicinity [6].
The need for corrosion control and mitigation is evident in the above-stated undesirable issues being faced by important sectors of the nation’s economy. There are two major methods used in the protection of steel from corrosion, namely cathodic protection and corrosion inhibitors. Cathodic protection works using the principle of a sacrificial anode, that is, making a metal to assume the work of a cathode in an electrochemical cell. With the aim of maximizing profit and reducing cost for various industries, the use of corrosion inhibitors has been set as the most effective means of addressing the corrosion of metals [7, 8].
The term eco-friendly (green) inhibitor is used to describe inhibitors that are compatible with nature while also performing their anticorrosion functions. This explains the reason why the recent research trends on corrosion inhibitors has shifted its focus from the use of certain chemicals that have hazardous effects on the environment. Furthermore, the availability, cost effectiveness of these green inhibitors and environmental legislation have favourably placed emphasis on the relevance and use of these green inhibitors [9].
One main property that an inhibitor must possess to be able to effectively protect a metal from corrosion is an excellent adsorption behaviour on the surface of the metal to be protected. Some plant extracts, that are being used as eco-friendly (green) corrosion inhibitors due to their biodegradability and non-toxic nature, have this adsorption behaviour [10]. Some active functional substances that research has shown to be the cause of effectiveness in corrosion inhibitors include tannins, pigments, steroids, saponins, anthraquinones and phenolic compounds are also present in these extracts [1, 11, 12].
With recent trends showing focus on the use of environmentally friendly plant extracts as effective corrosion inhibitors, this research focuses on aloe vera plant extract as corrosion inhibitor in acidic media of mild and stainless steels, both the gravimetric and electrochemical tests will be considered, with emphasis on the aloe vera inhibitor concentration range of (0–10) vol/vol% in the acidic medium. The novelty of this research work is in the establishment of the adsorption behaviour (in terms Gibbs free energy, ∆Gads, of the Langmuir isotherm) of both the mild steel and stainless steel in the acidic medium to determine the appropriate inhibitor concentration.
2 Materials and Methods
2.1 Aloe Vera and Metal Samples Preparation
The colourless liquid extract from clean and succulent aloe vera was collected in a clean air-tight container at room temperature. The extraction was obtained through simple hand press after cutting each strand of aloe vera plant into two [10, 13].
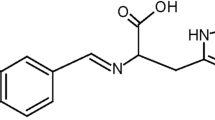
Figure 1 shows the main chemical compounds in aloe vera that are responsible for the inhibitive nature of the plant. These compounds possess aromatic structures with long aliphatic chains and free electron pairs available to bond easily with ions on the metal surface, exhibiting good corrosion protection efficiency [1, 10].
Each of the mild steel and stainless steel samples used was cut into the dimension of 15 × 15 × 5 mm. The samples were polished with emery papers of different grades, rinsed with distilled water and allowed to dry at the room temperature [4, 7]. The weights of the samples were taken and the samples were labelled accordingly, based on the experimental design employed. Varied concentration of the aloe vera extracted and 0.5 M H2SO4 acidic medium of 200 mL volume were used in the course of the experiments [13].
2.2 Corrosion Tests
The corrosion tests carried out are the gravimetric and electrochemical tests. The gravimetric test involves the immersion of the samples (for both mild steel and stainless steel) in 200 mL of 0.5 M H2SO4 solutions containing various concentrations of the aloe vera inhibitor and one without the inhibitor, for a period of 21 days at an ambient temperature 25 °C. Weight loss due to corrosion (with a 3–day interval) was determined. Also, inhibition efficiency (IE%) and surface coverage (θ) were determined using
where \(W\) and \({W}_{i}\) are weight loss in the absence of inhibitor and weight loss in the presence of inhibitor, respectively.
The electrochemical experiment was carried out at ambient temperature of 25 °C using Autolab PGSTAT 30 ECO CHIMIE potentiostat and electrode cell containing 200 mL of 0.5 M H2SO4 electrolyte (with and without varied concentrations of inhibitor). The electrochemical cell has a graphite rod (auxiliary electrode), Ag/AgCl (reference electrode) and the metal sample (working electrode), for both the mild steel and the stainless steel when used separately. The stabilization of the electrode at OCP was noted, potential range considered was − 2.0 V to + 1.0 V, at a scan rate of 0.00166 V/s, and current measurement (I) was taken.
The values of corrosion potential (Ecorr), corrosion current density, Icorr, (A/cm2), corrosion rate and polarization resistance were obtained using the experimental measurements from the Tafel plots of potential E (V) against log current I. Inhibition efficiency (IE%) and surface coverage (θ) were also determined using
where \({I}_{corr}\) and \({I}_{ocorr}\) are the current densities with and without inhibitor, respectively.
2.3 Adsorption Study of the Aloe Vera Inhibitor
The gravimetric data obtained from this research work were fitted into various adsorption isotherms. Based on the adsorption isotherms considered, the appropriate model was considered by the plot that gives a straight line that has a correlation coefficient of approximately one (1). The adsorption isotherm models employed in this research work is Langmuir adsorption isotherm which is expressed as
where K is the adsorption constant.
2.4 Thermodynamics Study of the Aloe Vera Inhibitor
The thermodynamic parameter, change in Gibbs free energy, (∆Gads) was evaluated from the data obtained from the adsorption isotherm plots, using the equation:
where Kads is the adsorption constant. The values of ∆Gads obtained were be used to validate the form of adsorption mechanism that aloe vera inhibitor employed during the corrosion process.
2.5 SEM Analysis
Quanta 200FEI Scanning equipment was used in carrying out the comparative morphological structures of the surface of the samples (with and without inhibitor) that suffered corrosion.
3 Results and Discussion
3.1 Gravimetric Test
Figure 2a and b shows the weight loss obtained from the variation of the aloe vera inhibitor, at different exposure time during gravimetric test. The weight loss rate of the stainless steel was observed to be lower than that of the mild steel, and this can be attributed to the combined alloy effect used in the make-up of stainless steels [13]. Also it could be seen that the weight loss rate decreased as the concentration of aloe vera inhibitor increased, an indication of the increased formation of a barrier layer of the inhibitor on the surface of the metal sample, thereby reducing corrosion reaction rate [10]. When no inhibitor was applied (control), the rate of weight loss was very high, and this established the performance of aloe vera as a corrosion inhibitor.
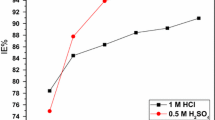
Figure 3a and b shows the inhibitor efficiency (%) obtained from the variation of the inhibitor concentration. For both mild steel and stainless steel, inhibition efficiency increase with increase in inhibitor concentration, but higher inhibitor efficiency was observed for stainless steel. The least value of efficiency was recorded when no inhibitor was introduced into the corrosion process. The results also show that the efficiency of the inhibitor slightly reduced with time, an indication that the inhibitive activity of the aloe vera inhibitor reduced with time.
Figure 4a and b shows the plots of the metal surface coverage by the aloe vera inhibitor against inhibitor concentration. The trends observed were similar to that of the trends obtained in the plots of inhibitor efficiency against inhibitor concentration: that is surface coverage increased with increased inhibitor concentration, an indication that the active sites on the metal samples were blocked by the inhibitor, thereby reducing the exposed surface area of the metal samples to the corrosive medium [12, 14].
3.2 Electrochemical Tests
Tables 1 and 2 show the electrochemical test parameters obtained for inhibited and uninhibited mild steel and stainless steel, respectively. It was observed that the increased concentration of the inhibitor in the corrosive medium reduced both the current density (jcorr) and corrosion rate significantly, and also indicated the non-uniformity of the corrosion process. These results are in conformity with the results obtained earlier in Figs. 2, 3 and 4: that is, an increase in the concentration of aloe vera inhibitor hindered the corrosion of both the mild and stainless steels. The decrease in jcorr values can also be attributed to the increased adsorption of the inhibitor on the mild steel metal surface: that is, the bond between the inhibitor molecules and the metal surface increased, thereby reducing the metal corrosion reaction rate [11, 12]. This fact is further justified by the increase in polarization resistance experienced as the inhibitor concentration increased.
The Ecorr values obtained from the experiment for both mild and stainless steel exhibit similar patterns. The Ecorr values define the potential at which the rate of oxidation is equal to the rate of reduction and at this point, both polarities of current are present. The values tend towards the more negative side of the Ecorr, then the cathodic current predominates at the expense of the anodic current. The little variations in the Ecorr values suggest a mixed-type behaviour in polarization of both steels. Although it did not fully assume either the negative or the positive type, it exhibited more of cathodic nature.[11, 12].
Figure 5 shows a steady decrease in the rate of corrosion with an increase in inhibitor concentration. This supports the weight loss data obtained from the gravimetric experiments. Also, Figs. 6 and 7 reflect increased inhibition efficiency and surface coverage as the inhibitor concentration increased. In all, the plots for both mild steel and stainless steel follow the same trend, and the values obtained indicated that stainless steel has higher corrosion resistance than the mild steel and the use of aloe vera (as inhibitor) was justified, as indicated in the Tafel plots (Figs. 8a and b).
Also, Tafel plots in Fig. 8a and b indicated the prevalence of the cathodic current due to the polarization of the sample in the negative direction: that is, aloe vera inhibitor exhibited more as cathodic inhibitor than anodic inhibitor.
3.3 Surface Morphology Analysis
The optical images of the mild steel and stainless steel after immersion in the acidic media (0.5 M H2SO4 solution) with and without aloe vera inhibitor are shown in Figs. 9 and 10. Minimal pits (indication of pitting corrosion) were observed in the case of stainless steel, while the major form of corrosion of the mild steel was uniform corrosion: that is, the degree of surface roughness in the case of mild was more than that of the stainless steel, an indication that mild steel suffered more corrosion attack by the aggressive medium (acid) compared to the corrosion level of the stainless. The morphological deterioration in each case of the metal samples was observed to reduce as the concentration of the aloe vera inhibitor increased, this implies increase in inhibitor concentration amounts to the formation of more passive film blocking the metal surface from further corrosion [15, 16]. In the case of the use of no inhibition, the corrosion impact (traceable to the level of roughness) of the sample was the highest, and the corrosion products formed (whitish substance) could be easily observed in this case.
3.4 Adsorption Studies on the Inhibitive Performance of Aloe Vera
The adsorption behaviour determines the nature and inhibitive effect of aloe vera. The data obtained from the experiments were tested to fit the Langmuir adsorption isotherm by plotting graphs. The data could not successively fit into Temkin and Freundlich adsorption isotherms. Figures 11 and 12 show Langmuir adsorption plots for mild steel and stainless steel, respectively.
From Figs. 11 and 12, it could be seen that as the inhibitor concentration increased, the inhibitor coverage on the metal surface increased: that is, the increased inhibitor coverage caused the bond between the inhibitor molecules and the metal surface to increase, thereby reducing the corrosion reaction rate on the metal surface [12]. The adsorption mechanism (physisorption or chemisorption) is described in terms of the values of the adsorption constant (Kads) and Gibbs free energy (∆Gads) obtained [20].
By plotting C/ Ө against C for different aloe vera inhibitor concentration, straight line graphs were obtained; this follows the principle of Langmuir adsorption isotherm as stated in Eq. 5 and also reported in literatures [17, 18]. The equilibrium adsorption constant (Kads) was also calculated.
From Table 3, the slope and the linear correlation coefficient (R2) of the fitted data were close to unity in each case, an indication that the adsorption of the inhibitor molecules obeys the Langmuir adsorption isotherm [10, 17, 19, 20].
3.5 Thermodynamics Studies on the Inhibitive Performance of Aloe Vera
The different values of Kads were used in calculating the ∆Gads values for Langmuir adsorption isotherms. From the calculated change in Gibbs free energy obtained (∆Gads), the validation of the form of adsorption mechanism that aloe vera inhibitor employed (as an anticorrosion agent) was established. Table 4 shows the thermodynamics properties obtained for mild steel and stainless steel, using aloe vera inhibitor.
The negative values of ∆Gads indicate that the reaction is spontaneous and a physisorption adsorption mechanism of the aloe vera inhibitor since the values of ∆Gads obtained are more than − 20 kJ/mol (the standard value for physisorption); this supports the consistent electrostatic interaction between charged molecules and a charged metal surface [20]. The lesser negative values of ∆Gads in the Langmuir isotherm indicate a better physisorption property of aloe vera as an inhibitor, and hence it is reasonable to claim that Langmuir isotherm is a suitable isotherm for the adsorption mechanism.
4 Conclusion
From this research work, the following conclusion can be made:
-
1.
Aloe Vera was found to be an effective inhibitor of mild and stainless steel as an increase in its concentration up to 10 vol/vol% led to an increase in inhibition efficiency.
-
2.
Aloe vera attained a maximum inhibition efficiency of 88.9% for the mild steel and 99.1% for stainless steel, both at an optimum concentration of 10 vol/vol% in H2SO4 medium.
-
3.
Langmuir adsorption isotherm was obeyed by the inhibition of mild and stainless steel using aloe vera in 0.5 M H2SO4 with the values of the regression coefficients near unity.
-
4.
The negative values of ∆Gads show the spontaneous adsorption of inhibitor unto the mild and stainless steel surfaces.
-
5.
The electrochemical potentiodynamic polarization studies show that aloe vera acted as mixed-type inhibitor, but more of cathodic inhibitor
-
6.
The overall results show that stainless steel has a higher corrosion resistance compared to mild steel.
References
Pallav S, Shruti A (2014) Aloe vera: a green corrosion inhibitor. Int J Res App Sci Eng Tech 2(5):14–17
Ayoola AA, Fayomi OSI, Popoola API (2018) Anticorrosion properties and thin film composite deposition of Zn-SiC-Cr3C2 coating on mild steel. DT. https://doi.org/10.1016/j.dt.2018.04.008
Ayoola AA, Fayomi OSI, Popoola API (2018) High temperature thermal treatment of Zn-10Nb2O5–10SiO2 crystal coatings on mild steel. Cog Eng 5:1–9. https://doi.org/10.1080/23311916.2018.1540026
Ayoola AA, Fayomi OSI, Ogunkanmbi SO (2018) Data on inhibitive performance of chloraphenicol drug on A315 mild steel in acidic medium. DIB 19:804–809
Orisanmi BO, Afolalu SA, Adetunji OR, Salawu EY, Okokpujie IP, Abioye AA, Abioye OP (2017) Cost of corrosion of metallic products in federal university of agriculture, abeokuta. Int J App Eng Res 12(24):14141–14147
Ashworth V (2000) A short introduction to corrosion and its control. In: Corrosion of metals and its prevention, vol 4. p 1–9
Fayomi OSI, Popoola API, Oloruntoba T, Ayoola AA (2017) Inhibitive characteristics of cetylpyridinium chloride and potassium chromate addition on type A513 mild steel in acid/chloride media. Cog Eng. https://doi.org/10.1080/23311916.2017.1318736
Fayomi OSI, Ayodeji AA, Omoniyi EB, Okolie ST (2018) Influence of aluminum silicate stabilizer on the coating structural composition and characteristics of multifunctional developed composite coating: a buildup for defense application. Int J Adv Manuf Tech. https://doi.org/10.1007/s00170-018-2655-9
Palou RM, Olivares-Xomelt O, Likhanova NV (2008) Environmentally friendly corrosion inhibitors. In: Cahn RW, Haasen P, Kramer EJ (eds) Materials science and technology: a comprehensive treatment: corrosion and environmental degradation, volumes I+II, I+II. https://doi.org/10.1002/9783527619306.ch9
Omotosho OA (2016) Inhibition evaluation of chemical and plant extracts on the corrosion of metallic alloys in acidic environment. PhD Thesis, Mechanical Engineering Department, Covenant University, Nigeria
Tessema D, Belete Y (2015) Investigation of the anti-corrosion activities of aloe vera extract on iron metal sheets. J Nat Sci Res 5(5):18–26
Singh AK, Mohapatra S, Pani B (2016) Corrosion inhibition effect of aloe vera gel: gravimetric and electrochemical study. J Ind Eng Chem 33:288–297
Vashi RT, Chaudhari HG (2017) The study of aloe-vera gel extract as green corrosion inhibitor for mild steel in acetic acid. Int J Innov Res Sci Eng Tech 6(11):22081–22091
Dwivedi D, Lepková K, Becker T (2017) Carbon steel corrosion: a review of key surface properties and characterization methods. RSC Adv 7:4580–4610
Perumal S, Muthumanickam S, Elangovan A, Karthik R, Mothilal KK (2017) Bauhinia tomentosa leaves extract as green corrosion inhibitor for mild steel in 1 M HCl medium. J Bio Tribo Corros 3(2):13
Fayomi OSI, Akande IG, Popoola API (2018) Corrosion protection effect of chitosan on the performance characteristics of A6063 alloy. J Bio Tribol Corros 4(73):72–78
Sudha R, Kalpana K, Rajachandrasekar T, Arivoli S (2007) Comparative study on the adsorption kinetics and thermodynamics of metal ions onto acid activated low cost pandanus carbon. J Chem 4(2):238–254
Uwah IE, Ugi BU, Ikeuba AI, Etuk KE (2013) Evaluation of the inhibitive action of eco-friendly benign costus afer stem extract on the corrosion of mild steel in 5 M HCl solution. Int J Dev Sust 2(4):1970–1981
Rocha J, Gomes JC, Elia ED (2010) Corrosion inhibition of carbon steel in hydrochloric acid solution by fruit peel aqueous extracts. Corr Sci 52(7):2341–2348
Loto RT, Loto CA, Popoola AP (2015) Inhibition effect of deanol on mild steel corrosion in dilute sulphuric acid. SA J Chem 68(1):105–114
Acknowledgements
The open access financial contribution of Covenant University CUCRID is highly appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This is to state that all authors agree that there is no conflict of interest in this research work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ayoola, A.A., Fayomi, O.S.I., Akande, I.G. et al. Inhibitive Corrosion Performance of the Eco-Friendly Aloe Vera in Acidic Media of Mild and Stainless Steels. J Bio Tribo Corros 6, 67 (2020). https://doi.org/10.1007/s40735-020-00361-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-020-00361-y