Abstract
Eucalyptus globulus (E. globulus) leave’s extract was tested for corrosion inhibition on the low carbon steel (LCS) in a 0.5 M sulphuric acid solution using weight loss, Tafel and EIS. The state of mixed adsorption with the potentiodynamic polarization effect is shown. E. globulus showed the strongest corrosion inhibition of 93.09% at a concentration of 600 mg/L. SEM and AFM are used to verify the formation of a protective layer on the surface of LCS. The adsorption phenomenon was verified using UV–Vis spectroscopic technique, whereas FT-IR confirmed the presence of several functional groups containing heteroatoms. Adsorption of the inhibitory molecules on the LCS surface followed the Langmuir adsorption isotherm. Theoretical studies were conducted to validate the study. All obtained results ensure that E. globulus leave’s extract can form an effective blocking layer and control the corrosion process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Low carbon steel (mild steel) is largely used for making a comprehensive variety of metallic structures and hardware. It is cheap and easily available in the market [1]. In industries when it comes in contact with the different industrial process like acid pickling, then corrosion takes place. The indirect and direct cost including common coatings of corrosion for India is US$ 26.1 billion or 2.4% and 49.75 billion or 4.5% of GDP [2]. However, in recent times, some synthetic corollary might be shown by the synthetic compounds, which may be pernicious for the environment as well as for the human health are preferred more to be used as corrosion inhibitors [3, 4]. An extensive class of corrosion inhibitors is constituted in natural products. The easy availability and non-toxicity give the plant extract a meaningful excellency to be used as a prohibition for corrosion [5,6,7]. The effectiveness of the corrosion process inhibited by plant extract on the LCS surface is established due to the presence of chemical constituents such as flavonoids, polyphenols and polysaccharides [8,9,10,11]. Natural products containing functional groups such as NH2, carbonyl and CHO group are known to have feasible inhibition power [12,13,14,15,16]. Many researchers have already reported different plants as a green corrosion inhibitor for the acidic medium with good inhibition efficiency. Table 1 shows the previously reported green corrosion inhibitors by other researchers [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. Although most of the previously reported green inhibitors showed good inhibition efficiencies, a higher inhibitor concentration was used there; this concern motivated us to conduct this investigation. To the best of author’s knowledge, this plant has not been used previously for corrosion inhibition study. Moreover, it provides good inhibition efficiency at low inhibitor concentration.
Eucalyptus globulus is an evergreen tree and generally grows up to 30–55 m (98–180 ft). This tree bears simple, alternate leaves 6–15 cm across. It belongs to the Myrtaceae family and known as eucalyptus, bluegum and Tasmanian bluegum. It is commonly found throughout India, Australia, Southern Africa, New Zealand and the western United States. Leaves of E. globulus are very easily available in the market. E. globulus contains eucalyptol, globulusin-A and globulusin-B as major phytochemical compounds [33, 34]. The image of E. globulus leaves and its major phytochemical constituents are represented in Fig. 1.
The present investigation is aimed to extract the E. globulus leaves and investigate the anti-corrosion properties for LCS in 0.5 M sulphuric acid, based on gravimetric measurements and electrochemical analysis. In addition, surface adsorption of the inhibitor molecules on the surface of LCS has been studied using atomic force microscopy (AFM) and scanning electron microscopy (SEM). For a better and deeper understanding of the adsorption phenomenon, ultraviolet–visible (UV–Vis.), Fourier-transform infrared (FT-IR) and nuclear magnetic resonance (NMR) spectroscopic techniques have been conducted. All the experimental outcomes were correlated with the theoretical data to better understand the adsorption phenomenon. The novelty of our investigation is to replace the existing synthetic inhibitors by natural inhibitors, and the use of the natural waste material as a green corrosion inhibitor makes it more novel and effective. The advances to use the natural extract as a green inhibitor are as follows: (1) Natural products are eco-friendly and non-hazardous to the environment as well as to the human being and (2) they convert waste products to a useful material with negligible production cost.
2 Experimental
2.1 Preparation of the Working Electrode, Inhibitor and Electrolytes
Fresh E. globulus leaves were validated by Dr. A. A. Bhat in the Department of Biotechnology, LPU, Punjab (INDIA). The crude material was thoroughly washed under running water followed by sterile distilled water and dried in the shade. They were grinded to convert in powder. The sample was approximately 100 g of the powder that was extracted in a 500-mL round bottom flask with 250 mL of solvent. The filtrate was collected and concentrated using a rotary evaporator under controlled temperature and pressure conditions. The extracts were concentrated to dryness to give a crude residue. Followed by this procedure, around 15% of extract yield with pH value of around 8 was obtained. 1 cm2 cubical-shaped LCS specimens were used in weight loss estimations and electrochemical analysis. The LCS specimens were abraded with emery paper of grade 100, 300, 600, 800, 1000 and 1200 then washed with distilled water and acetone, dried again and finally kept in a desiccator until used. The sulphuric acid was diluted to 0.5 M H2SO4 to use as the corrosive medium. The test solution with specific concentrations (100–600 mg/L) was obtained by diluting plant extract with 0.5 M sulphuric acid solution. The highest solvency of the E. globulus extract in 0.5 M sulphuric acid solution was observed up to 600 mg/L.
2.2 Weight Loss Studies
Weight loss measurements were conducted according to ASTM G 31–72 method at 298 K for 24 h and weighed accurately before and after the tests [35]. All the experiments were performed at a constant temperature (298 K) using PPI Fini X48 digital thermostat. For the precise weighing of the LCS samples, a Shimadzu BL-220H/D455006313 digital electronic weighing balance was used. The LCS specimens were immersed in the 0.5 M sulphuric acid solution containing different inhibitor concentrations at 298 K for 24 h. Weight loss or gravimetric measurement was conducted because that is an extensively used and trust-worthy method.
2.3 Electrochemical Studies
Electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization measurements (Tafel) were performed using the CHI760C electrochemical workstation. Experiments were performed at 298 K in 0.5 M sulphuric acid, including various concentrations (100–600 mg/L) of inhibitor. A traditional three-electrode system was used where LCS with a working area of 1 cm2 was used as working electrode. The platinum electrode used as an auxiliary electrode and the saturated calomel electrode (SCE) coupled to a luggin capillary was used as the reference electrode. Before each experiment, the working electrode was immersed in the test solution for 60 min to stabilized corrosion potential values (\({E}_{\mathrm{c}\mathrm{o}\mathrm{r}\mathrm{r}}\)). The polarization curves were recorded at ± 250 mV versus SCE with a scanning speed of 1 mV/s. The EIS spectra were scanned with a frequency range of 100 kHz to 0.01 Hz with a signal amplitude perturbation of 0.005 V. Electrochemical measurements were prepared repeated three times to get reliable values [36].
2.4 UV–Visible Spectroscopy
The UV–Visible spectra of the inhibitor solution before and after immersion of LCS sample for 24 h at 298 K were taken using Shimadzu UV-1800 UV–Visible absorption spectrophotometer at a wavelength of 500–200 nm. Then, they were used to explain the mechanism of inhibition.
2.5 FT-IR and 1H NMR Spectroscopy
For the identification of the presence of various functional groups, FT-IR analysis has been recorded with the help of Shimadzu 8400S spectrophotometer at a wavenumber range of 500–4000 cm−1. 1H NMR spectrum has been done to verify the presence of the prime phytochemical constituent in the plant extract. For NMR, Bruker Advance II 400 MHz system was used.
2.6 Surface Investigations
For the surface analysis, scanning electron microscope (SEM-Model: LEO-435 VP) and atomic force microscope (AFM-Model: NT-MDT-INTEGRA) images of pre-treated LCS specimen, LCS in 0.5 M sulphuric acid, without and with the inhibitor extract (600 mg/L) for 24 h at 298 K were taken out.
2.7 Quantum Chemical Calculations
Theoretical studies were carried out to a deeper understanding of the adsorption mechanism. Quantum chemical calculations were used as theoretical studies. It is a well-known fact that the extract of plants has several phytochemical constituents. For the theoretical studies, we followed the presence of three main phytochemical constituents in the plant extract E. globulus. Quantum chemical calculations were conducted using density functional theory (DFT) with Hyperchem 8.0 software. Key parameters were obtained from the optimized structures.
3 Results and Discussions
3.1 Gravimetric Measurements and Adsorption Isotherm
Gravimetric measurements were carried out as per ASTM G 31–72 method for 24 h at 298 K with different inhibitor concentrations in 0.5 M H2SO4 solution. LCS samples were used as per the ASTM method. These experiments were repeated three times to achieve more precise results. The elemental composition (wt%) of used LCS samples were Fe = 97.83%, Mn = 0.43%, P = 0.12%, Si = 0.39%, Cu = 0.43%, C = 0.08%, Ni = 0.27% and Cr = 0.45%. Corrosion rate was determined in the absence and presence of inhibitor using the following equation:
where W is the weight loss of LCS strip (g), CR is the corrosion rate (mmy−1), t is the immersion time (h), K is the constant equals to 8.76 × 104, ρ the density in g cm−3 which is 7.86 g cm−3 for mild steel according to ASTM G 31–72, and A is the surface area (cm2) of LCS. The surface area of the LCS can be calculated using the following equation:
The inhibition efficiency (IE %) and surface coverage (θ) values were calculated with the help of the following equations:
where \({C}_{\mathrm{R}}^{0}\) and \({C}_{\mathrm{R}}^{\mathrm{i}}\) are the corrosion rate of LCS in the absence and presence of inhibitor, respectively. θ is the surface coverage value. In Eq. (2), D and l are the diameters and the thickness of the specimen, respectively, and d is the diameter of the hole for holding.
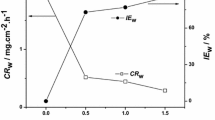
The results are presented in Table 2. Corrosion rate decreases with the increasing inhibitor concentration. In the absence of inhibitor, the highest corrosion rate (11.33 mmy−1) was observed, while in the presence of inhibitor (600 mg/L), the lowest corrosion rate (1.06 mmy−1) was observed. The highest value of inhibition efficiency achieved is 90.63% at 600 mg/L inhibitor concentration. The Gravimetric measurement values are used to plot the Langmuir adsorption isotherm. The Langmuir adsorption isotherm is a plot of C/θ vs C as represented in Fig. 2.
Adsorption of inhibitor molecules on the LCS surface by the Langmuir adsorption isotherm is provided by using the following equations:
where \({K}_{\mathrm{a}\mathrm{d}\mathrm{s}}\) is the equilibrium adsorption constant, C is the inhibitor concentration and θ is the surface coverage.
The slope and regression coefficient (R2) are near to 1 (Fig. 2), which indicates strong adsorption and formation of a monolayer coating on the LCS surface. Considering our previous investigation, Myristica fragrans shows \({K}_{\mathrm{a}\mathrm{d}\mathrm{s}}\) (9.95 Lg−1) with 83.27% IE at 500 mg/L, while in the present study the higher value of \({K}_{\mathrm{a}\mathrm{d}\mathrm{s}}\) (16.12 Lg−1) indicates better adsorption of the inhibitor molecule on the LCS surface with 90.63% IE at 600 mg/L.
3.2 UV–Visible Spectroscopy Analysis
To verify the adsorption phenomenon, UV–Visible spectroscopy technique has been used. UV–Vis analysis has been done in two modes, before and after the corrosion test. Figure 3 shows the UV–Vis spectra of inhibitor. In this figure, two spectra have been shown one for UV–Vis spectra before the corrosion test and the second one is after the corrosion test. Before the corrosion test, inhibitor shows two adsorption peaks at 269.53 nm and 291.02 nm. These peaks can be assigned as π–π* and n–π* transitions. With the help of Fig. 3, we conclude that the graph shows a low adsorption peak after the corrosion test. In the comparison between both the spectra, a significant change has been found in the adsorption band because of the inhibitor molecules get adsorbed on the LCS surface [37].
3.3 FT-IR and 1H NMR Studies
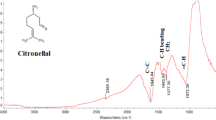
Fourier-transform infrared (FT-IR) spectroscopy technique has been carried out to investigate the presence of the various functional groups present in the phytochemical compounds. Figure 4 indicates the FT-IR spectrum of the pure leave’s extract of E. globulus. It was observed that the peak at 3382.36 cm−1 is due to O–H stretching of alcohol. The further peak at 2932.54 cm−1 due to the C–H stretching and the peak at 1071.98 cm−1 indicate C–O stretching.
For a deeper study of the adsorption phenomenon, 1H NMR of the pure extract was carried out: 1H NMR (CDCl3, 400 MHz/ppm), δ = 5.36 (–OH, phenolic), δ = 7.28 (–CH2, aromatic), δ = 3.68 (–O–CH2) and δ = 1.27 (–CH3). The presence of almost all the characteristic peaks is similar to the previously reported results [33, 34], which suggested the presence of reported major phytochemical constituents in the E. globulus extract. The phytochemicals containing heteroatoms and unsaturation has the polar function, so can be adsorbed on the metal surface as a protective layer. The adsorption of the polar atoms on the surface of the metal may occur through coordination between the lone pair or π-electron cloud and the metallic surface [38].
3.4 Potentiodynamic Polarization Measurements
The concentration effect of the E. globulus extract on the polarization character for LCS in 0.5 M H2SO4 at 298 K was analysed. The Tafel plots were recorded at different inhibitor concentration, which is represented in Fig. 5.
Surface coverage values increase with increasing inhibitor concentration. From Tafel plots, we got the values of the anodic and cathodic Tafel slopes (\({\beta }_{\mathrm{a}}\) and \({\beta }_{\mathrm{c}}\)), corrosion current density (\({i}_{\mathrm{c}\mathrm{o}\mathrm{r}\mathrm{r}}\)), corrosion potential (\({E}_{\mathrm{c}\mathrm{o}\mathrm{r}\mathrm{r}}\)) and corrosion restraint effectiveness (IE %) with the help of the following equation [39]:
where \({i}_{\mathrm{c}\mathrm{o}\mathrm{r}\mathrm{r}}^{\mathrm{i}}\) and \({i}_{\mathrm{c}\mathrm{o}\mathrm{r}\mathrm{r}}^{\mathrm{o}}\) represent the corrosion current density values with and without inhibitor, respectively. With the increase in inhibitor concentration, the polarization resistance (\({R}_{\mathrm{P}}\)) increases. These polarization resistance values were calculated from the slope of the potential–current plot,
where \({\beta }_{\mathrm{c}}\) and \({\beta }_{\mathrm{a}}\) are the cathodic and anodic Tafel slopes and \({i}_{\mathrm{c}\mathrm{o}\mathrm{r}\mathrm{r}}\) is the corrosion current density.
Table 3 shows the polarization parameters for LCS in 0.5 M H2SO4 without and with different concentrations of E. globulus extract at 298 K. As per Fig. 5, each anodic metal dissolution and cathodic hydrogen development response were inhibited, once the inhibitors were added to the corrosive medium. The values of \({E}_{\mathrm{c}\mathrm{o}\mathrm{r}\mathrm{r}}\) are within 85 mV with respect to the blank, which suggests that the studied inhibitor acts as a mixed type of corrosion inhibitor [40]. It should be noted that the addition of each concentration causes a remarkable decrease in anodic (\({\beta }_{\mathrm{a}})\) and cathodic (\({\beta }_{\mathrm{c}})\) Tafel slopes. But the higher variation in anodic Tafel slopes (\({\beta }_{\mathrm{a}})\) as compared to cathodic Tafel slopes (\({\beta }_{\mathrm{c}})\) indicates its anodic way of reaction. The values in Table 3 depict that, with the increase in E. globulus concentration, the corrosion current density decreases. It is already known that the adsorptive inhibitors hinder the release of hydrogen gas on the LCS surface and/or reduce the dissolution process of LCS into the aggressive solution by blocking the active sites on LCS surface or even can mechanically screen the covered part of the electrode and therefore protect it from the action of acid solution. Following this way, the inhibition efficiency is expected to increase with the increase in inhibitor concentration. As per our previous investigation, the M. fragrans reports the value of \({i}_{\mathrm{c}\mathrm{o}\mathrm{r}\mathrm{r}}\) (112.00 µA cm−2) with 87.42% IE at 500 mg/L, while in the present study, the lowest value of \({i}_{\mathrm{c}\mathrm{o}\mathrm{r}\mathrm{r}}\) (76.60 µA cm−2) indicates a better corrosion inhibition with 91.40% IE at 600 mg/L.
3.5 Electrochemical Impedance Spectroscopy (EIS) Measurements
EIS measurements were performed to study the impedance parameters of LCS in 0.5 M H2SO4 at various concentrations of E. globulus at 298 K. Before each test, the working electrode was immersed in the test solution for 1 h at 298 K to attain the steady-state potential. It is clear from Fig. 6 that \({E}_{\mathrm{O}\mathrm{C}\mathrm{P}}\) remains almost constant after 1 h of immersion, which indicates a steady state. The temperature is maintained constant (298 K) for all the tests. To analyse the impedance parameters from the experimental outcomes, the data were fitted to the electrical equivalent circuit. Figure 7a shows the used electrical equivalent circuit in the appearance and nonappearance of the inhibitor. In the equivalent circuit, \({R}_{\mathrm{s}}\) is the solution resistance, \({R}_{\mathrm{c}\mathrm{t}}\) is the charge exchange resistance, and \({C}_{\mathrm{d}\mathrm{l}}\) is the double-layer capacitance. The results are reported in Table 4.
The EIS curves (Nyquist and Bode plots) for LCS with and without E. globulus extract are shown in Fig. 7b, c. The inhibition efficiency based on impedance study can be calculated by using the following equation:
where \({R}_{\mathrm{c}\mathrm{t}}\) and \({R}_{\mathrm{c}\mathrm{t}}^{0}\) are the charge transfer resistance with and without the inhibitor, respectively.
As evident from Table 4, the values of \({C}_{\mathrm{d}\mathrm{l}}\) have decreased and \({R}_{\mathrm{c}\mathrm{t}}\) have increased by the increase in the inhibitor concentration. This may be attributed to the decreased in local dielectric constant and/or to the increase in the thickness of the \({C}_{\mathrm{d}\mathrm{l}}\). The dissolution mechanism could be predicted by the values of the phase shift (n) as an indicator. It is clear that a slight change in the value of n is observed after addition of various inhibitor concentrations. The almost invariable values of n indicate that the charge transfer process controls the dissolution mechanism in both the absence and the presence of various inhibitor concentrations. The inhibition efficiency was found to be improved with the increase in the concentration of E. globulus extract. The maximum inhibition efficiency was achieved at 600 mg/L inhibitor concentration. The molecules present in the plant extract can be adsorbed on the surface of the LCS by the mechanism of donating π-electrons of the aromatic rings or lone pair of electrons of the heteroatoms to the vacant orbital of iron [41,42,43,44]. These compounds can form a protective layer to protect the LCS surface from the corrosive medium. Although the graphs for the uninhibited and inhibited systems are similar, probably due to the similar mechanism of corrosion, the influence of the inhibit on the corrosion process is obvious. The effect, which is concentration-dependent, is manifested in the larger diameter of the capacitive loops, the displacement of the impedance and the phase angle to larger values in the extract-inhibited systems relative to the blank. This could be possible due to the obstruction of reaction points on the metal surface by adsorbed inhibitor molecules.
The results clearly demonstrate that E. globulus extract inhibits the corrosion of LCS at any concentration, and with the increase in the inhibitor concentration, inhibition effectiveness increases [45, 46]. As per our previous investigation, the M. fragrans reports the value of \({R}_{\mathrm{c}\mathrm{t}}\) (128.88 Ω cm2) with 87.81% IE at 500 mg/L, while in the present study, the highest value of \({R}_{\mathrm{c}\mathrm{t}}\) (227.48 Ω cm2) confirms the better corrosion inhibition with 93.09% IE at 600 mg/L.
3.6 Theoretical Studies
3.6.1 Quantum Chemical Calculations
Plant extracts have lots of phytochemical components. There are three main components of E. globulus selected for the theoretical study. E. globulus contains eucalyptol, globulusin-A and globulusin-B as the main phytochemical components. Figure 8 shows the optimized, HOMO and LUMO orbitals of the eucalyptol, globulusin-A and globulusin-B molecules. Following equations have been used to calculate different quantum chemical parameters [47]:
where \(\chi_{{{\text{inh}}}}\) and \(\chi_{{{\text{Fe}}}}\) demonstrate the electronegativity of inhibitor molecule and iron, respectively. \(\eta_{{{\text{inh}}}}\) and \(\eta_{{{\text{Fe}}}}\) means the hardness of the inhibitor molecule and iron, respectively. A value of 7 eV/mol has been taken for the \(\chi_{{{\text{Fe}}}}\), whereas a value of 0 eV/mol has been taken for the\({ \upeta }_{\mathrm{F}\mathrm{e}}\), as per the Pearson’s electronegativity scale. η is the softness value of the inhibitor molecule and σ is the chemical hardness of the inhibitor molecule. \({\Delta E}_{\mathrm{B}\mathrm{a}\mathrm{c}\mathrm{k} \mathrm{D}\mathrm{o}\mathrm{n}\mathrm{a}\mathrm{t}\mathrm{i}\mathrm{o}\mathrm{n}}\) (Eq. 18) intimates that, at η > 0 and < \({\Delta E}_{\mathrm{B}\mathrm{a}\mathrm{c}\mathrm{k} \mathrm{D}\mathrm{o}\mathrm{n}\mathrm{a}\mathrm{t}\mathrm{i}\mathrm{o}\mathrm{n}}\)0, the charge transfer to a molecule, took after by a back-donation starting with the molecule, is favoured energetically, and π is the chemical potential.
As per Table 5, the high value of EHOMO of eucalyptol (0.93 eV) indicates that the eucalyptol molecule has a fundamentally high affinity of charge donation to the LCS surface. Globulusin-B has the least ELUMO (− 0.41 eV), which shows the highest ability to accept electrons from the d-orbital of Fe. Globulusin-A has the lowest value of ΔE (0.01 eV),s which shows its highest reliability of forming the [Fe-globulusin-A] complex. Moreover, eucalyptol has a rather high value of µ (1.42 D), which indicates strong adsorption of eucalyptol molecule on the LCS surface [48].
3.7 SEM and AFM Analysis
Figure 9 highlights scanning electron microscopy (SEM) and atomic force microscopy (AFM) micrographs of LCS surface prior and afterwards the corrosion tests without and with the inhibitor. Figure 9a shows the SEM and AFM micrographs of abraded LCS surface. It shows a sleek surface with least surface roughness (2.99 nm) and maximum height (60 nm) values. Figure 9b shows the LCS surface after the corrosion test without the inhibitor. In this case, the LCS surface is highly corroded due to the acidic corrosive media, resulting in a rough micrograph with highest surface roughness (138.81 nm) and maximum height (2100 nm) values obtained. Figure 9c shows the LCS surface after the corrosion test with the inhibitor (600 mg/L). It shows the presence of the inhibitor covers the surface and prevents the corrosion process with much lower surface roughness (26.86 nm) and maximum height (300 nm) values. It is very less than the outcomes obtained in the case of without inhibitor. The decrease in height and average surface roughness of the LCS specimen is due to the adsorption of E. globulus inhibitor on the metal surface. It can be said that a protective layer is formed on the surface of the metal. It verifies E. globulus adsorb on the surface of the LCS creating a protective layer to prevent the corrosion process on the LCS surface.
3.8 The Proposed Mechanism of Action
To explain the inhibitory effect of E. globulus on LCS in 0.5 M H2SO4, it is important to evaluate the experimental and calculated results and correlate them with the structural, chemical and electronic properties of the inhibitory molecules. E. globulus contains several phytochemical compounds having a number of heteroatoms and aromatic rings. These compounds act as Lewis bases and form coordination bonds with the free d-orbital of Fe and adsorb on a LCS surface to form a protective layer on the LCS surface to protect against corrosive environments. The HOMO orbitals of the molecules studied indicate that a uniform pair of electrons are available for nucleophilic interaction (chemisorption) with the LCS surface. The LUMO in the heteroatoms causes the formation of dπ–dπ bonds, and the formation of superimposed three-dimensional electrons of iron atoms with a free spatial orbital heteroatom is possible. Also, the adsorption can be done by electrostatic interaction (physisorption) between heteroatoms and the Fe2+. Retro-donation can be done using π-electrons of aromatic rings. This phenomenon is also verified by theoretical studies and polarization measurements. Figure 10 shows the proposed mechanism for the adsorption phenomenon of globulusin B molecule adsorbed on a LCS surface.
4 Conclusion
The inhibition effect and adsorption behaviour of the leaves extract of E. globulus have been investigated using weight loss measurements, electrochemical studies, SEM and AFM surface investigations, FT-IR, 1H NMR and quantum chemical calculations. The conclusions of these investigations are as follows:
E. globulus leave’s extract was investigated as a sustainable and green corrosion inhibitor for LCS in 0.5 M H2SO4.
The gravimetric estimations and the electrochemical studies confirmed that the inhibition efficiency of E. globulus extract is > 93% at 600 mg/L.
Verification of various functional groups containing heteroatoms and unsaturation in the phytochemical constituents of the plant extract was done by using FT-IR and 1H NMR techniques.
UV–Visible technique verified the formation of coordination bonds between inhibitor molecules and Fe2+.
The prime phytochemical constituents were simulated using density functional theory (DFT) and the results support the experimental outcomes.
The surface adsorption of E. globulus extract was investigated by using scanning electron microscopy (SEM) supported by atomic force microscopy (AFM) technique.
References
Amin MA, Abd El-Rehim SS, El-Sherbini EEF, Bayoumi RS (2007) The inhibition of low carbon steel corrosion in hydrochloric acid solutions by succinic acid. Part I. Gravimetric measurements, polarization, EIS, PZC, EDX and SEM studies. Electrochim Acta 52:3588–3600
Koch G (2015) Trands in oil and gas corrosion research and technologies. Woodhead Publishing, Cambridge
Ahamad I, Prasad R, Quraishi MA (2010) Experimental and quantum chemical characterization of the adsorption of some Schiff base compounds of phthaloyl thiocarbohydrazide on the mild steel in acid solutions. Mater Chem Phys 124:1155–1165
Abd El-Lateef HM, Abo-Riya MA, Tantawy AH (2016) Empirical and quantum chemical studies on the corrosion inhibition performance of some novel synthesized cationic gemini surfactants on carbon steel pipelines in acid pickling processes. Corros Sci 108:94–110
Bagga MK, Gadi R, Yadav OS, Kumar R, Chopra R, Singh G (2016) Investigation of phytochemical components and corrosion inhibition property of Ficus racemosa stem extract on mild steel in H2SO4 medium. J Environ Chem Eng 4:4699–4707
Hassan KH, Khadom AA, Kurshed NH (2016) Citrus aurantium leaves extracts as a sustainable corrosion inhibitor of mild steel in sulfuric acid. S Afr J Chem Eng 22:1–5
Patel N, Rawat A, Jauhari S, Mehta G (2010) Inhibitive action on Bridelia restusa leaves extract on corrosion of mild steel in acidic media. Eur J Chem 1:129–133
Ibrahim T, Alayan H, Al Mowaqet Y (2012) The effect of Thyme leaves extract on corrosion of mild steel in HCl. Prog Org Coatings 75:456–462
Al-Otaibi MS et al (2013) The effect of temperature on the corrosion inhibition of mild steel in 1 M HCL solution by Curcuma longa extract. Int J Electrochem Sci 5:847–859
Vasudha VG, Shanmuga Priya K (2013) Polyalthia longifolia as a corrosion inhibitor for mild steel in HCl solution. Res J Chem Sci 3:21–26
Garai S, Garai S, Jaisankar P, Singh JK, Elango A (2012) A comprehensive study on crude methanolic extract of Artemisia pallens ( Asteraceae ) and its active component as effective corrosion inhibitors of mild steel in acid solution. Corros Sci 60:193–204
Rose K, Kim BS, Rajagopal K, Arumugam S, Devarayan K (2016) Surface protection of steel in acid medium by Tabernaemontana divaricata extract: Physicochemical evidence for adsorption of inhibitor. J Mol Liq 214:111–116
Oguzie EE, Chidiebere MA, Oguzie KL, Adindu CB, Momoh-Yahaya H (2014) BioLC-MS extracts for materials protection: Corrosion inhibition of mild steel in acidic media by Terminalia chebula extracts. Chem Eng Commun 201:790–803
Haldhar R, Prasad D, Saxena A, Singh P (2018) Valeriana wallichi root extract as a green & sustainable corrosion inhibitor for mild steel in acidic environments: experimental and theoretical study. Mater Chem Front 2:1225–1237
Saxena A, Prasad D, Haldhar R (2018) Use of Asparagus racemosus extract as green corrosion inhibitor for mild steel in 0.5 M H2SO4. J Mater Sci 53:8523–8535
Saxena A, Prasad D, Haldhar R (2016) Withania somnifera extract as green inhibitor for mild steel in 8 % H2SO4. Asian J Chem 28:2471–2474
Soltani N, Tavakkoli N, Khayatkashani M, Jalali MR, Mosavizade A (2012) Green approach to corrosion inhibition of 304 stainless steel in hydrochloric acid solution by the extract of Salvia officinalis leaves. Corros Sci 62:122–135
Haldhar R, Prasad D, Saxena A (2018) Myristica fragrans extract as an eco-friendly corrosion inhibitor for mild steel in 0.5 M H2SO4. J Environ Chem Eng 6:2290–2301
Rahim A, Rocca E, Steinmetz J, Kassim M, Adnan R, Sani Ibrahim M (2007) Mangrove tannins and their flavanoid monomers as alternative steel corrosion inhibitors in acidic medium. Corros Sci 49:402–417
Deng S, Li X (2012) Inhibition by Jasminum nudiflorum Lindl. leaves extract of the corrosion of aluminium in HCl solution. Corros Sci 64:253–262
Ostovari A, Hoseinieh SM, Peikari M, Shadizadeh SR, Hashemi SJ (2009) Corrosion inhibition of mild steel in 1 M HCl solution by henna extract: A comparative study of the inhibition by henna and its constituents (Lawsone, Gallic acid, alpha-d-Glucose and Tannic acid). Corros Sci 51:1935–1949
Li X, Deng S (2012) Inhibition effect of Dendrocalamus brandisii leaves extract on aluminum in HCl, H3PO4 solutions. Corros Sci 65:299–308
Torres VV, Amado RS, Sa CF, Fernandez TL, Riehl CAS, Torres AG, D'Elia E (2011) Inhibitory action of aqueous coffee ground extracts on the corrosion of carbon steel in HCl solution. Corros Sci 53:2385–2392
Okafor PC, Ikpi ME, Uwah IE, Ebenso EE, Ekpe UJ, Umoren SA (2008) Inhibitory action of Phyllanthus amarus extracts on the corrosion of mild steel in acidic media. Corros Sci 50:2310–2317
Radojčić I, Berković K, Kovač S, Vorkapić-Furač J (2008) Natural honey and black radish juice as tin corrosion inhibitors. Corros Sci 50:1498–1504
Deng S, Li X (2012) Inhibition by Ginkgo leaves extract of the corrosion of steel in HCl and H2SO4 solutions. Corros Sci 55:407–415
Njoku DI, Ukaga I, Ikenna OB, Oguzie EE, Oguzie KL, Ibisi N (2016) Natural products for materials protection: Corrosion protection of aluminium in hydrochloric acid by Kola nitida extract. J Mol Liq 219:417–424
Noor EA (2011) The impact of some factors on the inhibitory action of Radish fruits aqueous extract for mild steel corrosion in 1 M H2SO4 solution. Mater Chem Phys 131:160–169
Haldhar R, Prasad D, Bhardwaj N (2020) Surface adsorption and corrosion resistance performance of Acacia concinna pod extract: An efficient inhibitor for mild steel in acidic enviroment. Arab J Sci Eng 45:131–141
Li X, Deng S, Fu H (2012) Inhibition of the corrosion of steel in HCl, H2SO4 solutions by bamboo leaf extract. Corros Sci 62:163–175
Joseph O, Favomi O, Adenigba O (2017) Effect of Lecaniodiscus cupaniodes extract in corrosion inhibition of normalized and annealed mild steels in 0.5 M HCl. Energy Proc 119:845–851
Azzoui K, Mejdoubi E, Jodeh S (2017) Eco friendly green inhibitor Gum arabic (GA) for the corrosion control of mild steel in hydrochloric acid medium. Corros Sci 129:70–81
Maciel MV et al (2010) Chemical composition of Eucalyptus spp. essential oils and their insecticidal effects on Lutzomyia longipalpis. PhytochemistryPhytochemistry 167:1–7
Hasegawa T, Takano F, Takata T, Niiyama M, Ohta T (2008) Bioactive monoterpene glycosides conjugated with gallic acid from the leaves of Eucalyptus globulus. Phytochemistry 69:747–753
Saxena A, Prasad D, Haldhar R, Singh G, Kumar A (2018) Use of Saraca ashoka extract as green corrosion inhibitor for mild steel in 0.5 M H2SO4. J Mol Liq 258:89–97
Verma C, Quraishi MA, Ebenso EE, Obot IB, El Assyry A (2016) 3-Amino alkylated indoles as corrosion inhibitors for mild steel in 1M HCl: Experimental and theoretical studies. J Mol Liq 219:647–660
Saxena A, Prasad D, Haldhar R, Singh G, Kumar A (2018) Use of Sida cordifolia extract as green corrosion inhibitor for mild steel in 0.5 M H2SO4. J Environ Chem Eng 6:694–700
Kumar R, Yadav OS, Singh G (2017) Electrochemical and surface characterization of a new eco-friendly corrosion inhibitor for mild steel in acidic media: a cumulative study. J Mol Liq 237:413–427
Ahamad I, Quraishi MA (2010) Mebendazole: New and efficient corrosion inhibitor for mild steel in acid medium. Corros Sci 52:651–656
Daoud D, Douadi T, Hamani H, Chafaa S, Al-Noaimi M (2015) Corrosion inhibition of mild steel by two new S-heterocyclic compounds in 1 M HCl: Experimental and computational study. Corros Sci 94:21–37
Haldhar R, Prasad D, Saxena A, Kumar R (2018) Experimental and theoretical studies of Ficus religiosa as green corrosion inhibitor for mild steel in 0.5 M H2SO4 solution. Sustain Chem Pharm 9:95–105
Bhardwaj N, Prasad D, Haldhar R (2018) Study of Aegle marmelos as a green corrosion inhibitor for mild steel in acidic medium: Experimental and theoretical approach. J Bio-Tribo-Corros 4:1–10
Ashassi-Sorkhabi H, Asghari E (2008) Effect of hydrodynamic conditions on the inhibition performance of l-methionine as a ‘green’ inhibitor. Electrochim Acta 54:162–167
Zheng X, Zhang S, Li W, Yin L, He J, Wu J (2014) Investigation of 1-butyl-3-methyl-1H-benzimidazolium iodide as inhibitor for mild steel in sulfuric acid solution. Corros Sci 80:383–392
Mahdavian M, Attar MM (2009) Electrochemical behaviour of some transition metal acetylacetonate complexes as corrosion inhibitors for mild steel. Corros Sci 51:409–414
Hejazi S et al (2015) Electrochemical and quantum chemical study of Thiazolo-pyrimidine derivatives as corrosion inhibitors on mild steel in 1M H2SO4. J Ind Eng Chem 25:112–121
Salarvand Z, Amirnasr M, Talebian M, Raeissi K, Meghdadi S (2017) Enhanced corrosion resistance of mild steel in 1M HCl solution by trace amount of 2-phenyl-benzothiazole derivatives: Experimental, quantum chemical calculations and molecular dynamics (MD) simulation studies. Corros Sci 114:133–145
Morad MS (2000) An electrochemical study on the inhibiting action of some organic phosphonium compounds on the corrosion of mild steel in aerated acid solutions. Corros Sci 42:1307–1326
Acknowledgements
We are thankful to Prof. Gurmeet Singh, Department of Chemistry, University of Delhi, Delhi-110007, INDIA, for providing the lab facility for the research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Haldhar, R., Prasad, D. Corrosion Resistance and Surface Protective Performance of Waste Material of Eucalyptus globulus for Low Carbon Steel. J Bio Tribo Corros 6, 48 (2020). https://doi.org/10.1007/s40735-020-00340-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-020-00340-3