Abstract
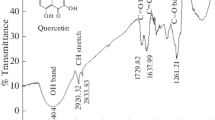
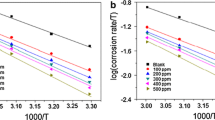
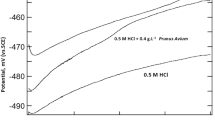
The adsorption mechanism and inhibitive action of the Eucalyptus plant leaf extract (Eu) on the corrosion of mild steel in 0.5 M H2SO4 and 0.5 M H3PO4 solutions were investigated by potentiodynamic polarization curves measurements and electrochemical impedance spectroscopy technique. Potentiodynamic polarization curves revealed that the Eucalyptus leaf extract acts as a mixed type inhibitor in both acidic solutions. The impedance responses indicated that the corrosion process occurs under activation control. Fourier transform infrared spectroscopy has been used to predict the possible major chemical constituent of the leaf extract. Four adsorption isotherms including Langmuir, kinetic–thermodynamic, Flory–Huggins and Temkin model were used to investigate the mode of inhibition of Eucalyptus leaf extract. The free energy of adsorption showed that the corrosion inhibition takes place by spontaneous physical adsorption of Eucalyptus leaf extract molecules on the mild steel surface. The obtained data indicated that Eucalyptus leaf extract is a more efficient inhibitor of mild steel corrosion in 0.5 M H2SO4 than in 0.5 M H3PO4 solutions. Thermodynamics activation parameters were also calculated and discussed.











Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.References
Khaled RY, Abdel-Gaber AM (2017) A comparative study of corrosion inhibition of steel and stainless steel in hydrochloric acid by N, N, N′, N′-tetramethyl-pphenylenediamine. Prot Met Phys Chem 53:956–960
Rahal HT, Abdel-Gaber AM, Younes GO (2016) Inhibition of steel corrosion in nitric acid by sulfur containing compounds. Chem Eng Commun 203:435–445
El Sayed MY, Abdel-Gaber AM, Rahal HT (2019) Safranin—a potential corrosion inhibitor for mild steel in acidic media: a combined experimental and theoretical approach. J Fail Anal Prev 19:1174–1180
Abdel-Gaber AM, Awad R, Rahal HT, Moussa D (2019) Electrochemical behavior of composite nanoparticles on the corrosion of mild steel in different media. J Bio Tribol Corros 5:49
Al-Moghrabi RS, Abdel-Gaber AM, Rahal HT (2019) Corrosion inhibition of mild steel in hydrochloric and nitric acid solutions using willow leaf extract. Prot Met Phys Chem 55:603–607
Saeed MT, Saleem M, Usmani S, Malik IA, Al-Shammari FA, Deen KM (2019) Corrosion inhibition of mild steel in 1 M HCl by sweet melon peel extract. J King Saud Univ-Sci (in press)
Nahle A, El-Hajjaji F, Ghazoui A, Benchat NE, Taleb M, Saddik R, Hammouti B (2018) Effect of substituted methyl group by phenyl group in pyridazine ring on the corrosion inhibition of mild steel in 1.0 M HCl. Anti-Corros Method 65:87–96
Okafor PC, Uwah IE, Ekerenam OO, Ekpe UJ (2009) Combretum bracteosum extracts as eco-friendly corrosion inhibitor for mild steel in acidic medium. Pigm Resin Technol 38:236–241
Saad IR, Abdel-Gaber AM, Younes GO, Nsouli B (2018) Thiourea and N-methylthiourea as corrosion inhibitors for steel in phosphoric acid. JFAP 18:1293–1299
Alaoui LM, Hammouti B, Bellaouchou A, Benbachir A, Guenbour A, Kertit S (2011) Corrosion inhibition and adsorption properties of 3-amino-1, 2, 3-triazole on mild steel in H3PO4. Der Pharma Chem 3:353–360
Laabaissi T, Lgaz H, Oudda H, Benhiba F, Zarrok H, Zarrouk A, Touir R (2017) Comparative study of corrosion inhibition effect of benzodiazepine derivative on the carbon steel surface in 2.0 M H3PO4 and 1.0 M HCl mediums: electrochemical, theoretical and Monte Carlo simulations studies. JMES 8:1054–1067
Abdel-Gaber AM, Abd-El-Nabey BA, Saadawy M (2009) The role of acid anion on the inhibition of the acidic corrosion of steel by lupine extract. Corros Sci 51:1038–1042
Abdel-Gaber AM, Abd-El-Nabey BA, Sidahmed IM, El-Zayady AM, Saadawy M (2006) Inhibitive action of some plant extracts on the corrosion of steel in acidic media. Corros Sci 48:2765–2779
Abdel-Gaber AM, Hijazi KM, Younes GO, Nsouli B (2017) Comparative study of the inhibitive action between the bitter orange leaf extract and its chemical constituent linalool on the mild steel corrosion in HCl. Quim Nova 40:395–401
Abd-El-Khalek DE, Abdel-Gaber AM (2012) Evaluation of nicotiana leaves extract as corrosion inhibitor for steel in acidic and neutral chloride solutions. Port Electrochim Acta 30:247–259
Abd-El-Nabey BA, Abdel-Gaber AM, Elawady GY, El-Houssein S (2012) Inhibitive action of some plant extracts on the alkaline corrosion of aluminum. Int J Electrochem Sci 7:7823–7839
Abd-El-Naby BA, Abdullatef OA, Abd-El-Gabr AM, Shaker MA, Esmail G (2012) Effect of some natural extracts on the corrosion of zinc in 0.5 M NaCl. Int J Electrochem Sci 7:5864–5879
Al-Moghrabi RS, Abdel-Gaber AM, Rahal HT (2018) A comparative study on the inhibitive effect of Crataegus oxyacantha and Prunus avium plant leaf extracts on the corrosion of mild steel in hydrochloric acid solution. IJIC 9:255–263
Hijazi KM, Abdel-Garber AM, Younes GO (2015) Electrochemical corrosion behavior of mild steel in HCl and H2SO4 solutions in presence of loquat leaf extract. Int J Electrochem Sci 10:4366–4380
Akalezi CO, Onyedika GO, Chahul HF, Oguzie EE (2016) Experimental and theoretical studies on the corrosion inhibition of mild steel in acidic media by Pentaclethra macrophylla plant extract. FUTOJNLS 2:265–280
Kalaiselvi K, Chung IM, Kim SH, Prabakaran M (2018) Corrosion resistance of mild steel in sulphuric acid solution by Coreopsis tinctoria extract: electrochemical and surface studies. Anti-Corros Method 65:408–416
Boumhara K, Harhar H, Tabyaoui M, Bellaouchou A, Guenbour A, Zarrouk A (2019) Corrosion inhibition of mild steel in 0.5 M H2SO4 solution by artemisia herba-alba oil. J Bio Tribol Corros 5:8
Tiwari P, Srivastava M, Mishra R, Ji G, Prakash R (2018) Economic use of waste Musa paradisiaca peels for effective control of mild steel loss in aggressive acid solutions. J Environ Chem Eng 6:4773–4783
Bhuvaneswari TK, Vasantha VS, Jeyaprabha C (2018) Pongamia Pinnata as a green corrosion inhibitor for mild steel in 1 N sulfuric acid medium. Silicon 10:1793–1807
Mourya P, Banerjee S, Singh MM (2014) Corrosion inhibition of mild steel in acidic solution by Tagetes erecta (Marigold flower) extract as a green inhibitor. Corros Sci 85:352–363
Haldhar R, Prasad D, Bhardwaj N (2019) Extraction and experimental studies of Citrus aurantifolia as an economical and green corrosion inhibitor for mild steel in acidic media. J Adhes Sci Technol 33:1169–1183
Ikeuba AI, Okafor PC (2019) Green corrosion protection for mild steel in acidic media: saponins and crude extracts of Gongronema latifolium. Pigm Resin Technol 48:57–64
Mohammed RA, AL-mammar DE (2019) Using natural materials as corrosion inhibitors for carbon-steel on phosphoric acid medium. IJIS 60:40–45
Victoria SN, Prasad R, Manivannan R (2015) Psidium guajava leaf extract as green corrosion inhibitor for mild steel in phosphoric acid. Int J Electrochem Sci 10:2220–2238
Ghareeb MA, Habib MR, Mossalem HS, Abdel-Aziz MS (2018) Phytochemical analysis of Eucalyptus camaldulensis leaves extracts and testing its antimicrobial and schistosomicidal activities. Bull Natl Res Cent 42:16
Tezeghdenti M, Dhouibi L, Etteyeb N (2018) Corrosion inhibition of carbon steel in 1 M sulphuric acid solution by extract of Eucalyptus globulus leaves cultivated in tunisia arid zones. J Bio Tribol Corros 1:16
Gualdrón AF, Becerra EN, Peña DY, Gutiérrez JC, Becerra HQ (2013) Inhibitory effect of Eucalyptus and Lippia Alba essential oils on the corrosion of mild steel in hydrochloric acid. JMES 4:143–158
Abdel-Gaber AM (2007) Effect of immersion time and temperature on the inhibition of the acid corrosion of zinc by fenugreek seeds extract. IJAC 3:161–174
Shams El Din AM, Mohammed RA, Haggag HH (1997) Corrosion inhibition by molybdate/polymaliate mixtures. Desal 114:85–95
Abdel-Gaber AM, Masoud MS, Khalil EA, Shehata EE (2009) Electrochemical study on the effect of Schiff base and its cobalt complex on the acid corrosion of steel. Corros Sci 51:3021–3024
Keddam M, Mattos OR, Takenouti H (1981) Reaction model for iron dissolution studied by electrode impedance II: determination of the reaction model. J Electrochem Soc 128:266–274
Epelboin I, Gabrielli C, Keddam M, Takenouti H (1981) The study of the passivation process by the electrode impedance analysis. Compr Treatise Electrochem 4:151–192
Epelboin I, Keddam M (1972) Kinetics of formation of primary and secondary passivity in sulphuric aqueous media. Electrochim Acta 17:177–186
Sherif EM, Park SM (2006) Effects of 1,4-naphthoquinone on aluminum corrosion in 0.50 M sodium chloride solutions. Electrochim Acta 51:1313–1321
Rahal HT, Abdel-Gaber AM, Awad R, Abdel-Naby BA (2018) Influence of nitrogen immersion and NiO nanoparticles on the electrochemical behavior of (Bi, Pb)-2223 superconductor in sodium sulfate solution. Anti-Corros Method 65:430–435
Rahal HT, Abdel-Gaber AM, Awad R (2017) Corrosion behavior of a superconductor with different SnO2 nanoparticles in simulated seawater solution. Chem Eng Commun 204:348–355
Nwabanne JT, Okafor VN (2012) Adsorption and thermodynamics study of the inhibition of corrosion of mild steel in H2SO4 medium using vernonia amygdalina. JMMCE 11:885–890
Gerengi H, Slepski P, Bereket G (2013) Dynamic electrochemical impedance spectroscopy and polarization studies to evaluate the inhibition effect of benzotriazole on copper-manganese-aluminium alloy in artificial seawater. Mater Corros 64:1024–1031
Abdel-Gaber AM, Khalil N, Abou El-Fetouh A (2003) The dissolution mechanism of steel in inorganic acids. Anti-Corros Method 50:442–447
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I, solid. J Am Chem Soc 38:2221–2295
El-Awady AA, Abd-El-Nabey BA, Aziz SG (1992) Kinetic-thermodynamic and adsorption isotherms analyses for the inhibition of the acid corrosion of steel by cyclic and open-chain amines. J Electrochem Soc 139:2149–2154
Florry PJ (1942) Thermodynamics of high polymer solutions. J Chem Phys 10:51–61
Temkin MI (1940) Adsorption equilibrium and the kinetics of processes on nonhomogeneous surfaces and in the interaction between adsorbed molecules. J Phys Chem 14:1153
Obot IB, Obi-Egbedi NO, Umoren SA (2009) Adsorption characteristics and corrosion inhibitive properties of clotrimazole for aluminium corrosion in hydrochloric acid. Int J Electrochem Sci 4:863–877
Cohen M (1959) The formation and properties of passive films on iron. Can J Chem 37:286–291
Muralidharan VS, Rajagopalan KS (1979) Kinetics and mechanism of corrosion of iron in phosphoric acid. Corros Sci 19:199–207
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdel-Gaber, A.M., Rahal, H.T. & Beqai, F.T. Eucalyptus leaf extract as a eco-friendly corrosion inhibitor for mild steel in sulfuric and phosphoric acid solutions. Int J Ind Chem 11, 123–132 (2020). https://doi.org/10.1007/s40090-020-00207-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40090-020-00207-z