Abstract
This study reports the performance of Hexamine as a useful corrosion inhibitor on Aluminium alloy in 3.65% NaCl at ambient temperature and a constant pH of 7. The corrosion-protective ability of Hexamine was investigated utilizing potentiodynamic polarization procedures, computational studies and mass loss estimates. The result from the research unveils that Hexamine hinders the corrosion of Aluminium alloy in sodium chloride solution. The inhibition effect was due to the blockage of the active site of the metal surface by the adsorbed molecule of Hexamine thereby forming a thin layer which minimizes the intrusion of chloride ion into the mobile sites of Aluminium alloy, leading to the reduction of corrosion current density. The deteriorations in mass by the inhibited Aluminium alloy were discovered to decrease as the mass of mass concentration of Hexamine increases. The outcome of the experiment indicated that Hexamine offered inhibition performance of 47.1%, which may likely increase as mass concentration increases. Polarization curve confirmed that Hexamine in 3.65% NaCl at ambient temperature behaved as a mixed-type inhibitor, reducing the corrosion rate and increasing the polarization potential. Adsorption of Hexamine molecules on the Aluminium alloy was attested to follow Langmuir adsorption isotherm with correlation regression coefficient (R2) value of 0.8408. The Morphology study via SEM micrograph affirms the adsorption of Hexamine molecules on the surface of the Aluminium alloy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The mechanism of corrosion investigations of aluminium and its alloy are still of essential interest because of their technological value and technical applicability, particularly in the machine, aerodynamics, household devices, packages and a photoelectric material [1]. Aluminium alloys are also predominantly used in marine applications where lighter weight materials and good mechanical properties are required [2]. Marine corrosion occurs with components of machinery and piping that are used in seawater or saline water. Exposure of parts can be constant or occasional. Vessels or ships, pipelines, maritime structures, are common cases of systems that encounter marine corrosion [3]. Degradation of aluminium and aluminium alloys has continued as discussion of several researchers due to their significance in contemporary development and vast industrial applications [4, 5]. Comparatively, aluminium and its alloy display better resistance to corrosion than iron and mild steel on exposure to a caustic environment, which was credited to a thin oxide layer that builds on them [6, 7]. Nevertheless, copious subjects linked to the corrosion of Aluminium and its alloy in a saline environment unveil that they are predisposed to damages by corrosion on exposure to chloride atom [8,9,10,11,12,13]. Consequently, adequate corrosion assuring means should be employed. Example of these practical measures is the application of inhibitors. A number of investigations have been conducted to present inhibitors suitable for diverse destructive media [14,15,16,17], however, some of the tested inhibitors have been found to have an awkward impact on the climate [18]. Thus, it is necessary to examine the makeup of an inhibitor before using. It is worthy of note that, a huge measure of chemical inhibitors have been discovered to exhibit strong inhibitive action amidst moderate toxicity, however, the bulk of them are high-priced compared to natural or organic corrosion inhibitors.
Organic inhibitors like Hexamine have grown in relevance in recent years because of their environmentally friendly nature. Hexamine as an antibiotic finds various engineering applications [19]. Metal corrosion defence by the inhibitive organic compounds is a function of their ability to adsorb on the base metal surface creating barrier layers against corrosive species in the media [20]. Organic inhibitors protective strength in corrosion reduction is a function of numerous determinants such as the quality of the metal surface, the nature of eroding media and the chemical composition of the inhibitor [21, 22]. Those determinants make it imperative for prudent selection of inhibitors for metals. Corrosion inhibition by Hexamine had been widely used. The study of corrosion inhibitory performance of Hexamine on cast iron pipes in aqueous salt solution of 2% NaCl revealed that Hexamine minimized the pipes corrosion, offering maximum corrosion efficiency of 52.9% after 288 h of exposure [23]. Mild steel embedded in concrete with 0.5% of Hexamine after 180 days of exposure was discovered to have been inhibited by 43.52% [24]. The choice of Hexamine is as a result of its inhibitory action which could be linked with various factors such as the structure of its molecules, adsorption ability, distribution and synergy of molecules on the metallic surface [4, 25]. This study examines the inhibitive capacity of Hexamine on the corrosion of Aluminium alloy in seawater 3.65% NaCl solution. The performance of Hexamine on the inhibition of Aluminium alloy was examined applying potentiodynamic polarization methods, mass loss techniques, computational comparisons and SEM micrographs.
2 Experimental Procedures
2.1 Sample Preparation
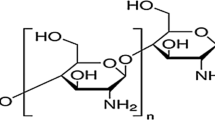
The Aluminium alloy coupons utilized for these studies are of the dimension of (20 × 20 × 2) mm. The synthetic composition in (wt %) is given in Table 1. These coupons were later cleaned with sandpapers of varying degrees and washed with distilled water. All specimens’ mass was registered and specified accordingly. 3.65% NaCl solution was prepared using doubled distilled water which served as the corrosive medium. The mass of the Hexamine whose molecular composition is shown in Fig. 1 was varied in 3.65% NaCl simulated solution for each potentiodynamic polarization analysis conducted. 200 ml of solution was prepared for all the corrosion and mass loss measurement.
Molecular structure of Hexamine [26]
2.2 Potentiodynamic Polarization Experiment
Autolab PGSTAT 101 Metrohm potentiostat including NOVA software of version 2.1.2 was employed for this electrochemical experiment. The Aluminium alloy coupon was fused to cable and installed on a resin. Aluminium alloy served as the practical electrode. A graphite rod was adopted as the counter electrode and silver chloride electrode (SCE) serves as the reference electrode. Potentiodynamic polarization curves were acquired from the potential of − 2.0 V to − 0.5 V vs. open circuit potential at a sweep rate (scan rate) of 0.005 m/s.
The practical electrode (Aluminium alloy) was dipped into the electrolyte (3.65% NaCl solution) for 10 min to accomplish the steady-state potential. The same technique was carried out with four different Aluminium alloy specimens altering the concentration of Hexamine in four different 200 ml of 3.65% NaCl solution and the outcomes were recorded. To ensure reproducibility each experiment was carried out four times. The polarization potential (Ecorr), and current density (jcorr) data were estimated from the Tafel plots. The surface coverage (θ) and the percentage inhibition efficiency (% IE) were computed from Eqs. 1 and 2 [27,28,29,30].
jcorr, inhibited corrosion current densities. \(j_{{{\text{corr}}}}^{{\text{o}}},\) uninhibited corrosion current density.
3 Results and Discussion
3.1 Potentiodynamic Polarization Measurement
Potentiodynamic polarization data of uninhibited and inhibited Aluminium alloy in 3.65% NaCl are shown in Table 2. Their equivalent polarization curves are presented in Fig. 2, implying that polarization had occurred. This was affirmed by the presence of both cathodic and anodic branch [31, 32]. Tafel extrapolation of the current–potential lines was used to obtain jcorr; the corrosion current densities and the corresponding values of corrosion potentials (Ecorr) as presented in Table 2. The values of corrosion rate (Cr) and polarization potential (Pr) were likewise generated from the Tafel curve. Notably, the presence of Hexamine produced a reduction in corrosion rate by pushing the anodic and cathodic polarization curves in the region of the lower values of current densities. The reduction in current density could be attributed to the blockage of active sites of the Aluminium alloy [32]. However, there was a slight disparity in Ecorr values on altering the mass concentration of Hexamine as observed in Fig. 2, confirming that Hexamine is a mixed-type inhibitor in 3.65% NaCl at room temperature [29, 33, 34].
3.2 Open Circuit Potential (OCP) Measurement
The OCP is the potential in a working electrode relative to the electrode in reference while there is no current or potential existing in the cell. The change in the open circuit potential results in polarization. This is due to the flowing current across the electrode/electrolyte interface [35]. Figure 3 presents the open circuit potential (OCP) versus time curves for Aluminium alloy in 3.65% NaCl. Meticulous analysis of the OCP vs. time curves shows that Hexamine moves the steady-state potential toward a more negative path. The contradictory shift of the curves with respect to the uninhibited Aluminium alloy intimates that the cathodic reaction was predominant. More so, there are striking variations in the characteristics of the curved compared to the uninhibited sample. The OCP values for the inhibited samples were between − 0.99V and − 0.86V within the first 5 s. This later moved to less negative values between 0.89 and − 0.85. The OCP vs. time curve for the samples were close to straight line indicating the achievement of steady-state potential [36, 37].
3.3 Mass Loss Measurement and Corrosion Rate
Measurement of mass loss after the electrochemical test was carried out using the OHAUS pioneer TMPA1214 model are presented in Table 3. Mass loss effects show that introduction of Hexamine into the 3.65% NaCl medium minimizes corrosion. The mass loss of Aluminium alloy diminishes slightly as the mass concentration of Hexamine increases. This behaviour is an indication that the mass loss rate had been altered. Figure 4 reveals the effect of Hexamine on the corrosion rate of Aluminium alloy. In accordance with the work of author ref [38], the rate of corrosion reduced as the concentration of Hexamine increases. As presented in Fig. 4 the corrosion rate of the uninhibited Aluminium alloy has the highest corrosion rate.
3.4 Mechanism of Inhibition Efficiency and Adsorption Study
The values of corrosion current (jcorr) in Table 2 were found to reduce with a rise in the concentration of Hexamine, symbolizing the adsorption of Hexamine on the exterior of Aluminium alloy [39]. The adsorption minimizes the effect of the Chloride ion. This is the reason for the increase in corrosion inhibition efficiency of Hexamine as the mass concentration increases. As shown in Fig. 5 the maximum inhibition efficiency was 47.1% for the 1.2 g/l inhibited sample.
An adsorption mechanism necessitated the calculation of C/θ and C for the potentiodynamic polarization system using Langmuir adsorption isotherm and a linear correlation [40,41,42]. Equation 3 shows the Langmuir isothermal adsorption act. This provides an extensive understanding of metal inhibitor synergy and the metallic-complex actions in the coverage region. The Langmuir isothermal plot in Fig. 6 on the surface characteristics presents a linear correlation with the increase in the concentration of the inhibitor revealing the continuous adsorption of the inhibitor on the surface of Aluminium alloy. The value of R2 for Langmuir absorption isothermal was 0.8408. This R2 value is a similar range as those of ref [42, 43]. This revealed that the corrosion protection of Aluminium alloy by Hexamine had been accomplished because R2 is close to unity.
The Langmuir adsorption isothemal law,
C, concentration of the corrosion inhibitor; θ, degree of surface coverage; k adsorption equilibrium constant.
3.5 SEM Micrograph Studies
The SEM micrographs of the Hexamine inhibited samples after the corrosion experiment are shown in Fig. 7a–d. The micrographs show the surface topography and morphological deterioration of the Hexamine inhibited samples as a result of the chemical reaction of the corrosive ion in the chloride medium. Flakes indicating corrosion products such as oxides and hydroxide of Aluminium can be observed [44]. Notably, the flakes on the surface of Fig. 7d were found to be less. The reduction in flake could be as a result of the increase in the mass concentration of Hexamine. Comparatively, minimal breakdown of passive film was noticed with Fig. 7d; however, the thin layer covering formed by Hexamine molecules gives protection against corrosion in all the cases. The combined effort of the heteroatom of Hexamine enables its adsorption on the interface of aluminium via physical and chemical reaction mechanism [45].
The inhibitory action of Hexamine could also be traceable to the availability of four amine groups with lone pair, donor nitrogen atoms in the molecule of Hexamine which adsorb on the surface of Aluminium alloy to resist its corrosion. More so, the presence of methylene group closer to the lone pair donor atom improves the electron density of Nitrogen atom and via inductive effect, strengthens Hexamine [46].
4 Conclusions
-
The corrosion studies of Aluminium alloy were carried out at ambient temperature using 3.65% NaCl and the result shows that Hexamine enhanced the corrosion resistance of the alloy in saline environment. The inhibitory efficiency increases with increase in mass concentration of Hexamine.
-
The adsorption of Hexamine molecule on the Aluminium alloy surface follows Langmuir adsorption isotherm. The correlation regression coefficient of R2 = 0.8408 was obtained in 3.65% NaCl solution. The value of R2 is close to unity which shows that the inhibitor was effectively adsorbed.
-
The potentiodynamic polarization investigations show that Hexamine acted as a mixed-type inhibitor. This was confirmed by the Tafel curves.
-
The morphology study shows the formation of thin film on the surface of Aluminium alloy immersed in 3.65% NaCl in the presence of Hexamine. The adsorption of Hexamine molecules was confirmed by the SEM micrographs
References
Rathod KN, Vashi RT (2016) Inhibition effect of ammonium dichromate on the corrosion of aluminium in phosphoric acid. IJCS 4(1):37–42
Ezuber H, El-Houd A, El-Shawesh F (2008) A study on the corrosion behavior of aluminum alloys in seawater. Mater Design 29(4):801–805
Rosliza R, Nik WW (2010) Improvement of corrosion resistance of AA6061 alloy by tapioca starch in seawater. Curr Appl Phys 10(1):221–229
El-Dahan HA, Soror TY, El-Sherif RM (2005) Studies on the inhibition of aluminum dissolution by hexamine–halide blends: part I. Weight loss, open circuit potential and polarization measurements. Mater Chem Phys 89(2–3):260–267
Fayomi OSI, Akande IG, Popoola API (2018) Corrosion protection effect of chitosan on the performance characteristics of A6063 alloy. J Bio- Tribo-Corros 4(4):73
Fayomi OSI, Abdulwahab M, Popoola AP, Asuke F (2015) Corrosion resistance of AA6063-type Al–Mg–Si alloy by silicon carbide in sodium chloride solution for marine application. J Marine Sci Appl 14(4):459–462
Krishnaveni K, Ravichandran J (2014) Effect of aqueous extract of leaves of Morinda tinctoria on corrosion inhibition of aluminium surface in HCl medium. Trans Nonferrous Metals Soc China 24(8):2704–2712
Halambek J, Berković K, Vorkapić-Furač J (2013) Laurus nobilis L. Oil as green corrosion inhibitor for aluminium and AA5754 aluminium alloy in 3% NaCl solution. Mater Chem Phys 137(3):788–795
Moore KL, Sykes JM, Hogg SC, Grant PS (2008) Pitting corrosion of spray formed Al–Li–Mg alloys. Corros Sci 50(11):3221–3226
Sherif ES (2011) Corrosion and corrosion inhibition of aluminum in Arabian Gulf seawater and sodium chloride solutions by 3-amino-5-mercapto-1,2,4-triazole. Int J Electrochem Sci 6(5):1479–1492
Pyun SI, Na KH, Lee WJ, Park JJ (2000) Effects of sulfate and nitrate ion additives on pit growth of pure aluminum in 0.1 M sodium chloride solution. Corrosion 56(10):1015–1021
Fayomi OSI, Abdulwahab M (2012) Degradation behaviour of aluminium in 2M HCl/HNO3 in the presence of arachis hypogeae natural oil. Int J Electrochem Sci 7:5817–5827
Blücher DB, Svensson JE, Johansson LG (2003) The NaCl-induced atmospheric corrosion of aluminum the influence of carbon dioxide and temperature. J Electrochem Soc 150(3):B93–B98
Umoren SA, Eduok UM (2016) Application of carbohydrate polymers as corrosion inhibitors for metal substrates in different media: a review. Carbohydr Polymers 140:314–341
Ameh PO, Eddy NO (2014) Commiphora pedunculata gum as a green inhibitor for the corrosion of aluminium alloy in 0.1 M HCl. Res Chem Intermed 40(8):2641–2649
Akin M, Nalbantoglu S, Cuhadar O, Uzun D, Saki N (2015) Juglans regia L. Extract as green inhibitor for stainless steel and aluminium in acidic media. Res Chem Intermed 41(2):899–912
Wang Y, Chen Y, Zhao Y, Zhao D, Zhong Y, Qi F, Liu X (2017) A Reinforced organic–inorganic layer generated on surface of aluminium alloy by hybrid inhibitors. J Mol Liq 225:510–516
Winkler DA, Breedon M, White P, Hughes AE, Sapper ED, Cole I (2016) Using high throughput experimental data and in silicon models to discover alternatives to toxic chromate corrosion inhibitors. Corros Sci 106:229–235
Berland K, Hyldgaard P (2010) Structure and binding in crystals of cage like molecules: hexamine and platonic hydrocarbons. J Chem Phys 132(13):134705
Umoren SA, Obot IB, Ebenso EE, Okafor PC, Ogbobe O, Oguzie EE (2006) Gum arabic as a potential corrosion inhibitor for aluminium in alkaline medium and its adsorption characteristics. Anti-Corros Methods Mater 53(5):277–282
Fayomi OSI, Akande IG, Oluwole OO, Daramola D (2018) Effect of water-soluble chitosan on the electrochemical corrosion behaviour of mild steel. Chem Data Collect 17–18:321–326
Elgahawi H, Gobara M, Baraka A, Elthalabawy W (2017) Eco-friendly corrosion inhibition of AA2024 in 3.5% NaCl using the extract of Linum usitatissimum seeds. J Bio- Tribo-Corros 3(4):55
Issa HM, Alshatteri AH (2018) Corrosion prevention of cast iron industrial water pipes: a preliminary comparative study of hexamine and aniline inhibitors. J Garmian Univ 5(2): 67–83
Quraishi MA, Nayak DK, Singh BN, Kumar V, Pandey KK (2016) Experimental studies on effects of sodium citrate, calcium nitrite and hexamine as corrosion inhibitor in concrete. J Steel Struct Construct 2(2):1–5
Vashi RT, Naik D (2010) Hexamine as corrosion inhibitors for zinc in phosphoric acid. J Chem 7(S1):S1–S6
Taghdiri M, Zamani N (2013) Hexamine adsorption study on activated carbon from aqueous solutions for application in treatment of hexamine industrial wastewater. Int J Environ Sci Technol 10(1):19–26
Haque J, Verma C, Srivastava V, Quraishi MA, Ebenso EE (2018) Experimental and quantum chemical studies of functionalized tetrahydropyridines as corrosion inhibitors for mild steel in 1 M hydrochloric acid. Results Phys 9:1481–1493
Zakaria K, Negm NA, Khamis EA, Badr EA (2016) Electrochemical and quantum chemical studies on carbon steel corrosion protection in 1 M H2SO4 using new eco-friendly Schiff base metal complexes. J Taiwan Instit Chem Engineers 61:316–326
Dohare P, Chauhan DS, Hammouti B, Quraishi MA (2017) Experimental and DFT investigation on the corrosion inhibition behavior of expired drug lumerax on mild steel in hydrochloric acid anal. Bioanal Electrochem 9:762
Anejjar A, El Mouden OI, Batah A, Bouskri A, Rjoub A (2018) Corrosion inhibition potential of ascorbic acid on carbon steel in acid media. Appl J Environ Eng Sci 3(1):3–1
Solomon MM, Gerengi H, Kaya T, Umoren SA (2017) Enhanced corrosion inhibition effect of chitosan for St37 in 15% H2SO4 environment by silver nanoparticles. Int J Biol Macromol 104:638–649
Fayomi OSI, Atayero AA, Mubiayi MP, Akande IG, Adewuyi PA, Fajobi MA, Ayara WA (2019) Mechanical and opto-electrical response of embedded smart composite coating produced via electrodeposition technique for embedded system in defence application. J Alloy Compd 773:305–313
Perumal S, Muthumanickam S, Elangovan A, Karthik R, Mothilal KK (2017) Bauhinia tomentosa leaves extract as green corrosion inhibitor for mild steel in 1M HCl medium. J Bio- Tribo-Corros 3(2):13
Abd-El-Nabey BA, Goher YM, Fetouh HA, Karam MS (2015) Anticorrosive properties of chitosan for the acid corrosion of aluminium. Portugaliae Electrochimica Acta 33(4):231–239
Norsworthy R. Understanding corrosion in underground pipelines: basic principles. Underground Pipeline Corros. 2014; 3:34
Gupta RK, Malviya M, Verma C, Quraishi MA (2017) Aminoazobenzene and diaminoazobenzene functionalized graphene oxides as novel class of corrosion inhibitors for mild steel: experimental and DFT studies. Mater Chem Phys 198:360–373
Akande IG, Oluwole OO, Fayomi OSI (2018) Optimizing the defensive characteristics of mild steel via the electrodeposition of Zn–Si3N4 reinforcing particles. Defence Technol. https://doi.org/10.1016/j.dt.2018.11.00
Chitra S, Anand B (2017) Surface morphological and FTIR spectroscopic information on the corrosion inhibition of drugs on mild steel in chloride environment. J Chem Pharm Sci 10:453–456
Niouri W, Zerga B, Sfaira M, Taleb M, Touhami ME, Hammouti B, Mcharfi M, Al-Deyab SS, Benzeid H, Essassi EM (2014) Electrochemical and chemical studies of some benzodiazepine molecules as corrosion inhibitors for mild steel in 1 M HCl. Int J Electrochem Sci 9:8283–8298
Fayomi OSI, Abdulwahab M, Durodola BM, Joshua TO, Alao AO, Joseph OO, Inegbenebor AO (2013) Study of the electrochemical behavior and surface interaction Of AA6063 Type Al–Mg–Si alloy by sodium molybdate in simulated sea water environment. Int J Manag Inf Technol Eng 1(3):159–166
Fayomi OSI (2014) The inhibitory effect and adsorption mechanism of roasted Elaeis guineensis as green inhibitor on the corrosion process of extruded AA6063 Al–Mg–Si alloy in simulated solution. Silicon 6(2):137–143
Verma C, Chauhan DS, Quraishi MA (2017) Drugs as environmentally benign corrosion inhibitors for ferrous and nonferrous materials in acid environment: an overview. J Mater Environ Sci (JMES) 8(11):4040–4051
Hameed RA, Al-Shafey HI, Abu-Nawwas AH (2014) 2-(2,6-Dichloranilino) phenyl acetic acid drugs as eco-friendly corrosion inhibitors for mild steel in 1M HCl. Int J Electrochem Sci 9:6006–6019
Gao B, Zhang X, Sheng Y. Studies on preparing and corrosion inhibition behaviour of quaternized polyethylenemine for low carbon steel in sulphuric acid. Mater Chem Phys 2008;108(2–3): 375–381
Fayomi OSI, Bamgboye OA, Durodola BM, Inam WA, Daniyan AA (2017) Adsorption and corrosion inhibition properties of floxapen compound on the electrochemical characteristics of type-A5-series aluminium in sodium chloride solution. Int J Microstruct Mater Prop 12(5–6):391–401
Kumari M (2017) Use of hexamine as corrosion inhibitor for carbon steel in hydrochloric acid. Int J Adv Educ Res 2(6):224–235
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fayomi, O.S.I., Akande, I.G. Corrosion Mitigation of Aluminium in 3.65% NaCl Medium Using Hexamine. J Bio Tribo Corros 5, 23 (2019). https://doi.org/10.1007/s40735-018-0214-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-018-0214-4