Abstract
The corrosion inhibition behavior of praziquantel for mild steel in 1 M HCl was studied by theoretical and electrochemical measurements. Tafel polarization measurement indicates that the praziquantel inhibitor acts as a mixed type. The corrosion rate decreases with the increasing concentration of inhibitor in 1 M HCl. The effect of inhibition is attributed due to the adsorption of inhibitor molecule on it and obeys the Langmuir adsorption isotherm. Adsorption parameters reveal that the adsorption process is exothermic and spontaneous. The quantum chemical parameters support the result obtained by electrochemical techniques. The surface morphology as SEM images shows the formation of a passive layer over the metal surface is an indication of the reduction in the corrosion rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Mild steel has many structural and industrial applications because of its natural availability, toughness, and most economical viability. This is used under various aggressive conditions such as alkaline, acid and salt solution in industrial applications. The aggressive corrosive environment enhances the corrosion of steel due to the presence of chlorides sulfate and nitrate ions in it. Corrosion process can be controlled by painting, cathodic protection, metal coating or by the use of corrosion inhibitors.
Therefore, the use of corrosion inhibitors is a well-known method to control the corrosion of metal in aggressive corrosive media. Usually, most of the famous inhibitors are organic/inorganic compounds containing nitrogen, sulfur and oxygen atoms [1,2,3,4,5,6]. Hence, the inhibitor molecules get adsorbed on metal surface, which retards the corrosion. This adsorption of corrosion inhibitor depends mainly on physicochemical properties of the molecule such as functional groups, steric factor, molecular size, molecular weight, molecular structure, aromaticity, the electron density of the donor atoms and p-orbital character of donating electrons [7,8,9,10,11]. Along with this, it also depends on the electronic structure of the molecules [12, 13].
The existing inhibitors are toxic. There is a need to develop the eco-friendly inhibitors. Few compounds such as ketosulfone [14], rhodanine azo sulfa drugs [15], metol [16], hydralazine [17], aspirin [18], torsemide and furosemide [19], ciprofloxacin [20], sulfa drugs [21], lamotrigine [22], rhodanine azo sulfa drugs [23] and risperidone [24] are reported to be useful inhibitors for metals.
The present study was focused on the inhibition efficiency of praziquantel for the corrosion of mild steel in 1 M HCl in a temperature range of 303–333 K. Praziquantel molecule is a heterocyclic aromatic compound, which consisting of electron-rich nitrogen, oxygen atoms and π-bonds in its structure might be favorable for its adsorption on the metal surface. Hence, its study as corrosion inhibitors was carried out for mild steel in 1 M HCl solution.
2 Experimental
2.1 Materials and Methods
Mild steel strips with dimensions of 6 cm × 1 cm × 0.1 cm were used for weight loss method, and the same strips with an exposed area of 1 cm2 were used for electrochemical studies. The strips are abraded with emery paper from grade no. 80 up to 2000 and washed thoroughly by using double-distilled water.AR-grade hydrochloric acid and double-distilled water were used to prepare the 1 M HCl corrosive medium for all the experiments.
2.2 Inhibitor
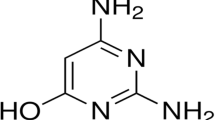
Praziquantel is an anthelmintic veterinary drug, which is effective against flatworms. It is white-colored and soluble in alcohol. IUPAC name of the praziquantel is [(RS)-2-(cyclohexylcarbonyl)-1,2,3,6,7,11b-hexahydro-4H-pyrazino [2,1-a] isoquinolin-4-one]. Praziquantel is first dissolved in 1 cm3 of ethanol and then added into HCl media. The selected molecule is a heterocyclic compound, which contains two oxygen and two nitrogen atoms. Due to the presence of these electroactive elements, it is expected to act as a good inhibitor. The range of concentrations of inhibitor used is from 50 ppm to 250 ppm. The structure of the inhibitor molecule is shown in Fig. 1.
2.3 Weight Loss Measurements
The weight loss analysis is a practical and chemical process of corrosion inhibition study. In this present work, different mild steel strips were taken and passed through a pre-treatment process. The mild steel strips were weighed accurately by digital balance with an accuracy ± 0.1 g. Different mild steel specimens were immersed in a solution with the absence and presence of various inhibitor concentrations for different periods at 303 K. After the immersion process, the mild steel strips were removed and washed using double-distilled water, dried at room temperature and weighed accurately. The weight was recorded, and weight difference before and after the immersion process was calculated. The following expression was used to calculate the corrosion rate.
where W0 and W are the weight of mild steel strip in the absence and presence of inhibitor in 1 M hydrochloric acid, respectively. ν is the corrosion rate, S and T are the surface area of the steel strip and the time of immersion in hours, respectively. The inhibition efficiency (\(\eta_{\text{w}}\)) is calculated as follows,
where \(\nu\) and \(\nu_{i}\) are a weight loss of the mild steel strip in the absence and presence of inhibitor solution, respectively. The corrosion parameters were computed and recorded.
2.4 Electrochemical Tafel Polarization Measurements
An electrochemical reaction can explain kinetics of corrosion process. In electrochemical Tafel polarization analysis, mild steel strip which is in contact with the corrosive medium assumes a potential which refers to corrosion potential (Ecorr) [25]. This corrosion current polarizes both the anodic and cathodic current on the mild steel surface. For this polarization, current can be measured with applied potential at a specified scan rate. In this, corrosion measurement parameter, corrosion current density (icorr) and corrosion rate (CR) are computed by the Tafel plot analysis. The inhibition efficiency can be calculated as follows:
where \(i_{\text{corr}}\) and \(i_{\text{corr}}^{0}\) are the corrosion current density in the presence and absence of the inhibitor concentration from the bulk of the solution, respectively.
2.5 Electrochemical Impedance Spectroscopy (EIS) Measurement
Impedance measurements were done by using AC signals with an amplitude of either scan rate 0.01 mV/s. It was at steady state corrosion potential (Ecorr) in the frequency range of 10 k Hz to 0.1 Hz.
The obtained EIS data were fit into an equivalent circuit by using Z-simp 3.21 software. The EIS parameters such as polarization resistance (RP) and double-layer capacitance (Cdl) were calculated by using the Nyquist plot. The following expression was used to calculate the inhibition efficiency \((\eta_{Z} )\)
where RP and R 0P are the polarization resistance in the presence and absence of inhibitor from the bulk of the solution, respectively.
2.6 Adsorption Isotherm and Thermodynamic Parameters
To learn about the mode of adsorption of praziquantel on mild steel surface in 1 M HCl at different temperatures, attempts were made to fit experimental data with several adsorption isotherms such as Tempkin, Freundlich and Langmuir’s adsorption isotherm. By using these data, thermodynamic parameters were calculated using standard equations.
2.7 Activation Parameters
To study the activation parameters on the corrosion inhibition of mild steel, electrochemical impedance spectroscopy (EIS) measurements were taken at different temperatures from 303 to 333 K in the absence and presence of various concentrations of praziquantel.
2.8 Quantum Chemical Studies
Quantum chemical calculations were performed to analyze the adsorption and inhibition mechanism of praziquantel inhibitor on the mild steel surface in the gas phase by using Parametric Method 3 (PM3). These computations were carried out using the Hyperchem 7.5 package program.
2.9 Scanning Electron Microscopic (SEM) Studies
The mild steel strips surface morphology was recorded after immersion in 1 M HCl in the absence and presence of praziquantel for 4 h by using scanning electron microscope (JEOL JSM-840A model).
3 Results and Discussion
3.1 Weight Loss Measurements
The weight loss experiment for the corrosion of mild steel in 1 M HCl in the absence and presence of different concentration of the praziquantel at 303 K is conducted, and corrosion parameters are discussed. The computed ρ and \(\eta_{\text{W}}\) values from the weight loss method are reported in Table 1
Results obtained from weight loss method show that the corrosion rate decreases with praziquantel. From the observation of Table 1, we find the maximum efficiency of 85.71% at 250 ppm concentration of inhibitor. This clearly shows that weight loss decreases significantly with the addition of praziquantel inhibitor. The decrease in the rate of corrosion with an increase in concentration of praziquantel is attributed due to the surface coverage of metal increases as it adsorbs inhibitor molecules [26].
3.2 Electrochemical Tafel Polarization Measurements
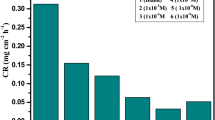
The polarization measurement is carried out to analyze the nature and effect of the inhibitor on the kinetics of the anodic and cathodic reactions [27]. Figure 2 shows the Tafel polarization plots of mild steel in the absence and presence of various concentrations of praziquantel in 1 M HCl. The computed corrosion parameters from the polarization method are reported in Table 2.
From the close observation of Fig. 2, we find that the addition of praziquantel shifts both the anodic and cathodic polarization curves toward a lower Ecorr value. This phenomenon is attributed to the adsorption of praziquantel on the mild steel surface, which inhibits anodic dissolution of mild steel and cathodic hydrogen liberation reaction. In general, if the displacement of corrosion potential (Ecorr) value is greater than ± 85 mV concerning the corrosion potential of the blank, the inhibitor can be considered either as anodic or cathodic type. In our work, Ecorr value is lesser than ± 85 mV (48 mV), which indicates that the inhibitor acts as a mixed type of inhibition [28]. There is no significant variation in \(\beta_{a}\) and \(\beta_{c}\) values. This confirms that the presence of praziquantel in corrosive media acts as an adsorptive inhibitor for mild steel. It retards both anodic and cathodic reactions by blocking the active corrosion sites on the surface [29, 30].
The observation of Table 2 suggests that the corrosion rate decreases and inhibition efficiency increases with the increase in inhibitor concentration. A maximum value of 80.44% at 250 ppm of praziquantel is observed [31]. The mechanism of inhibition is by the formation of a protective film on the surface of mild steel [32].
3.3 Electrochemical Impedance Spectroscopy (EIS) Measurements
EIS for mild steel in the absence and presence of various concentrations of praziquantel in 1 M HCl are presented as Nyquist plots in Fig. 3. An equivalent circuit model is used to fit the EIS results shown in Fig. 4. EIS parameters such as charge transfer resistance which is equal to polarization resistance (RP), double-layer capacitance (Cdl), surface coverage \((\theta )\) and inhibition efficiency \((\eta_{Z} )\) are calculated and reported in Table 3.
The Nyquist plots (Fig. 3) consist of semicircles with their centers on the real axis. These are described as polarization resistance (RP). The RP value increases with the increase in the inhibitor concentrations. These semicircles are not perfect, and there is frequency dispersion effect due to the roughness and inhomogeneity nature of the working electrode (mild steel). The increase in RP value is an indication of the corrosion inhibition of mild steel due to the formation of protective layer by the adsorption of praziquantel molecule onto the surface of the mild steel. The decreasing in the Cdl values (Table 3) with the addition of inhibitor is attributed due to the decrease in dielectric constant or increase in the electric double layer on metal surface [33]. Inhibition efficiency obtained from EIS method is in good agreement with weight loss and Tafel polarization method and is shown in Fig. 5.
3.4 Adsorption Isotherm and Thermodynamic Parameters
Adhesion of dissolved inhibitor molecules on a metal surface from the inhibited solution is referred to as adsorption. The inhibition effect is attributed to the adsorption of inhibitor molecule on the surface of mild steel as a protective film, which reduces the corrosion rate [34]. The adsorption on the corroding surfaces never reaches the real equilibrium and tends to enter a steady adsorption state. When corrosion rate is sufficiently decreased in the presence of an inhibitor, the steady adsorption state manages to attain quasi-equilibrium state. Hence, it is reasonable to consider quasi-equilibrium adsorption in a thermodynamic way using the appropriate adsorption isotherm. The degree of surface coverage (θ) for inhibitor is obtained from EIS measurement data.
The linear regression coefficient (R2) of Langmuir adsorption isotherm is found more close to unity. Hence, it can be said that the adsorption of praziquantel molecule on mild steel surface in 1 M HCl solution obeys the Langmuir’s adsorption isotherm (Fig. 6). This isotherm assumes that the adsorbed molecule occupies only one site, and it does not interact with other adsorbed species. Table 4 shows the calculated values of \(K_{\text{ads}}\) and \(\Delta G_{\text{ads}}^{0}\).
A plot of ΔG 0ads /T v/s 1000/T is provided in Fig. 7, and the computed ΔH 0ads and ΔS 0ads values are reported in Table 4.
The adsorption of the inhibitor takes place on the steel surface by replacement of water molecules with praziquantel molecules, and it can be explained by the following expression [35],
The value of Kads indicates a strong adsorption ability of praziquantel on the surface of the mild steel. The higher is its value, the higher is the ability of adsorption. This adsorption process can take place in two ways such as physisorption and chemisorption. Physisorption involves an electrostatic interaction of inhibitor molecules with the metal surface. Chemisorption involves a chemical interaction (i.e., the interaction of nonbonding electrons with vacant d-orbital of metal) between the inhibitor molecule and the metal surface [36].
In general, if the value of \(\Delta G_{\text{ads}}^{0}\) is less than − 20 kJ/mol, the adsorption process occurs through physisorption. If the \(\Delta G_{\text{ads}}^{0}\) value is greater than − 40 kJ/mol, it is chemisorption [37]. The negative sign of \(\Delta G_{\text{ads}}^{0}\) indicates the spontaneity of the adsorption (Table 4). In this study, \(\Delta G_{\text{ads}}^{0}\) values are found in a range of − 32.71 to − 34.52 kJ/mol. This shows that the adsorption of praziquantel on mild steel surface at different temperatures (303–333 K) involves both physisorption and chemisorption [38].
Further, the negative sign of \(\Delta H_{\text{ads}}^{0}\) indicates that the adsorption of praziquantel on mild steel surface is exothermic. This exothermic adsorption may involve physisorption, chemisorption or both. In an exothermic process, physisorption can be distinguished from chemisorption by considering the absolute \(\Delta H_{\text{ads}}^{0}\) value. The \(\Delta H_{\text{ads}}^{0}\) value which is lesser than − 40 kJ/mol involves physisorption processes. For chemisorption, this value approaches − 100 kJ/mol. In the present work, ΔH 0ads is − 63 kJ/mol, and it suggests that praziquantel gets adsorbed on the mild steel surface, predominately by chemisorption [39, 40].
The negative value of entropy (∆S 0ads ) indicates that the reaction suffers a loss in the degree of freedom during the complexation process [41]. Also, as the adsorption process is exothermic, it should be accompanied by a decrease in entropy [42].
3.5 Activation Parameters
The temperature plays a vital role in the mechanism of corrosion and its inhibition. The effect of temperature is investigated using Arrhenius and transition state theory. Electrochemical Tafel data are fitted to study the activation parameters.
Figure 8 represents the Arrhenius plot of ln νcorr against 1/T for the mild steel corrosion in 1 M HCl solution in the absence and presence of praziquantel. The computed \(E_{a}^{*}\) and A values are reported in Table 5.
The values of apparent activation energy (\(E_{a}^{*}\)) are higher in inhibited solution than those of the uninhibited solution. Thus, activation parameters mainly control the corrosion rate of mild steel. The increase in apparent activation energy for the mild steel dissolution in inhibited solution may be interpreted as physical adsorption [43]. Szauer and Brand have explained that as the temperature increases, the increase in activation energy can be attributed to an appreciable decrease in the adsorption of the inhibitor on the mild steel surface in 1 M HCl solution [44].
According to the Arrhenius equation, the corrosion rate (υcorr) is affected by both apparent activations energy (\(E_{a}^{*}\)) value and Arrhenius pre-exponential factor (A). The Arrhenius pre-exponential factor (A) in the Arrhenius equation for corrosion process and different reactions are related to the number of active centers. In the present work, \(E_{a}^{*}\) value of the inhibited solution is higher than that of the uninhibited solution. This is an indication that the inhibitor is adsorbed on the active adsorption sites, and corrosion process occurs predominantly on the other active sites of higher energy. Values of \(E_{a}^{*}\) and A obtained in the presence of praziquantel are higher than in the blank acid solution. This means that the presence of praziquantel results in a high number of active centers remaining uncovered with the inhibitor (Fig. 9).
The calculated ΔH* and ΔS* values are listed in Table 5. The positive values of ΔH* indicate that the dissolution reaction is an endothermic process and difficult. The negative value of ΔS* suggests that the formation of the activated complex in the rate-determining step represents an association rather than a dissociation step. Therefore, this shows that the decrease in disorder takes place during the transition from reactants to activated complex [45].
3.6 Quantum Chemical Studies
Quantum chemical calculation deals with molecular orbital energy calculation based on Parametric Method 3(PM3) in Hyperchem 7.5 package program. This review is performed on praziquantel to get its structural and electronic properties and co-relate these features with its inhibition efficiency [46]. According to the Frontier molecular orbital theory, the formation of a transition state is due to an interaction between the frontiers orbitals [HOMO and LUMO].
Figures 10 and 11 show the HOMO and LUMO Frontier energy distribution of the molecule, respectively. The calculated quantum chemical parameters such as the energy of highest occupied molecular orbital (EHOMO), the strength of lowest unoccupied molecular orbital (ELUMO), energy gap in between HOMO and LUMO (ΔEHOMO–LUMO), total energy, binding energy and dipole moment (μ) are reported in Table 6.
According to the Frontier molecular orbital theory, transition states are formed due to the interactions between HOMO and LUMO of the inhibitor molecule. HOMO is directly related to the ionization potential and gives the electron-donating ability of the inhibitor molecule. Higher energy values of HOMO indicate that it provides the π-electrons to the vacant d-orbital of the metal atom. This high value also facilitates the adsorption of the inhibitor on the metal surface. The energy of LUMO is related to electron affinity, and lower ELUMO value indicates the acceptance of electrons by the metal from the inhibitor. Due to these donor–acceptor, electronic interaction takes place to give rise to better adsorption of the inhibitor on the surface of the metal. Large HOMO–LUMO gap implies high stability for the molecule. A decrease in energy gap usually leads to easier polarization of the molecule. The lower ΔE (ELUMO − EHOMO) values give the higher inhibition because the excitation energy gap is more polarizable and is associated with chemical reactivity [47]. The energy required for removing an electron from the last occupied orbital will be low as well. For the praziquantel molecule system, ΔE value is found as − 7.815 eV. This small energy gap of an inhibitor is expected to be a useful corrosion inhibitor.
The binding energy of the praziquantel is found to be a negative value of − 4311 k cal/mol, which suggests that the inhibitor is very stable and less prone to split apart. Hence, the inhibitor gets spontaneously adsorbed on the mild steel surface with higher stability to reduce the corrosion rate.
Dipole moment (μ) is the measure of the polarity of a polar covalent bond. Higher dipole moments increase the adsorption of inhibitors on the mild steel surface, thus improving the inhibition efficiency [48, 49]. The calculated dipole moment (μ) of praziquantel is 1.074.
3.7 Scanning Electron Microscopy (SEM) Analysis
The surface investigation of the mild steel in 1 M HCl solution in the absence and presence of praziquantel by using scanning electron microscopy (SEM) is as shown in Fig. 12. From close observation of Fig. 12, a damaged surface is obtained whenever mild steel is immersed in an uninhibited 1 M HCl solution because of the attack by corrosion. However, in the presence of an inhibitor, the surface is improved markedly regarding smoothness. Usually, a protective film gets coated on the mild steel surface, which reduces the corrosion process considerably.
4 Conclusions
-
Praziquantel acts as an active corrosion inhibitor for the corrosion of mild steel in 1 M HCl solution. Inhibition efficiency values increase with an increase in the inhibitor concentration.
-
Praziquantel acts as a mixed-type inhibitor.
-
Corrosion inhibition mechanism is achieved by adsorption of praziquantel molecule on the mild steel surfaces. Adsorption isotherm obeys the Langmuir model.
-
The decrease in enthalpy and entropy is the driving force for the adsorption of praziquantel on the surface of the mild steel.
-
Quantum chemical parameters strengthen the experimental result of this study.
References
Emregul KC, Atakol (2003) Corrosion inhibition of mild steel with schiff base compounds in 1 M HCl. Mater Chem Phys 82:188–193
Raicheva SN, Aleksiev BV, Sokolova EI (1993) The effect of the chemical structure of some nitrogen- and sulfur-containing organic compounds on their corrosion inhibiting action. Corros Sci 34:343–350
Arab ST, Noor EA (1993) Inhibition of acid corrosion of steel by some S-alkylisothiouronium iodides. Corrosion 49:122–129
El Sayed A (1997) Phenothiazine as inhibitor of the corrosion of cadmium in acidic solutions. J Appl Electrochem 27:193–200
Cheng XL, Ma HY, Chen S, Yu R, Chen X, Yao ZM (1998) Corrosion of stainless steels in acid solutions with organic sulfur-containing compounds. Corros Sci 41:321–333
Abd EL, Rehim SS, Magdy AM, Ibrahim Khaled KF (1999) 4-Aminoantipyrine as an inhibitor of mild steel corrosion in HCl solution. J Appl Electrochem 29:593–599
Khamis E (1990) The Effect of temperature on the acidic dissolution of steel in the presence of inhibitors. Corrosion 46:476–484
Stupnisek Lisac E, Podbrscek S, Soric T (1994) Non-toxic organic zinc corrosion inhibitors in hydrochloric acid. J Appl Electrochem 24:779–784
Schmitt G, Bedbur K (1985) Investigations on structural and electronic effects of acid inhibitors by AC impedance. Werkst Korros 36:273–280
Rosenfeld IL (1981) Corrosion inhibitors. Mc Graw-Hill, New York
Stupnisek-Lisac E, Metikos-Hukovic M (1993) Electrochemical study of substituted pyrroles for protection of iron during pickling Br. Corros J 28:74–76
Granese SL, Rosales BM, Oviedo C, Zerbino JO (1992) The inhibition action of heterocyclic nitrogen organic compounds on Fe and steel in HCl media. Corros Sci 33:1439–1453
Granese SL (1998) Study of the inhibitory action of nitrogen-containing compounds. Corrosion 44:322–329
Prasanna BM, Praveen BM, Hebbar Narayana, Venkatesha TV, Tandon HC (2014) Ketosulfone drug as a green corrosion inhibitor for mild steel in acidic medium. Ind Eng Chem Res 53:8436–8444
Shylesha BS, Venkatesha TV, Praveen BM, Nataraja SE (2012) Acid corrosion inhibition of steel by Lamotrigine. Int Schol Res Netw ISRN Corros 932403:1–8
Praveen BM, Venkatesha TV (2009) Metol as a corrosion inhibitor for steel. Int J Electrochem Sci 4:267–275
Prasanna BM, Praveen BM, Hebbar Narayana, Venkatarangaiah TV (2014) Anticorrosion potential of hydralazine on mild steel in 1 M hydrochloric acid solution. J Fundam Appl Sci 7:222–243
Prasanna BM, Praveen BM, Hebbar Narayana, Venkatesha TV, Tandon HC, Abd Hamid SB (2017) Electrochemical study on the inhibitory effect of Aspirin on mild steel in 1 M hydrochloric acid. J Assoc Arab Univ Basic Appl Sci 22:62–69
Pongsak L, Dusit U, Pakawadee S (2010) Tryptamine as a corrosion inhibitor of mild steel in hydrochloric acid solution. Corros Sci 52:30–36
Kumar SH, Karthikeyan S (2013) Torsemide and Furosemide as green inhibitors for the corrosion of mild steel in hydrochloric acid medium. Ind Eng Chem Res 52:7457–7469
Akpan IA, Offiong NAO (2013) Inhibition of mild steel corrosion in hydrochloric acid solution by Ciprofloxacin drug. Int J Corros 301689:1–5
Abdallah M (2009) Antibacterial drugs as corrosion inhibitors for corrosion of aluminum in hydrochloric solution. Corros Sci 46:1981–1996
Shylesha BS, Venkatesha TV, Praveen BM, Nataraja SE (2012) Acid Corrosion Inhibition of Steel by Lamotrigine. Int Schol Res Netw ISRN Corros 932403:1–8
Nataraja SE, Venkatesha TV, Tandon HC (2012) Computational and experimental evaluation of the acid corrosion inhibition of steel by tacrine. Corros Sci 60:214–223
Princeton, Applied Research, Oak Ridge, TN
Emregul KC, Atakol O (2003) Corrosion inhibition of mild steel with schiff base compounds in 1 M HCl. Mater Chem Phys 82:188–193
Pavithra MK, Venkatesha TV, Punith Kumar KM, Anantha NS (2016) Electrochemical, gravimetric and quantum chemical analysis of mild steel corrosion inhibition by colchicines in 1 M HCl medium. Res Chem Intermed 42:2409–2428
Ayyannan G, Karthikeyan K, Vivekananthan SS, Gopiraman M, Rathinavelu A (2013) Chemical and electrochemical investigations of high carbon steel corrosion inhibition in 10% HCl medium by quinoline chalcones. Ionics 19:919–932
Bobina M, Kellenberger A, Millet J, Muntean C, Vaszilcsin N (2013) Corrosion resistance of carbon steel in weak acid solutions in the presence of l-histidine as a corrosion inhibitor. Corros Sci 69:389–395
Amin MA, Khaled KF, Mohsen Q, Arida HA (2010) A study of the inhibition of iron corrosion in HCl solutions by some amino acids. Corros Sci 52:1684–1695
Safak S, Duran B, Yurt A, Turkoglu G (2012) Schiff bases as a corrosion inhibitor for aluminum in HCl solution. Corros Sci 54:251–259
John S, Joseph A (2012) Electro analytical and theoretical investigations of the corrosion inhibition behavior of bis-1,2,4-triazole precursors EBATT and BBATT on mild steel in 0.1 N HNO3. Ind Eng Chem Res 51:16633–16642
Ivanov ES (1986) Inhibitors for metal corrosion in acidic media. Metallurgy, Moscow
Qu Q, Jiang SA, Bai W, Li L (2007) Effect of ethylenediamine tetraacetic acid disodium on the corrosion of cold rolled steel in the presence of benzotriazole in hydrochloric acid. Electrochim Acta 52:6811–6820
Ferreira ES, Giancomelli C, Giacomelli FC, Spinelli A (2004) Evaluation of the inhibitory effect of L-ascorbic acid on the corrosion of mild steel. Mater Chem Phys 83:129–134
Hebbar N, Praveen BM, Prasanna BM, Venkatesha TV (2014) Inhibition effect of an anti-HIV drug on the corrosion of zinc in acidic medium. Trans. Ind. Inst. Met. 68:543–551
Arukalam IO, Madu IO, Ijomah NT, Ewulonu CM, Onyeagoro GN (2014) Acid corrosion inhibition and adsorption behavior of ethyl hydroxyethyl cellulose on mild steel corrosion. J Mat 101709:1–11
Badiea AM, Mohana KN (2009) Effect of temperature and fluid velocity on corrosion mechanism of low carbon steel in presence of 2- hydrazino-4,7-dimethylbenzothiazole in industrial water medium. Corros Sci 51:2231–2241
Pavithra MK, Venkatesha TV, Punith Kumar MK (2012) Inhibition of mild steel corrosion by Rabeprazole sulfide. Corros Sci 60:104–111
Wankasi D, Tarawou T (2008) Studies on the effect pH on the sorption of Pb(II) and Cu(II) ions from aqueous media by nipa palm (nypa fruit cans wurmb). J Appl Sci Environ Manag 12:87–94
Mu G, Li X, Liu G (2005) Synergistic inhibition between tween 60 and NaCl on the corrosion of cold rolled steel in 0.5 M sulfuric acid. Corros Sci 47:1932–1952
Thomas JM, Thomas WJ (1981) Introduction to the principles of heterogeneous catalysis, Vth edn. Academic Press, London
Obot IB, Obi-Egbedi NO (2011) Anti-corrosive properties of xanthone on mild steel corrosion in sulphuric acid: experimental and theoretical investigations. Curr Appl Phys 11:382–392
Ostovari A, Hoseinieh SM, Peikari M, Hashemi S (2009) Corrosion inhibition of mild steel in 1 M HCl solution by henna extract: a comparative study of the inhibition by henna and its constituents (Lawsone, Gallic acid, α-d-Glucose and Tannic acid). Corros Sci 51:1935–1949
Ghasemi O, Danaee I, Rashed GR, Rashvand Avei M, Maddahy MH (2013) Inhibition effect of a synthesized N, N′-bis(2-hydroxybenzaldehyde)-1,3-propandiimine on corrosion of mild steel in HCl. J Cent South Univ 20:301–311
Obi-Egbedi NO, Obot IB, El-Khaiary MI (2011) Quantum chemical investigation and statistical analysis of the relationship between corrosion inhibition efficiency and molecular structure of xanthenes and its derivatives on mild steel in sulphuric acid. J Mol Struct 86:1–3
Awad MK, Mustafa MR, Elnga MMA (2010) Computational simulation of the molecular structure of some triazoles as inhibitors for the corrosion of metal surface. Theochem 959:1–3
Li X, Deng S, Fu H (2010) Adsorption and inhibition effect of vanillin on cold rolled steel in 3.0 M H3PO4. Prog Org Coat 67:420–426
Khalil N (2003) Quantum chemical approach of corrosion inhibition. Electrochim Acta 48:2635–2640
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Praveen, B.M., Prasanna, B.M., Hebbar, N. et al. Experimental and Theoretical Studies on Inhibition Effect of the Praziquantel on Mild Steel Corrosion in 1 M HCl. J Bio Tribo Corros 4, 21 (2018). https://doi.org/10.1007/s40735-018-0137-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-018-0137-0