Abstract
The corrosion inhibitive effect of 3-(3-oxo-3-phenyl-propenyl)-1H-quinolin-2-one (PPQ) and 3-(3-oxo-3-phenyl-propenyl)-1H-benzoquinolin-2-one (PPBQ) on high carbon steel (HCS) in 10 % HCl media was evaluated by chemical (weight loss) and electrochemical (electrochemical impedance spectroscopy and potentiodynamic polarization technique) measurements. The inhibition efficiencies obtained from weight loss and electrochemical measurements were in good agreement. The inhibition efficiency was found to increase with the increase in inhibitor concentration but decreased with rise in temperature. Potentiodynamic polarization studies revealed the mixed mode inhibition of inhibitors. The adsorption behavior of these inhibitors on the HCS surface was found to obey the Langmuir adsorption isotherm. The thermodynamic parameter values of free energy of adsorption (∆G ads) and enthalpy of adsorption (∆H ads) revealed that the inhibitor was adsorbed on the HCS surface via both chemisorption and physisorption mechanisms. The adsorption mechanism of inhibition was supported by spectroscopic techniques (UV–visible, FT-IR, and wide-angle X-ray diffraction), surface analysis (SEM–EDS), and adsorption isotherms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carbon steel is extensively used as a constructional material in many industries due to its excellent mechanical properties and low cost [1–4]. Certainly, the acid solutions are also widely used in many industries for various applications including chemical cleaning and process, acid pickling of steel and iron, oil well acidification, and the petrochemical processes [5,6]. The corrosion of carbon steel mainly in acidic environments and its inhibition constitutes a complex progressive problem [7]. Therefore, inhibitors are often used in these industrial processes to control the corrosion of metals. Most of the well-known acid corrosion inhibitors are organic compounds containing heteroatoms (nitrogen, sulfur, and oxygen atoms) and aromatic rings [8–12]. Particularly thiourea derivatives [13,14] and benzimidazole derivatives [15,16] have been extensively studied as metal corrosion inhibitors in acid solutions by many authors. However, due to high operational hazard, high cost, and low stability of these organic compounds, there is still a continuous search for better corrosion inhibitors. Hence, investigating and exploring new classes of corrosion inhibitors for the corrosion inhibition of metals in acid solutions is very important.
Generally, organic compounds containing heteroatom can act as good corrosion inhibitors because it can adsorb easily on the surface of steel. According to Adamson et al. [17], adsorption can be described by two main types, namely (1) physisorption (involves electrostatic forces between ionic charges on the adsorbed species and the electric charge at the metal/solution interface) and (2) chemisorption (involves charge sharing or charge transfer from the heteroatom of inhibitor molecules to the metal surface).
In the present study, we introduce quinoline chalcones as a new class of inhibitors to prevent the corrosion of high carbon steel (HCS) in 10 % HCl. In order to reveal the inhibiting nature of quinoline chalcones, chemical and electrochemical measurements were carried out. The choice of these compounds is based on molecular structure considerations, i.e., these are organic compounds having more heteroatoms (nitrogen and oxygen) with lone pairs of electrons and multiple phenyl rings with delocalized π electrons (aromaticity) which can serve as centers for adsorption. These factors favor both the chemical and physical type of interactions of inhibitor molecules with the metal surface. In addition, to study the mechanism of inhibition, UV–visible, FT-IR, WAXD, and SEM–EDS were carried out for the scraped samples.
Experimental
Specimens and electrolytes
HCS specimens having nominal composition of 2.68 % C, 0.36 % Si, 0.24 % Cr, and 96.72 % Fe were used. HSC specimens with dimensions of 3 × 2 × 0.5 cm were used for chemical measurements. Prior to all measurements, the HCS specimens were mechanically abraded with different grades of emery papers and washed with acetone then rinsed by double distilled water. Analytical reagent-grade 37 % hydrochloric acid was purchased from Merck, and all other chemicals were purchased from Aldrich and used as received. The maximum solubility of these inhibitors in 100 mL of 10 % HCl was found to be 400 ppm. The concentration range of inhibitors employed was varied from 50 to 400 ppm and the electrolyte used was 100 mL per each experiment. In order to attain the good solubility of the present inhibitors in 10 % HCl solution, prior to the all measurements, the test solutions (100 mL of 10 % HCl containing different concentrations of inhibitors) were sonicated for 1 h followed by stirring at room temperature for 1 day.
Chemical measurements
After accurately weighing, three parallel HCS specimens were completely immersed in an open beaker containing 100 mL of 10 % HCl with and without different concentrations of inhibitors at different temperatures (300, 310, and 320 K) for 2 h. Then, the HCS specimens were removed, washed with distilled water, dried, and the mass of the HCS specimen was determined using an analytical balance. The experiments were done in triplicate, and the weight loss values were averaged. The inhibition efficiency [E w, (%)] and degree of surface coverage (θ) were calculated using the following equations [18,19]:
where W corr and W corr/inh are the weight loss of the HCS in the absence and presence of inhibitors, respectively.
Electrochemical measurements
Electrochemical measurements were carried out in a conventional three-electrode cylindrical glass cell using a CH electrochemical analyzer Model 604B electrochemical workstation. HCS specimens with an exposed surface area of 1 cm2 were used for these electrochemical measurements. Prior to the measurement, the electrode was polished properly with different grades of emery papers and degreased with acetone then dried in vacuo. A platinum disc was used as a counter-electrode (CE), saturated calomel electrode (SCE) was used as the reference electrode, and HCS was used as the working electrode. The working electrode was immersed in a test solution for 2 h until a steady-state open-circuit potential was obtained. The linear Tafel polarization segments of the anodic and cathodic curves were extrapolated to the corrosion potential to obtain the corrosion current densities (I corr). The anodic and cathodic polarization curves were recorded by a sweep rate 0.5 mV s−1. Inhibition efficiency [E p (%)] was calculated from I corr using the following equation [20]:
where I′ corr and I corr are corrosion current densities in the absence and presence of inhibitors, respectively.
Electrochemical impedance measurements were carried out at the range from 100 kHz to 10 mHz at an amplitude of 10 mV [20]. The impedance diagrams are given in Nyquist representation. The electrical equivalent circuit for the system is shown in Fig. 1. In the given electrical equivalent circuit, R s is the solution resistance, R ct is the charge transfer resistance, and CPE is the constant phase element. Inhibition efficiency [E i (%)] and double-layer capacitance (C dl) were calculated from the charge transfer resistance (R ct) using the following equations [21,22]:
where R ct and R′ct are charge transfer resistance of HCS in the absence and presence of an inhibitor, and f max is the frequency at which the imaginary component of the impedance (−Zmax) is maximal.
FT-IR and UV–visible spectroscopic studies
The HCS specimens were immersed in 100 mL of 10 % HCl solution with the inhibitors at a concentration of 400 ppm for 2 h at room temperature. After termination of the experiment, the HCS specimens were air dried and surface film was scratched carefully with a knife and then the resultant powder (scraped sample) was used to record FT-IR spectra using a PerkinElmer FT-IR spectrophotometer and UV–vis spectra using PG instrument model T90+ spectrophotometer in DMF.
Wide angle X-ray diffractrometer
To confirm the layer formation of the inhibitors on the HCS surface, the scraped samples obtained as described in the above subsection were subjected to wide-angle X-ray diffraction (WAXD) analysis using a Rotaflex RTP300 X-ray diffractometer (Rigaku Co., Tokyo, Japan). The X-ray diffractometer was operated at 50 kV and 200 mA. Nickel-filtered Cu Kα radiation was used for the measurements with an angular range of 5° < 2θ < 75° at room temperature.
Surface analysis
The HCS specimens were immersed in 100 mL of 10 % HCl with the inhibitor concentration of 400 ppm for 2 h. After termination of the experiment, the HCS specimens were washed with double distilled water, dried, and examined for their surface morphology using SEM S-3000N (Hitachi, Japan).
Results and discussion
Synthesis of inhibitors
Quinoline chalcones namely 3-(3-oxo-3-phenyl-propenyl)-1H-quinolin-2-one (PPQ) and 3-(3-oxo-3-phenyl-propenyl)-1H-benzoquinolin-2-one (PPBQ) were prepared according to the method described by Muhammad et al. [23]. The chemical structure and abbreviations of inhibitors (PPQ and PPBQ) are shown in Fig. 2.
PPQ
A solution of 2.0 g of NaOH in 19.0 mL of water and 12.0 mL of ethanol was stirred for 30 min at 10 °C. To the above reaction mixture, the alkaline solution of 12.0 mL of acetophenone was added dropwise with constant stirring for 5 h at room temperature. Then 2-oxo-3-formyl quinoline (0.1 mol) was added to the above reaction mixture with constant stirring for 1 h at 25 °C. The yellow solid of 3-(3-oxo-3-phenyl-propenyl)-1H-quinolin-2-one (PPQ) was separated and washed well with double distilled water. Then the obtained PPQ was recrystallized using a mixture of hexane and ethanol (4:1) (Scheme 1). The yield was 81 %. The purity of the resultant compound was checked by TLC and the structure was confirmed by analytical and spectral (UV–vis and FT-IR) techniques. The elemental analysis for C18H13NO2 is as follows: calc. (found): C, 78.53 (78.51); H, 4.76 (4.79); N, 5.09 (5.7); UV–vis (DMF), nm: λ 238, 362, 396; FT-IR (KBr disc), cm−1: ν N–H 3,025, ν C=O 1,676.
PPBQ
A solution of NaOH (2.0 g) in 19 mL of water and 12 mL of ethanol was stirred for 30 min at 10 °C. Then the alkaline solution of 12 mL of acetophenone was added with constant stirring for 5 h at room temperature. To the above reaction mixture, 2-oxo-3-formyl benzoquinoline (1.0 mol) was added with constant stirring for 1 h at 25 °C. The yellow solid of 3-(3-oxo-3-phenyl-propenyl)-1H-benzoquinolin-2-one (PPBQ) was separated and washed well with double distilled water. Then the obtained PPBQ was recrystallized using a mixture of hexane and ethanol (4:1) (Scheme 2). The yield was 85 %. The purity of the resultant compound was checked by TLC and structure was confirmed by analytical and spectral (UV–vis and FT-IR) techniques. Elemental analysis for C22H15NO2 is as follows: calc. (found): C, 81.21 (81.19); H, 4.65 (4.64); N, 4.30 (4.30); UV–Vis (DMF), nm: λ 237, 364, 410; FT-IR (KBr disk), cm−1: ν N–H 3,045, ν C=O 1,677.
Chemical measurements
Effect of inhibitor concentration
The values of inhibition efficiency [E w (%)], weight loss, and surface coverage (θ) were obtained from weight loss measurements with addition of different concentration of inhibitors (PPQ and PPBQ) after 2 h of immersion in 10 % HCl at 300 K. The data are summarized in Table 1. The inhibition efficiency as functions of concentration (Fig. 3a) and surface coverage (Fig. 3b) are shown in Fig. 3. The anodic dissolution of steel in acidic medium and the corresponding cathodic reaction have been reported in pervious literatures [24,25]:
These reactions give information including the high solubility of the corrosion products and the weight loss of metal (corrosion of metal) in the acid medium. Hence, to find the weight loss of HCS in 10 % HCl solution in the absence and presence of different concentrations of inhibitors, this weight loss measurement was carried out. The calculated weight loss values are presented in Table 1. It can be seen that both inhibitors efficiently inhibit the corrosion of HCS in 10 % HCl solutions. When increasing the inhibitors concentration, inhibition efficiency was increased, which may be due to the increased availability of heteroatoms and aromatic rings present in inhibitors [26]. In addition, the protonated inhibitor species may also assist the interaction of the inhibitors with Clads − ions which are adsorbed on the HCS surface. Inhibitor PPBQ provided the highest inhibition efficiency, whereas inhibitor PPQ provided the lowest inhibition efficiency. The variation in the inhibition efficiency might be due to the substituents and molecular sizes of the inhibitors. Accordingly, for PPBQ, the higher inhibition efficiency was achieved because of the presence of additional aromatic rings [27].
Effect of temperature
The values of inhibition efficiency [E w (%)], weight loss, and surface coverage (θ) were obtained from weight loss measurements at different temperatures (300–320 K). The data are summarized in Table 2. The inhibition efficiency as functions of concentration (Fig. 4a) and surface coverage (Fig. 4b) are shown in Fig. 4.
In Table 2, it can be seen that inhibition efficiency of inhibitors was found to decrease with increasing temperature. In fact, adsorption and desorption of inhibitor molecules continuously occur at the metal surface and an equilibrium exists between two processes at a particular temperature. At higher temperature (320 K), the equilibrium between adsorption and desorption shifted towards desorption until equilibrium is reestablished [28]. Hence, by increasing the temperature, inhibition efficiency was decreased, which may be due to desorption of the weakly adsorbed inhibitor molecules from HCS surface. Hence, the lower inhibition efficiencies were obtained at higher temperatures, which also revealed that the mechanism of adsorption of the inhibitor is physisorption [28].
Electrochemical impedance spectroscopy measurements
Figure 5 shows the Nyquist plots for HCS in 10 % HCl solution in the absence and presence of inhibitors (PPQ and PPBQ) at 300 K, and the electrochemical corrosion kinetic parameters, viz., the charge transfer resistance (R ct) and double-layer capacitance (C dl), were derived from these plots. The data are summarized in Table 3. Additionally, the inhibition efficiency as functions of concentration (Fig. 6a) and surface coverage (Fig. 6b) are shown in Fig. 6. Previously reported [29,30] circuit models (Fig. 1) have been used to fit the experimental results.
As seen from Fig. 5, the Nyquist plots contain a depressed semi-circle, with the center below the real X-axis in which size is increased by increasing the inhibitors (PPQ and PPBQ) concentration, indicating that the corrosion inhibition of HCS in 10 % HCl is mainly a charge transfer process [31]. From Table 3, the values of R ct increased with increasing inhibitor concentration. On the other hand, the values of C dl decreased with increasing inhibitor concentration, which is most probably due to the decrease in local dielectric constant and/or increase in thickness of the electrical double layer, suggesting that the inhibitors (PPQ and PPBQ) act via adsorption at the metal/solution interface [16,32]. Moreover, the value of C dl decreased with increasing inhibitor concentration, which is probably due to the gradual replacement of water molecules by adsorption of inhibitor molecules on the electrode surface, which decreases the extent of the metal dissolution [32].
In addition, Bode plots are also presented in Fig. 7. A new phase angle shift at higher frequency range and a continuous increase in the phase angle shift with increasing concentration of inhibitors were observed. According to Tan et al. [33], this phase angle shift is due to the formation of inhibitor film which changes the electrode interfacial structure. The continuous increase in the phase angle shift is obviously correlated with the progress of surface coverage by the inhibitors.
Potentiodynamic polarization measurements
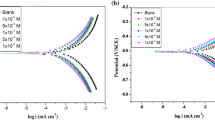
Figure 8 shows the anodic and cathodic polarization curves for HCS in 10 % HCl solution in the absence and presence of inhibitors (PPQ and PPBQ) at 300 K. The electrochemical parameters such as corrosion potential (E corr), corrosion current density (I corr), cathodic Tafel constant (b c), anodic Tafel slope (b a), and inhibition efficiency [E p (%)] were calculated from these curves and data are summarized in Table 4. In addition, the inhibition efficiency as functions of concentration (Fig. 9a) and surface coverage (Fig. 9b) are shown in Fig. 9.
As can be seen from Table 4, the value of I corr decreased with increasing inhibitor concentration reveals the inhibiting nature of the inhibitors [14]. Moreover, in Fig. 6, the anodic (b a) and cathodic Tafel slopes (b c) remain almost unchanged, which indicate that the adsorptive nature of inhibitor molecules is not due to the interference on the reactions of metal dissolution and reduction of protons [34]. Hence, it confirms that the inhibitors (PPQ and PPBQ) act as adsorptive inhibitors, i.e., they decrease anodic dissolution and also retard the hydrogen evolution reaction via blocking the active reaction sites on the HCS surface [34]. Generally, if the displacement in corrosion potential is more than ±85 mV with respect to the corrosion potential of the blank, the inhibitor can be considered as a distinctive cathodic or anodic type. In our study, the displacement was not more than ±85 mV, which suggests mixed type inhibition behavior of the inhibitors and retards corrosion by blocking the active sites of the metal [13]. The minimum inhibition efficiency of 93.2 % was achieved by PPQ and maximum inhibition efficiency 94.9 % was achieved by PPBQ, which correlates well with the chemical and electrochemical impedance measurements.
Adsorption isotherms
Basic information on the interaction between inhibitor molecules and metal surfaces can be provided from the adsorption isotherm [35]. Hence, in order to explain the types of interactions (chemisorption and physisorption) of inhibitor molecules (PPQ and PPBQ) on HCS surface, different adsorption isotherms such as Langmuir, Frumkin, Temkin, and Freundlich were fitted. The adsorption of inhibitor molecule obeys Langmuir adsorption isotherm only.
Generally, adsorption of organic molecules can occur through the interactions by replacement of the adsorbed water from the iron surface:
where “n” is the number of water molecules removed from the metal surface for each molecule of inhibitor adsorbed.
A correlation between surface coverage (θ) and inhibitor concentration (C inh) in the electrolyte can be represented by the Langmuir adsorption isotherm [36]:
where K ads is the adsorption constant.
Surface coverage values (θ) for the inhibitors are presented in Table 4. The best-fit straight line was obtained for the plot of C inh/θ (mol/L) vs. C inh (mol/L) with a slope of around unity. The correlation coefficient (r 2) was used to choose the isotherm type that best fit the experimental data. These plots suggest that the adsorption of inhibitors (PPQ and PPBQ) onto a HCS surface follows the Langmuir adsorption isotherm (Fig. 10). From the intercepts of the straight lines with the C inh/θ axis, the values of K ads were calculated and data are summarized in Table 5. The standard free energy of adsorption (ΔG°ads) and the adsorption constant (K ads) are related by the equation [37]:
where T is temperature and the constant value of 55.5 represents the concentration of water in solution (mol/dm3).
In Table 5, the negative values of ΔG°ads reveal the spontaneity of the adsorption process and stability of the adsorbed layer on the HCS surface. In general, if the magnitude of ∆G°ads is around −20 kJ mol−1 or less negative, it is understood for electrostatic interactions existing between inhibitor and the charged metal surface (i.e., physisorption), whereas if the values are around −40 kJ mol−1 or more negative, then it is a charge transfer type of interaction that exists between inhibitor and the charged metal surface (i.e., chemisorption) [38]. In our study, ∆G°ads was around −28 kJ mol−1 for inhibitors (PPQ and PPBQ), which reveals that the inhibitor molecules adsorb on the HCS surface by both electrostatic (л electrons of aromatic rings) and charge transfer (C=O and –NH) process. The skeletal representation of adsorption of the inhibitors molecule (PPQ and PPBQ) on the HCS surface is shown in Fig. 11.
The other important thermodynamic functions can also be calculated from the following equation:
where ∆H°ads and ∆S°ads are the enthalpy and entropy of adsorption, respectively.
The calculated values of free energy of adsorption (∆G°ads) were plotted against temperature; straight lines were obtained as shown in Fig. 12. It is obvious from the ∆G°ads values at 320 K, on increasing the temperature the mode of interaction is most likely chemisorption. ∆H°ads was found from the graph and the calculated values are given in Table 5. The negative value of ∆H°ads reflects the exothermic nature of the adsorption process on HCS [39]. In addition, the negative values for the entropy of activation are in line with an associatively activated process [34].
FT-IR spectrum
In order to investigate the interactions between inhibitors and HCS surface in 10 % HCl solution, FT-IR spectra were recorded for inhibitors (PPQ and PPBQ) and scraped samples (Fe-PPQ and Fe-PPBQ). In Fig. 13, FT-IR spectra of the inhibitors show C=O stretching peak at 1,676 and 1,677 cm−1 for PPQ and PPBQ, respectively, and N–H stretching peak at 3,025 and 3,045 cm−1 for PPQ and PPBQ, respectively. FT-IR spectrum of the scraped samples (Fig. 13b) reveals the presence of functional groups whose absorption peaks correspond with C=O (shifted from 1,676 to 1,659 cm−1 for PPQ and from 1,677 to 1,655 cm−1 for PPBQ) and N–H (shifted from 3,460 to 3,407 cm−1 for PPQ and from 3,450 to 3,411 cm−1 for PPBQ). The shift in the absorption frequencies for the scraped samples strongly suggests that the inhibitors (PPQ and PPBQ) are strongly adsorbed on the metal surface through the C=O and N–H groups [40]. Moreover, broadening of the C=O band at 1,670 cm−1 and the N–H band at 3460 cm−1 suggests that the inhibitors make coordination bond with the metal surface [41]. Moreover, several modifications in the peaks for scraped samples revealed that the inhibitors (PPQ and PPBQ) are strongly adsorbed on the HCS surface.
UV–visible spectrum
UV–visible spectra were recorded for inhibitors (PPQ and PPBQ) and the scraped samples (Fe-PPQ and Fe-PPBQ) (Fig. 14). In Fig. 14, inhibitor showed two spectral bands at around 240 nm and 370 nm which can be assigned to π→π* and n→π* transitions, respectively. However, in the case of scraped samples (Fe-PPQ and Fe-PPBQ), the bands around 420 nm completely disappeared, which was ascribed to charge transfer process probably from inhibitor molecule (C=O and N–H) to HCS surface. This result gives a strong support to the possibility of the chemisorbed layer formation on HCS surface [42].
Wide angle X-ray diffractometer
The wide angle X-ray diffractograms were carried out for inhibitors (PPQ and PPBQ) and scraped samples (Fe-PPQ and Fe-PPBQ) (Fig. 15). Figure 15 shows very high relative intensity peaks in the 5 to 25° region for inhibitors, suggesting the crystalline nature of inhibitors (PPQ and PPBQ). However, for scraped samples, broad peaks were observed in the 5 to 25° region, suggesting the productive film formation on HCS surface via adsorption by inhibitor molecules (PPQ and PPBQ) and the resulting amorphous nature of the inhibitors [43].
Surface analysis
SEM images were recorded (Fig. 16) in order to establish the interactions of the inhibitors on the HCS surface in 10 % HCl solution. Figure 16a shows the finely polished characteristic surface of HCS and shows some scratches which had arisen during polishing. Figure 16b reveals that the surface of HCS was severely corroded due to the aggressive attack by 10 % HCl solution. Figure 16c and d shows the SEM images of HCS surface immersed for the same period of time in 10 % HCl solution containing 400 ppm of inhibitors, revealing the formation of a protective film by the inhibitors on the HCS surface [44]. Moreover, even if the HCS surface was well covered by inhibitors in 10 % HCl solution, still the SEM images of HCS specimens after immersed in inhibitors showed some scratches, which may be due to the formation of corrosion products on HCS surface.
The EDS spectrum was recorded (Fig. 16, inserts) in order to determine the percentage of chloride present on the HCS surface in the presence and absence of inhibitors (PPQ and PPBQ). Figure 16a and Table 6 show the EDS spectrum of HCS, which revealed that the surface was chlorine free in the absence of 10 % HCl. However, for HCS with 10 % HCl solution (Table 6), very high chloride content (4.05 %) was observed due to aggressive attack by 10 % HCl solution. Figures 16c and d and Table 6 shows EDS spectrum of the HCS immersed for the same period of time in 10 % HCl solution containing 400 ppm of inhibitors. This result revealed that the inhibitors protect HCS surface from 10 % HCl which shows very low chloride content (0.22 % for PPQ and 0.19 % for PPBQ) whereas the HCS after immersion in 10 % HCl solution showed high chlorine content of 4.05 %, which revealed that the inhibitor effectively inhibits corrosion of HCS in 10 % HCl [45].
Conclusion
In conclusion, the inhibitors (PPQ and PPBQ) acted as effective corrosion inhibitors for HCS in 1.0 M HCl. The inhibition efficiency of the studied inhibitors was increased when the concentration of inhibitors was increased. Good agreement was observed between the results obtained from chemical and electrochemical measurements. Electrochemical polarization measurements revealed the mixed mode of inhibition of inhibitors. Inhibitors inhibit the corrosion of HCS in acid medium by both chemisorption and physisorption mechanisms, and the adsorption of the compound on the metal surface obeys the Langmuir adsorption isotherm. FT-IR, WAXD, and UV–visible spectrum were evidence for the chemisorption mechanism and weight loss at different temperature method was evidence for the physisorption mechanism. SEM–EDS images provided information of protective film formation on HCS surface. Thermodynamic adsorption parameters (ΔH°ads, ΔS°ads, and ΔG°ads) revealed that the adsorbed process of inhibitors on HCS surface was an exothermic and spontaneous process.
References
Fantana MG (1987) Corrosion engineering. In: Mater Lett, 3rd edn. McGraw-Hill, New York
Hashimoto K, Masumoto T, Herman H (1981) Ultrarapid quenching of liquid alloy. Academic, New York
Hashimoto K, Luborsky FE (1983) Amorphous metallic alloys. Butterworths, London
Gilman JJ, Ashbrook RL (1983) Rapid solidification technology source book. ASM, Metals Park
Abd El-Maksoud SA, Fouda AS (2005) Some pyridine derivatives as corrosion inhibitors for carbon steel in acidic medium. Mater Chem Phys 93:84–90
Migahed MA, Nassar IF (2008) Corrosion inhibition of Tubing steel during acidization of oil and gas wells. Electrochim Acta 53:2877–2882
Wang HL, Fan HB, Zheng JS (2002) Corrosion inhibition of mild steel in hydrochloric acid solution by a mercapto-triazole compound. Mater Chem Phys 77:655–661
Dhayabaran VV, Lydia IS, Merlin JP, Srirenganayaki P (2004) Inhibition of corrosion of commercial mild steel in presence of tetrazole derivatives in acid medium. Ionics 10:123–125
Brozendra NB, Mollah MYA, Susan ABH (2012) Potentiodynamic studies on corrosion of copper by chloride ions and its inhibition by inorganic and organic ions in aqueous buffer solution. Ionics 18:189–195
Gopiraman M, Sakunthala P, Kanmani R, Alex Ramani V, Sulochana N (2011) Inhibitive action of Clematis gouriana extract on the corrosion of mild steel in acidic medium. Ionics 17:843–852
Gopiraman M, Sathya C, Vivekananthan S, Kesavan D, Sulochana N (2012) Influence of 2,3-dihydroxyflavanone on corrosion inhibition of mild steel in acidic medium. J Mater Eng Perform 16:240–246
Prabavathi N, Sulochana N (2004) Electrochemistry of 4′-hydroxy and acetoxy flavones CV and NPP study. Ionics 10:109–112
Ashok Kumar SL, Gopiraman M, Saravana Kumar M, Sreekanth A (2011) 2-Acetylpyridine-N(4)-morpholine thiosemicarbazone (HAcpMTSc) as a corrosion inhibitor on mild steel in HCl. Ind Eng Chem Res 50:7824–7832
Gopiraman M, Selvakumaran N, Kesavan D, Karvembu R (2012) Adsorption and corrosion inhibition behaviour of N-(phenylcarbamothioyl)benzamide on mild steel in acidic medium. Prog Org Coat 73:104–111
Bentiss F, Lebrini M, Lagrenee M (2005) Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in mild steel/2,5-bis(n-thienyl) 1,3,4-thiadiazoles/hydrochloric acid system. Corros Sci 47:2915–2931
Khaled KF (2003) The inhibition of benzimidazole derivatives on corrosion of iron in 1 M HCl solutions. Electrochim Acta 48:2493–2503
Adamson AW (1990) Physical chemistry of surfaces. Wiley, New York
Ismail AA, Nabel AN (2009) Some Schiff base surfactants as steel-corrosion inhibitors. J Surfactant Deterg 12:313–319
Gopiraman M, Sakunthala P, Kesavan D, Alexramani V, Kim IS, Sulochana N (2012) An investigation of mild carbon steel corrosion inhibition in hydrochloric acid medium by environment friendly green inhibitors. J Coat Technol Res 9(1):15–26
Aljourani J, Raeissi K, Golozar MA (2009) Benzimidazole and its derivatives as corrosion inhibitors for mild steel in 1 M HCl solution. Corros Sci 51:836–1843
Hosseini SMA, Azimi A (2009) The inhibition of mild steel corrosion in acidic medium by 1-methyl-3-pyridin-2-yl-thiourea. Corros Sci 51:728–732
Xianghong L, Shudan D, Hui F, Guannan M (2009) Inhibition effect of 6-benzylaminopurine on the corrosion of cold rolled steel in H2SO4 solution. Corros Sci 51:620–634
Muhammad A, Munawar AM, Hamid L (2007) Antimicrobial activity and synthesis of quinoline-based chalcones. J Appl Sci 7(17):2485–2489
Evans UR (1937) Metallic corrosion passivation and protection. Arnold, London
Uhlig HH (1963) Corrosion and corrosion control. Wiley, New York
Nabel AN, Mohamad FZ, Mounir AIS (2009) Synthesis and evaluation of 4-diethyl amino benzaldehyde Schiff base cationic amphiphiles as corrosion inhibitors for carbon steel in different acidic media. J Surfactant Deterg 12:321–329
Abiola OK, James AO (2010) The effects of Aloe vera extract on corrosion and kinetics of corrosion process of zinc in HCl solution. Corros Sci 52:661–671
El-Etre AY (2008) Inhibition of C-steel corrosion in acidic solution using the aqueous extract of zallouh root. Mater Chem Phys 108:278–284
Stanly Jacob K, Parameswaran G (2010) Corrosion inhibition of mild steel in hydrochloric acid solution by Schiff base furoin thiosemicarbazone. Corros Sci 52:224–228
Arab ST, Emran KM (2008) Structure effect of some thiosemicarbazone derivatives on the corrosion inhibition of Fe78B13Si9 glassy alloy in Na2SO4 solution. Mater Lett 62:1022–1032
Bentiss F, Lagrenee M, Traisnel M, Hornez JC (1999) The corrosion inhibition of mild steel in acidic media by a new triazole derivative. Corros Sci 41:789–803
Bentiss F, Lebrini M, Lagrenée M (2005) Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in mild steel/2,5-bis(n-thienyl)-1,3,4-thiadiazoles/hydrochloric acid system. Corros Sci 47:2915–2931
Tan YJ, Bailey S, Kinsellal B (1996) An investigation of the formation and destruction of corrosion inhibitor films using electrochemical impedance spectroscopy (EIS). Corros Sci 38:1545–1554
Gopiraman M, Selvakumaran N, Kesavan D, Kim IS, Karvembu R (2012) Chemical and physical interactions of 1-benzoyl-3,3-disubstituted thiourea derivatives on mild steel surface: corrosion inhibition in acidic media. Ind Eng Chem Res 51:7910–7922
Metikos HM, Babic R, Gruba Z, Brinic S (1996) Impedance investigation of corrosion inhibition of Armco iron by thiourea. J Appl Electrochem 26:443–449
Sheng X, Ting YP, Pehkonen SO (2007) Evaluation of an organic corrosion inhibitor on abiotic corrosion and microbiologically influenced corrosion of mild steel. Ind Eng Chem Res 46:7117–7125
Emregül KC, Atakol O (2004) Corrosion inhibition of iron in 1 M HCl solution with Schiff base compounds and derivatives. Mater Chem Phys 83:373–379
Bouyanzer A, Hammouti B, Majidi L (2006) Pennyroyal oil from Mentha pulegium as corrosion inhibitor for steel in 1 M HCl. Mater Lett 60:2840–2843
Taqui MM, Shukla RS (1992) Kinetic and spectroscopic study of the formation of an intermediate ruthenium (III) ascorbate complex in the oxidation of L-ascorbic acid. Polyhedron 10:2711–2721
Gaber AMA, Nabey BAAE, Saadawy M (2009) The role of acid anion on the inhibition of the acidic corrosion of steel by lupine extract. Corros Sci 51:1038–1045
Abdel-Gaber AM, Abd-El-Nabey BA, Saadawy M (2009) The role of acid anion on the inhibition of the acidic corrosion of steel by lupine extract. Corros Sci 51:1038–1042
Soliman AA, Khattab MM, Reissner M, Weinberger P, Werner F, Linert W (2007) Synthesis, structure, spectroscopic and magnetic characterization of a novel spin-crossover iron (II) complex with 1-cyclopropyltetrazole ligands. Inorg Chim Acta 360:3987–3996
Ping WK, Xiang CZ, Yu Z, Pei WP, Hong WJ, Quan DL (2008) Molecular weight and proposed structure of the Angelica sinensis polysaccharide–iron complex. Chin J Chem 26:1068–1076
Zhao P, Liang Q, Li Y (2005) Electrochemical, SEM/EDS and quantum chemical study of phthalocyanines as corrosion inhibitors for mild steel in 1 mol/l HCl. Appl Surf Sci 252:1596–1607
Ostovari A, Hoseinieh SM, Peikari M, Shadizadeh SR, Hashemi SJ (2009) Corrosion inhibition of mild steel in 1 M HCl solution by henna extract: a comparative study of the inhibition by henna and its constituents (lawsone, gallic acid, a-D-glucose and tannic acid). Corros Sci 51:1935–1949
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ayyannan, G., Karthikeyan, K., Vivekananthan, S.S. et al. Chemical and electrochemical investigations of high carbon steel corrosion inhibition in 10 % HCl medium by quinoline chalcones. Ionics 19, 919–932 (2013). https://doi.org/10.1007/s11581-012-0812-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-012-0812-y