Abstract
Corrosion behavior of Ti–15Mo alloy in artificial saliva containing variation in pH (7.2, 3.5) and varying concentrations of fluoride ions (2500, 5000 and 10,000 ppm) was evaluated using potentiodynamic polarization, electrochemical impedance spectroscopy studies to ascertain its suitability for dental implant applications. The study reveals that there is a strong dependence of the corrosion resistance of Ti–15Mo alloy on the concentration of fluoride ions in the electrolyte medium. Surface morphological characterization was carried out using SEM–EDAX to investigate the corrosion resistance in artificial saliva containing sodium fluoride at various pH conditions, and it was found that the surface roughness of the specimens was highly increased at pH 3.5. In spite of the active dissolution, the Ti–15Mo alloy exhibits passivity at anodic potentials at all concentrations of the fluoride ions studied. The results suggest that Ti–15Mo alloy can be a suitable alternative for dental implant applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In dentistry, metallic materials are used as implants in reconstructive oral surgery to replace a single or an array of teeth in the fabrication of dental prosthesis such as metal plates for complete and partial dentures, essentially inpatients requiring hypoallergenic materials. Nowadays, titanium and its alloys have been widely used for dental restorative applications such as crown, bridge, framework and dentures. These alloys are the most commonly used metal for dental implants and prosthesis owing to its excellent mechanical properties, good corrosion resistance and good adhesion, in biological fluids with good biocompatibility [1].
The stability of titanium under corrosion conditions is essentially due to the formation of a stable and tightly adherent thin protective oxide layer on its surface, and the passive film stability depends on it structure and composition. For instance, pH is known to have a strong influence on the corrosion resistance of titanium and its alloys. For a metal to be used in oral environment, it should be biocompatible and have high corrosion resistance. Among the various types of titanium alloys, Ti–6Al–4V has become the metals of choice for endosseous parts of presently available dental implants [1,2,3]. Since the oral environment could involve fluoride medium, the degree of corrosion resistance offered by the Ti alloys in fluoride-containing medium becomes an important criterion in the selection of a metallic biomaterial to be used in the oral medium.
Titanium alloys developed in the early stage are mainly α + β-type ones. In general, the biocompatibility is an important criterion for the selection of any biomaterial. Therefore, the research and development on β-type titanium alloys, which are considered to be advantageous in terms of biocompatibility, are increasing [4, 5]. Several β-phase Ti alloys, having Nb, Ta, Zr and Mo as alloying elements (β-stabilizer elements), were developed. Nag et al. [6, 7] have recommended it as one of the promising biocompatible Ti alloys. Daniel et al. [8] have investigated the corrosion behavior of β-Ti20Mo alloy in artificial saliva, while Ho et al. [9] and Oliveira et al. [10]. have studied the structure and properties of a series of binary Ti–Mo alloy with Mo content ranging up to 20 wt% [11].
The biocompatibility of Ti and its alloys for the dental implant is decided based upon the osseointegration response and cell adhesion behavior. The contact between the metallic implant and the receiving living tissues is made through the oxide layer on the implant surface, which allows the osseointegration process [12]. However, corrosion of titanium and its alloys can still occur in some clinical circumstances. The corrosion resistance of dental alloys in an acidic oral environment is surely a crucial subject when biocompatibility is of concern.
Titanium and its alloys are used in the dental field, and the oral environment is exposed to fluoride-containing medium which becomes an important property for their use in dentistry. On the other hand, when the fluoride-containing mouth rinse, toothpaste or prophylactic gel (NaF: around (0.1–2%) are used in the oral environment where proteins are naturally present in the saliva, the corrosion or electrochemical behavior of dental alloys in the oral environment with fluoride ions and proteins should be taken into account. In addition, some reports have shown the negative influence of fluoride on the corrosion resistance of titanium alloys [13, 14].
Reclaru et al. [14] have studied the effect of fluorides on titanium and other dental alloys for dentistry applications. The results revealed the development of oxide layer with one or more fluoride ion containing electrolytes. Al-Mobarak et al. [15] have investigated the effect of hydrogen peroxide on the electrochemical behavior of Ti and its alloys for dental applications and found that the presence of H2O2 leads to decreased corrosion resistance of Ti and its alloys and an enhanced dissolution/oxidation rate and the effect of H2O2 depends on its concentration as well as pH of the solution.
Nakagawa et al. [16] have examined the effect of fluoride concentration and pH on corrosion behavior of titanium, and the results revealed that the fluoride concentrations and pH values on corrosion environments where the fluoride concentration and pH value are known. Nakagawa et al. [17] have also studied the corrosion behavior of pure titanium and titanium alloys in fluoride-containing solutions. The results proved that the corrosion resistance of Ti–0.2Pd alloy was greater than Cp–Ti, Ti–6Al–4V and Ti–6Al–7Nb alloys in the wide range of pH and fluoride concentrations. Nakagawa et al. [18] have also investigated the effect of fluoride and dissolved oxygen concentrations on the corrosion behavior of pure titanium and titanium alloys.
Huang [19] examined the effects of fluoride and albumin concentration on the corrosion behavior of Ti–6Al–4V alloy and reported that the protectiveness of the TiO2 passive film formed on Ti–6Al–4V alloy was destroyed by fluoride ions via the formation of Na2TiF6 when the NaF concentration was increased up to 0.1%. Various researchers have also reported on the corrosion behavior of influence of fluoride ion on the Cp–Ti and its alloys for dental implant applications [20,21,22,23,24,25,26,27,28,29].
The objective of this present investigation is to study the interaction of Ti–15Mo alloy in artificial saliva with a high concentration of fluoride ions in simulated oral environments with varying fluoride concentrations at various pH conditions to improve the bioactivity. Surface morphological studies were carried out using scanning electron microscopy to evaluate the surface roughness at various pH conditions.
2 Materials and Methods
2.1 Specimen Preparation
Ti –15Mo alloy (Kobe Steel Ltd., Japan) was used in the present study with the size of dimension 1 × 1 cm2, and its chemical composition is presented in Table 1. The specimens were ground using abrasive SiC paper from 600 to 1200# grade. Final polishing was done using alumina powder (0.5 μm in size) in order to produce a mirror finish surface followed by rinsing with distilled water and degreased with acetone. Further the specimens were ultrasonicated in acetone for about 20 min. Finally the specimens were washed in distilled water and dried in an oven.
2.2 Test Electrolyte Media
For all the experiments, AR-grade chemicals/reagents were used and solutions were prepared in double-distilled water. The testing medium and the electrolyte solution were artificial saliva [15], and its chemical composition is presented in Table 2. Electrolyte medium used was the artificial saliva enriched with sodium fluoride (NaF) with different concentrations of 2500, 5000 and 10,000 ppm. This particular concentrations of the artificial saliva were prepared similar to that of the fluoride concentrations obtained in a commercial fluoride-containing mouth rinse and toothpaste. The pH (7.2, 3.5) was lowered by adding lactic acid. The pH was measured with an XC601-type glass electrode (Radiometer Analytical, Villeurbanne, France) convected to a PHM 220-type pH meter. The temperature was maintained at 37 ± 1 °C for all the experiments.
2.3 Electrochemical Characterization
Electrochemical experiments were performed using a conventional three electrode cell assembly with potentiostat (model PGSTAT 12 with FRA, Autolab, the Netherlands B.V.) controlled by a personnel computer with dedicated software; viz., General Purpose Electrochemical System (GPES version 6.0) was used for conducting the polarization experiments. A saturated Ag/AgCl electrode served as a reference electrode, platinum sheet acted as a counter electrode and the test specimens (Ti–15Mo alloy) acted as the working electrode with the exposed area of 1 cm2. Potentiodynamic polarization studies were carried out in the potential range of − 1 to 2 V at a scan rate of 1 mV/s in an aerated medium. Electrochemical impedance spectroscopic studies (EIS) were carried out using an electrochemical system frequency response analyzer (FRA), and the impedance spectra were acquired in the frequency range of 104 to 10−2 Hz with a 10 mV amplitude sine wave generated by FRA. The impedance spectra (Bode plots) were fitted using a nonlinear least square (NLLS) method. In order to obtain reliable results, all the experiments were triplicated for concordant values.
2.4 Surface Morphological Characterization
Surface morphology and elemental composition of the Ti–15Mo specimens were examined using (Hitachi model S-3400) scanning electron microscope (SEM) coupled with an energy-dispersive X-ray analysis system (EDAX).
3 Results and Discussion
3.1 Electrochemical Characterization
3.1.1 Potentiodynamic Polarization Results (PDP)
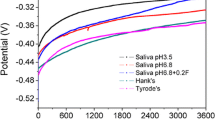
Potentiodynamic polarization studies of Ti–15Mo were conducted in the potential range of − 1 to 2 V at a scan rate of 1 mV/s to observe the effect of fluoride ions at various pH conditions. Figure 1 shows the polarization curves of Ti–15Mo in artificial saliva containing different fluoride concentrations (2500, 5000 and 10,000 ppm) at pH (7.2, 3.5), respectively. It can be observed that there is a cathodic shift in corrosion potential range (E corr) from − 0.107 to − 0.398 V vs. SCE with increase in fluoride ion concentration from 0 to 10,000 ppm. Also the shape of the curves is quite similar; the active region of the curves is extended to higher current region in the presence of fluoride ions. For a given pH value, the corrosion current density of Ti–15Mo increases with increase in fluoride concentration and for a given fluoride concentration, and pH decrease leads to an increase in the corrosion current density. The shift in the active region of the polarization curves toward higher current region suggests the negative influence of fluoride ions on the corrosion resistance of Ti–15Mo alloy. The negative influence of fluoride ions has been confirmed by various researchers [13,14,15,16,17,18,19,20]. Nakagawa et al. [16] have also reported such occurrence at 30 ppm NaF. Hence, the increase in the active region in the presence of fluoride ions is due to the formation of a porous defective oxide layer which reduces the corrosion protection. Huang et al. [19] evaluated the active–passive transition of Ti–6Al–4V alloy in acidic artificial saliva when the NaF concentration is 0.5%. Kwon et al. [20] have also evaluated the effect of acidic fluoride solution on β-titanium alloy wire. Oshida et al. [21] have reported that commercially available fluoride treatment agent cause discoloration of Ti–6Al–4V alloy. Schutz and Thomas [22] evaluated that 20 ppm of NaF may destroy the protective oxide layer on Cp–Ti.
The anodic region of the polarization curve (Fig. 1b) exhibits an active–passivation transition observed for pH 3.5. The active–passive transition observed in the anodic region both in the absence and in the presence of fluoride ions suggested that the presence of fluoride ions in artificial saliva did not hinder the formation of passive oxide film on the surface of Ti–15Mo alloy. It can be identified that for pH 3.5, the E corr shifts in the negative direction, the fluoride ions interfere in the titanium oxide formation which causes the changes in the protective passive layer of the metal [23, 24]. As the NaF concentration is increased from 2500 to 10,000 ppm, the higher current density was obtained due to the dissolution of protective oxide film and the influence of fluoride ions may affect the properties of the oxide layer, causing the E corr to shift in the negative direction. Hence, the lower pH can enhance the activity of fluorine ions in the electrolyte medium and significantly decreased the corrosion resistance of the oxide film [25].
3.1.2 Electrochemical Impedance Spectroscopic (EIS) Studies
EIS studies of Ti–15Mo were performed in artificial saliva for various fluoride concentration/pH conditions, in order to observe the influence of both parameters with the impedance response. Figures 2 and 3 show the Bode phase angle and Bode impedance plots for Ti–15Mo in artificial saliva containing (2500, 5000 and 10,000 ppm) at pH (7.2, 3.5), respectively. It can be noted that the impedances measured for the various concentrations leading to an active state of Ti with a reduction in corrosion resistance of Ti–15Mo with both fluoride concentration increase and pH decrease, which is also reflected in the change in phase angle behavior suggests the stable passive film formation on Ti–15Mo [25]. At higher fluoride concentration (10,000 ppm), the phase angle values shifts to decrease in its values which may be due to the dissolution of passive oxide film formation consisting oxides of titanium and molybdenum. Satendra et al. [26, 27] evaluated that the passive oxide film formed above 2500 ppm of fluoride ions may not be stable for a longer time and starts dissolving due to the negative effect of higher concentration of fluoride ions. It can also be observed that the width of the phase angle decreased significantly with the increase in the fluoride concentration. The resistance value of the passive film was initially high, and then, it decreased with decrease in pH values which may be due to the dissolution of barrier layer formed on the metal surface. Further the phase angle value drops slightly toward the lower values in the low-frequency range indicating the contribution of surface film resistance to the impedance, i.e., the resistance of the surface film decreases [28].
The results obtained were in good agreement with the impedance values obtained on fitting curve using the nonlinear least square fit developed by boukamp. Figure 4 shows the equivalent circuit diagram used to evaluate the electrochemical impedance parameters for Ti–15Mo alloy in artificial saliva. The proposed equivalent circuit model R s (R b Q b) was used to fit the spectra obtained for Ti–15Mo alloy for the blank (Fig. 4a) where R s represents the ohmic or solution resistance, and R b and Q b represent the charge transfer resistance and double-layer capacitance of the barrier layer. This represents the presence of a single layer on the metal surface possessing resistance as well as capacitance. The surface film is considered to be a parallel circuit of a resistor due to the ionic conduction through the film and a capacitor due to its dielectric properties [29, 30]. The equivalent circuit proposed for the Ti–15Mo alloy with the variation of pH and fluoride concentrations is R s(R p Q p) (R b Q b) (as shown in Fig. 4b) where R p and Q p represents the charge transfer resistance and double-layer capacitance of the porous layer and is characterized by two parallel combination of resistance and capacitance in series with the solution resistance. It indicated the formation of two layers, viz. inner barrier and outer porous layers, respectively [31].
The electrochemical impedance parameters by fitting with the equivalent circuit in artificial saliva with different fluoride concentrations at various pH values are shown in Table 3. It was observed that the resistance of the barrier film decreased with increase in concentration of fluoride and the variation of pH, whereas the capacitance increased. The R p and Q p values were determined from the Bode plots which are presented in Table 4. The changes in the charge transfer resistance and double-layer capacitance of the porous layer of Ti–15Mo in artificial saliva with varying fluoride and pH concentrations are presented in Fig. 5. It can be observed that the Rp values decreased from 4773 to 4632 kΩ cm2 for pH 7.2 and from 4773 to 4184 kΩ cm2 for pH 3.5, whereas the Q p values increased from 4.64 to 5.27 µF cm−2 for pH 7.2 and from 3.26 to 4.23 µF cm−2 for pH 3.5, respectively, with increase in fluoride ion concentration from 0 to 10,000 ppm.
As from the electrochemical results, it can be inferred that the active–passive transition is observed in the presence of all concentrations of fluoride ions at different pH values. In spite of the active dissolution in the presence of fluoride ions, the Ti–15Mo exhibits passivity at anodic potentials. Increase in fluoride ion concentration increases the i corr, Q p and Q b values, and causes a cathodic shift in E corr and a decrease in R p and R b values, suggesting the negative influence of fluoride ion and a decrease in corrosion protective ability of Ti–15Mo alloy.
3.2 Surface Morphological Characterization
Surface morphological behavior on Ti–15Mo in artificial saliva with varying fluoride concentrations at various pH conditions was examined, and the obtained images are presented in Fig. 6. The surface morphology for the blank surface (BARE) at pH 7.2 showed a smooth uniform surface (Fig. 6a). A smooth surface with white crystal deposits (Fig. 6b) was observed for the 2500 ppm of fluoride concentration on Ti–15Mo alloy at pH 7.2. When there is an increase in the concentration of fluoride ions of 5000 ppm, a rough surface of white particles with small blisters (Fig. 6c) was exhibited due to the fluoride ions attack over the surface of Ti–15Mo. The aggressive actions of fluoride anions are hindered when a thick oxide layer is formed after long exposure period with a very rough surface of pores (Fig. 6d) was observed for 2500 ppm of fluoride at pH 3.5 and (Fig. 6e) for 5000 ppm of fluoride at pH 3.5 which evinced that the rate of fluoride ions can cause more corrosive attack on Ti–15Mo surfaces.
4 Conclusions
Corrosion behavior of Ti–15Mo alloy in artificial saliva by varying with fluoride ions and pH conditions was evaluated. Electrochemical results revealed that the increase in fluoride ion concentration increases the i corr, Q p and Q b values, which causes a cathodic shift in E corr and a decrease in R p and R b values, suggesting the negative influence of fluoride ion to decrease the corrosion protective ability of surface of Ti–15Mo. Morphological studies exhibited a smooth uniform surface for blank, whereas a rough surface with blisters, indicates the increase in surface roughness of the oxide layer was observed at acidic condition (pH 3.5). The surface also gets damaged due to the increase of the fluoride ions. Hence, based on the results obtained, Ti–15Mo alloy can be a used for dental implants.
References
Barry M, Kennedy D, Keating K, Schauperl Z (2005) Design of dynamic test equipment for the testing of dental implants. Mater Des 26:209–216
Okazaki Y (2002) Dental casting properties of Ti–15Zr–4Nb–4Ta alloy. Mater Trans 43:3134–3141
Al-Mayouf AM, Al-Swayih AA, Al-Mobarak NA, Al-Jabab AS (2004) Corrosion behavior of a new titanium alloy for dental implant applications in fluoride media. Mater Chem Phys 86:320–329
Kuroda D, Niinomi M, Morinaga M, Kato Y, Yashiro T (1998) Design and mechanical properties of new β type titanium alloys for implant materials. Mater Sci Eng, A 243:244–249
Ahmed T, Long M, Silvestri J, Ruiz C, Rack HJ (1996) A new low modulus, biocompatible titanium alloy. In: Blenkinsop PA, Evans WJ, Flower HM (eds) Titanium’95: science and technology. The Institute of Materials, London, p 1760
Nag S, Banerjee R, Fraser HL (2005) Comparison of microstructural evolution in Ti–Mo–Zr–Fe and Ti–15Mo biocompatible alloys. J Mater Sci Mater Med 16:679–685
Nag S, Banerjee R, Fraser HL (2005) Microstructural evolution and strengthening mechanisms in Ti–Nb–Zr–Ta, Ti–Mo–Zr–Fe and Ti–15Mo biocompatible alloys. Mater Sci Eng C 25:357–362
Daniel M, Romeu C, Ioan D, Doina-Margareta G, Thierry G (2010) Corrosion behaviour of β–Ti20Mo alloy in artificial saliva. J Mater Sci Mater Med 21:2907–2913
Ho WF, Ju CP, Chern Lin H (1999) Structure and properties of cast binary Ti–Mo alloys. Biomaterials 20:2115–2122
Oliveira NTC, Aleixo G, Caram R, Guastaldi AC (2007) Development of Ti–Mo alloys for biomedical applications: microstructure and electrochemical characterization. Mater Sci Eng A 452–453:727–731
Huang HH (2003) Surface characterization of passive film on NiCr-based dental casting alloys. Biomaterials 24:1575–1582
Rincic N, Baucic I, Miko S, Papic M, Prohic E (2003) Corrosion behaviour of the Co–Cr–Mo dental alloy in solutions of different composition and different pH values. Coll Antropol 27:99–106
Wilhelmsen W, Grande AP (1987) The influence of hydrofluoric acid and fluoride ion on the corrosion and passive behavior of titanium. Electrochim Acta 32:1469–1474
Reclaru L, Meyer JM (1998) Effects of fluorides on titanium and other dental alloys in dentistry. Biomaterials 19:85–92
Al-Mobarak NA, Al-Mayouf AM, Al-Swayih AA (2006) The effect of hydrogen peroxide on the electrochemical behavior of Ti and some of its alloys for dental applications. Mater Chem Phys 99:333–340
Nakagawa M, Matsuya S, Shiraishi T, Ohta M (1999) Effect of fluoride concentration and pH on corrosion behavior of titanium for dental use. J Dent Res 78:1568–1572
Nakagawa M, Matsuya S, Udoh K (2001) Corrosion behavior of pure titanium and titanium alloys in fluoride-containing solutions. Dent Mater J 20:305–314
Nakagawa M, Matsuya S, Udoh K (2002) Effect of fluoride and dissolved oxygen concentrations on the corrosion behavior of pure titanium and titanium alloys. Dent Mater J 21:83–92
Huang HH (2003) Effects of fluoride and albumin concentration on the corrosion behavior of Ti–6Al–4V alloy. Biomaterials 24:275–282
Kwon YH, Seol HJ, Kim H, Hwang KJ, Lee SG, Kim KH (2005) Effect of acidic fluoride solution on β–titanium alloy wire. J Biomed Mater Res Part B Appl Biomater 73:285–290
Oshida Y, Sellers CB, Mirza K, Farzin-Nia F (2005) Corrosion of dental metallic materials by dental treatment agents. Mater Sci Eng C 25:343–348
Schutz RW, Thomas DE (1987) Corrosion of titanium and titanium alloys, Metals handbook, vol 13. ASM International, Metals Park, p 669
Horasawa N, Marek M (2010) Effect of fluoride from glass ionomer on discoloration and corrosion of titanium. Acta Biomater 6:662–666
Pan J, Thierry D, Leygraf C (1996) Electrochemical impedance spectroscopy study of the passive oxide film on titanium for implant applications. Electrochim Acta 41:1143–1153
Souto MR, Laz MM, Reis RL (2003) Degradation characteristics of hydroxyapatite coatings on orthopaedic TiAlV in simulated physiological media investigated by electrochemical impedance spectroscopy. Biomaterials 24:4213–4221
Kumar S, Sankara Narayanan TSN (2008) Corrosion behavior of Ti–15Mo alloy for dental implant applications. J Dent 36:500–507
Kumar S, Sankara Narayanan TSN, Kumar SS (2010) Influence of fluoride ion on the electrochemical behaviour of β–Ti alloy for dental implant application. Corros Sci 52:1721–1727
Karthega M, Tamilselvi S, Rajendran N (2006) Effect of pH on the corrosion behaviour of Ti–6Al–4V alloy for dental implant application in fluoride media. Trend Biomater Artif Org 20:31–34
Tamilselvi S, Murugaraj R, Rajendran N (2007) Electrochemical impedance spectroscopic studies of titanium and its alloys in saline medium. Mater Corros 58:113–120
Sasikumar Y, Rajendran N (2017) Effect of acid treatment on the surface modification of Ti–6Al–7Nb and Ti–5Al–2Nb–1Ta and its electrochemical investigations in simulated body fluid. J Bio Tribo Corros 3:41
Sasikumar Y, Rajendran N (2013) Influence of surface modification on the apatite formation and corrosion behavior of Ti and Ti–15Mo alloy for biomedical applications. Mater Chem Phys 138:114–123
Acknowledgements
The authors acknowledge the Indian Council for Medical Research (ICMR), New Delhi, for their financial support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sasikumar, Y., Rajendran, N. Effect of Fluoride Concentration and pH on Corrosion Behavior of Ti–15Mo in Artificial Saliva. J Bio Tribo Corros 4, 3 (2018). https://doi.org/10.1007/s40735-017-0119-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-017-0119-7