Abstract
Purpose of review
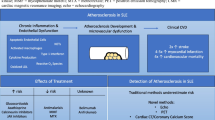
Systemic sclerosis (SSc) is a systemic inflammatory, autoimmune disorder characterized by diffuse fibrosis of the skin and visceral organ involvement. Endothelial dysfunction and microvascular injury dominate the pathophysiology and clinical manifestations of the disease, while the impact of macrovascular atherosclerotic disease on cardiovascular (CVD) morbidity and mortality is yet to be established. In this article, we aim to review current knowledge about CVD as well as cardiac complications in SSc and discuss the potentially implicated pathogenetic mechanisms.
Recent findings
Systemic inflammation has been identified as an important trigger and contributor for the development and progression of atherosclerosis, closely associated with high cardiovascular mortality in patients with autoimmune disorders, such as rheumatoid arthritis. A close interplay between traditional risk factors and factors related to the disease, including inflammation, endothelial injury, and immune-mediated cytotoxicity, sharing common pathogenetic features with microvasculopathy, may be responsible for large-vessel involvement and promotion of atherosclerosis in SSc. Cardiac complications, including heart failure due to impairment of coronary microcirculation and myocardial fibrosis, are listed among the primary cause of death in SSc. Evaluation of indirect surrogate markers of CVD, namely, arterial stiffness, carotid media thickness, and flow-mediated dilation, in small studies has provided inconsistent results regarding the association between SSc and atherosclerosis, highlighting the need for further research on this field. In this article, we aim to review current knowledge about large-vessel involvement and CVD in SSc and discuss the potentially implicated pathogenetic mechanisms.
Summary
SSc conveys a higher risk for CVD associated with both vascular and fibrotic complications during the course of the disease. Increasing attention is given on the use of vasodilators, immunosuppressants, and more recently antifibrotic drugs that potentially improve myocardial function and reduce atherosclerotic disease burden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic sclerosis (SSc) is a rare, multiorgan, connective tissue disease, characterized by fibrosis of the skin and internal organs and significant deterioration of life quality. Autoimmune activation, endothelial dysfunction, and extensive tissue fibrosis form the pathogenetic triad of the disorder, culminating in a heterogenous spectrum of manifestations with varying prognosis [1]. Based on the extent of skin involvement, SSc is classified in two major subtypes, limited and diffuse, with the latter representing the more aggressive type with severe visceral organ involvement [2]. Despite better understanding in the pathogenesis of SSc, the disease remains a field of increasing interest in terms of precise comprehension of its complex pathogenesis and optimal medical management.
Cardiac involvement in SSc encapsulates a wide spectrum of clinical indices including myocardial fibrosis and dysfunction, pericarditis, myocarditis, and valvulopathies as well as pulmonary hypertension and accounts for the majority of SSc-related deaths in this population [3]. While small-vessel disease represents a pathognomonic feature of SSc, it remains unclear whether common pathogenetic mechanisms, including endothelial dysfunction, systematic inflammation, oxidative stress, and impaired coagulation, may lead to large-vessel involvement and, particularly, development of accelerated atherosclerosis predisposing patients with SSc to high risk for CVD [4, 5]. Similarly to what occurs in other systemic autoimmune disorders such as rheumatoid arthritis and systemic lupus erythematosus with well-establish excess burden of CVD, interrelations between traditional risk factors, autoimmune dysregulation, and systemic inflammation may account for the progression of accelerated atherosclerosis in SSc [6]. Non-invasive assessments of vascular function such as carotid intima-media thickening (cIMT) and flow-mediated dilatation (FMD) have demonstrated impaired endothelial function suggesting a significant degree of atherosclerotic macrovascular disease in this population[7].
The aim of this review is to discuss the various guises of cardiovascular morbidity in SSc with a special focus on potential pathogenetic mechanisms of macrovascular involvement. Aspects of disease-specific CVD such as myocardial dysfunction and pulmonary arterial hypertension (PAH) are also discussed.
Macrovascular atherosclerotic disease in SSc hypotheses
Connective tissue diseases are correlated with increased morbidity and mortality related mainly to cardiovascular events and progression of subclinical atherosclerosis [8]. SSc, is a chronic multisystem disorder characterized by widespread vascular impairment and accelerated fibrosis of the skin and internal organs with broad patient-to-patient variability [9] Microvascular injury is the pathognomonic feature of SSc culminating in manifestations such as Raynaud’s phenomenon, digital ulcers, pulmonary arterial hypertension, and renal crisis. Besides well-characterized microvascular involvement, a number of recent reports suggest a degree of macrovascular implication, as revealed by the detection of abnormalities in morphological and functional indices of vascular evaluation, namely, aortic wall stiffness, cIMT, and FMD [4, 10, 11]. Additionally, SSc patients may develop atherosclerotic alterations more frequently compared to the general population [7, 10]. Atherosclerotic wall damage in SSc is recognized as a multifactorial process, where not only traditional CVD risk factors but also endothelial damage, autoimmunity, and chronic inflammation play an important pathogenetic role [12].
Traditional and disease-related risk factors in SSc
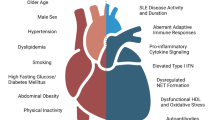
CVD is associated with the greatest burden of morbidity and mortality worldwide in both developed and developing countries. Traditional CVD risk factors such as smoking, elevated cholesterol levels, and high blood glucose are significant contributors to CVD in the general population [13] but their role in autoimmune diseases appears to be less prominent [14]. Renal crisis was the leading cause of death in SSc in the past; however, during the recent decades, the death rates due cardiopulmonary complications including CVD have substantially increased [15]. Nevertheless, conflicting evidence exists regarding the involvement of atherosclerosis in SSc patients [4].
Studies of small size have suggested that patients with SSc have similar or lower prevalence of traditional CVD risk factors compared with controls [16,17,18,19]. Man et al. [17] demonstrated no difference in frequencies of obesity, hyperlipidemia, hypertension, and diabetes between cases and controls [17]. Blood pressure occurrence was shown to be similar between SSc and controls [20]. Another study investigating 48 SSc patients and 46 healthy controls suggested only a slight increase in blood pressure and fasting glucose as well as a lower body mass index in SSc patients [21]. However, a recent Danish nationwide cohort study including 2778 SSc patients reported higher rates of hypertension and treated dyslipidemia among SSc patients compared to controls at baseline [22], indicating for first-time classic CVD risk factors as potential contributors to atherosclerosis in SSc disease setting.
In view of lipid profile alterations, the results of previous studies in SSc population have also been inconclusive. In a case-control study, the lipid levels of 31 female SSc patients were generally similar to those of 33 matched healthy controls [23]. In another study, Mok et al. demonstrated that SSc was significantly correlated with lower low-density lipoprotein levels, high-density lipoprotein levels, and body mass index compared to controls [24]. Additionally, in an older study by Ho et al., there were no significant differences in conventional cardiovascular risk factors, including lipid and glucose levels, as well as systolic and diastolic blood pressure, between SSc patients and controls [25].
Taking everything together, there are insufficient data to support that traditional CVD risk factors are more often in SSc patients compared to general population and large-scale studies are needed to define the precise impact of these parameters on increased CVD morbidity and mortality in this population. Given that—similarly to other systemic inflammatory diseases—classic CVD factors cannot explain on their own the heightened CVD risk; it has been suggested that disease itself represents a predisposing factor coupled with high levels of inflammation especially during the first year of the disease [26].
It is well recognized that vascular impairment in SSc is ubiquitous and occurs early in the course of the disease. It is characterized by endothelial cell activation and altered vascular tone. Raynaud’s phenomenon is one of the earliest signs of vasculopathy reflecting morphological changes in capillaries [27]. An impairment of endothelium-dependent vasodilation seems to take place before the beginning of clinical atherosclerosis in SSc [5]. Endothelial activation, the main initiating factor of vascular abnormalities and excess matrix accumulation typical for SSc, is likely to be triggered via a number of various reasons, including oxidative stress, hypoxia, and infection [27]. Activated endothelial cells induce the recruitment of adhesion molecules leading to increased perivascular inflammatory infiltrates, intimal fibrosis, and fibroblast overproduction. As a result, the balance between turnover and deposition of extracellular proteins is disrupted. Moreover, the overexpression of adhesion molecules contributes to a substantial endothelium homeostasis imbalance by promoting the synthesis of vasoconstrictors, predominantly endothelin 1, and by downregulating the production of vasodilation mediators, such as nitric oxide and prostacyclin [28].
Dysfunction of platelets also play an important role in the pathophysiology of vascular injury in SSc via the promotion of fibroblast activation and accumulation of vasoactive mediators, thus contributing to derangement of vascular hemostasis and the promotion of damage in micro- and macrovascular beds. Endothelial cell apoptosis in SSc is a significant factor of vascular impairment, and it is induced by antibody-dependent cell-mediated cytotoxicity via Fas-dependent pathway [29].
Autoimmune dysregulation is also actively implicated in derangement of vascular homeostasis as anticentromere antibodies have been linked to plaque formation and ischemic arterial events [30]. Antiphospholipid antibodies have also been associated with pulmonary arterial hypertension in a group of SSc patients [10]. Last but not least, there is mounting evidence that novel markers of atherosclerotic risk, such as homocysteine [31], lipoprotein[a] [23], and oxidized low-density lipoprotein [32], are more prevalent in SSc, but these results have not been evaluated in large-scale studies.
Atherosclerosis and coronary artery disease in SSc
The presence of macrovascular disease in SSc is not well established and definitely less studied compared to other complications such as visceral organ fibrosis, and the question whether atherosclerosis has an impact on morbidity in SSc remains to be answered. However, growing amount of evidence indicates that macrovascular atherosclerotic disease might lead to increased (20–40%) morbidity and mortality in SSc [9, 33, 34]. In a study with 5860 SSc patients, it was demonstrated that pulmonary fibrosis, pulmonary arterial hypertension, heart failure, and arrhythmias were the primary causes of death in this group of patients [9].
Coronary arteries pathology in SSc was initially evaluated by D’ Angelo et al. in 1969 in an autopsy-based study [35]. They found significant increase in atherosclerotic lesions in small coronary arteries and arterioles, whereas no difference was described in medium-size coronary arteries between SSc patients and controls. Later, Hesselstrand et al. reported that almost 20% of deaths in a Swedish cohort of SSc patients were correlated to CVD [36]. Table 1 shows the summary of the studies assessing the prevalence of coronary artery disease (CAD) and the risk of CVD events in SSc.
A recent nationwide Danish study suggested that SSc is a major cardiovascular risk factor for the composite endpoint of stroke, myocardial infarction (MI), and cardiovascular death, as well as for all-cause mortality [37]. Another recent investigation based on US Nationwide Inpatient Sample indicated that hospitalized SSc patients with atherosclerotic CVD are 1.3 times more likely to die compared to patients with SSc without atherosclerotic CVD [34]. The Australian Scleroderma Cohort Study, including 850 SSc as well as 15,787 and 8802 individuals as controls from the National Health Survey and the Australian Diabetes, Obesity, and Lifestyle Study, respectively, suggested that there is a greater than threefold increased prevalence of coronary artery disease defined as history of percutaneous coronary intervention, coronary artery bypass grafting, angina, or MI in SSc compared with the general population 15]. Interestingly enough, the findings were not associated with the presence of traditional CVD risk factors most of which were less prevalent in SSc patients. Similarly, a nationwide population-based prospective study found that SSc patients had a 2.45-fold risk for developing acute MI compared with the general population independently of relevant risk factors [9, 18].
Moreover, Man et al. [17] reported that the incidence of MI and stroke in patients with SSc was increased approximately 2-fold compared with healthy controls. The associations did not change substantially after adjustment for various CVD risk factors and after excluding patients with other autoimmune diseases. In line with the previous findings, it was documented that patients with SSc had an increased risk of MI and stroke in comparison to the general population [25].
The majority of the studies mentioned above support an increased prevalence of atherosclerotic CAD among SSc patients, which constitutes a rather novel observation. However, the aforementioned studies showed difference in the prevalence of CAD, which probably reflects the great heterogeneity regarding methodology, definition of cardiovascular events, patient sample, and predefined outcomes. Consequently, there is a great need for large-scale well-designed studies in order to evaluate the contribution of large-vessel disease to high CVD morbidity and mortality in SSc patients.
Table 1 summarizes the comparative studies between SSc patients and controls in view of CVD outcomes.
Micro- and macrovascular disease in SSc sclerosis: two sides of the same coin?
In view of the recent developments and the better understanding of vascular complications in SSc, a number of studies have attempted to investigate possible correlations between micro- and macrovascular dysfunction. Nailfold videocapillaroscopy is an established, non-invasive, and reproducible method for the assessment of microcirculation with expanding implications in the assessment of SSc patients ranging from the diagnostic approach to the follow-up and prognostic utility regarding internal organ complications [39, 40]. Non-invasive assessments of endothelial function including pulse wave velocity (PWV) for the measurement of arterial stiffness, cIMT, and FMD are validated, reliable techniques for the evaluation of vascular health [41]. Only a handful of small studies have explored whether micro- and macrovascular injury in SSc are interrelated. In a case-control study of 39 SSc Korean patients, it was found that FMD levels did not differ significantly between the early/active and late capillary groups, although PWV is found to be already elevated in SSc patients with early NCV changes [42]. Another case-control study of 43 SSc patients reported that FMD was already reduced in SSc patients with early pattern, and that lower FMD values are found in patients with the late pattern compared to those with active and early patterns [43]. cIMT was significantly associated with the presence of avascular areas in a cohort of 115 SSc patients [44]. A recent study of 37 consecutive SSc patients found a significant correlation between Augmentation Index (AIx) and the average number of capillaries/mm2 (r = − 0.34, p = 0.047) and between AIx and the capillaroscopic skin ulcer risk index (CSURI) (r = 0.35, p = 0.44). This study suggests that microvascular vasculopathy is associated with higher wave reflections, indicating an association between atherosclerotic disease and microvascular injury in SSc patients [45]. Collectively, these reports point towards a possible connection between distinct aspects of vascular involvement in SSc, indicating significant correlations between progressive microvasculopathy and parameters of atherosclerotic macrovascular disease. Whether micro- and macrocirculation constitute different forms of endothelial dysfunction in patients with SSc remains to be addressed in large, longitudinal studies.
Primary myocardial involvement in SSc
Cardiac involvement in SSc is usually asymptomatic, but when clinically apparent, it is associated with poor prognosis [46]. Primary scleroderma heart involvement (SHI)—in contrast to secondary SHI due to lung or kidney involvement—is the result of myocardial fibrosis accompanied by disturbance of coronary microcirculation due to functional and structural vascular damage [47]. Observational data suggest that SHI is more common in the diffuse cutaneous form of the disease [48]. Regarding its association with scleroderma-specific autoantibodies, SHI is associated with antibodies against Ku antigen (anti-Ku) [49]; anti-histone antibodies [50] and anti-RNA polymerase antibodies I, II, and III [51]; antibodies against topoisomerase I (anti-Scl70); and anti-fibrillarin antibodies (anti-U3-RNP) [52].
Manifestations of cardiac involvement in SSc patients may involve all heart sub-structures, leading to myocardial ischemia and hypertrophy, conduction system abnormalities, pericardial effusion, and heart failure. The hallmark of heart disease in SSc is myocardial fibrosis with patchy fibrotic deposits, equally distributed throughout left and right ventricular myocardium [7]. Results from previous studies suggest that a degree of myocardial perfusion abnormalities may be reversible, whereas others remain unchanged leading to the hypothesis of the coexistence of ischemic lesions accessible to reperfusion after small coronary vasospasm and irreversible lesions (i.e., organic vessel disease and myocardial fibrosis) [53,54,55,56]. Myocardial blood flow disturbances in SSc may be the result of microvascular alterations, but not from the traditional atherosclerotic coronary disease. The “myocardial Raynaud’s phenomenon” (vasospasm of the small coronary arteries and arterioles) is thought to be involved in the development of structural vascular alterations resulting from the early scleroderma-related ischemic myocardial changes with subsequent ischemia reperfusion injury [48]. Subsequently, this phenomenon might be more relevant in the preclinical phase of myocardial involvement, while in the course of the disease, collagen deposition and damage of the coronary capillary bed becomes more evident [57]. Another characteristic feature of myocardial involvement is foci of contraction band necrosis in all parts of the myocardium, including the immediate subendocardial area, usually spared in the atherosclerotic disease [58, 59]. Contraction band necrosis along with severe fibrosis is most frequent in primary SHI rather than secondary SHI [60, 61]. In an autopsy study, MI was found in patients with normal coronary arteries [62]. Furthermore, hemosiderin myocardial deposits are not typically seen in SSc-related myocardial pathology but are evident in the atherosclerotic process [48]. One of the main characteristics of myocardial fibrosis is diastolic left ventricular dysfunction with well-preserved ejection fraction, and less frequently systolic dysfunction, which may be both asymptomatic [63]. Worsening of myocardial function has been associated with several indices of disease progression, namely, age, active digital ulcer, lung fibrosis, muscle weakness, and elevated CRP in large cohort of 1451 SSc individuals [64]. In addition, renal crisis is usually associated with considerable deterioration of heart function leading to heart failure and pulmonary edema [65]. Pericardial disease is another cardiac manifestation, symptomatic in a low percentage of patients [66]. Constrictive pericarditis can also be present. Clinical manifestation may be due to both constrictive pericarditis and restriction because of myocardial fibrosis. Other cardiac manifestations, such as conduction abnormalities, are a consequence of conduction system fibrosis and rarely correlated with myocardial involvement [48].
Table 2 describes the main pathognomonic features of myocardial involvement in SSc.
Cardiac rhythm disturbances are common among patients with SSc, and 6% of the cardiovascular mortality was secondary to the aforementioned according to the 2010 EULAR Scleroderma Trial and Research group (EUSTAR) database [9]. Arrhythmias are attributed mostly to myocardial fibrosis, and the consequent electrical instability is considered to play the key role in arrhythmogenesis [67]. Different forms of atrial and ventricular arrhythmias may occur [57]. Sudden cardiac death is more likely among SSc patients with arrhythmias, especially ventricular, compared with the arrhythmia-free patients in the Genetics versus Environment in Scleroderma Outcome Study (GENISOS) cohort study [68]. A useful tool in the prediction of ventricular arrhythmias and sudden cardiac death may be the measurement of certain parameters of the 12-lead surface electrocardiogram, more specifically the prolongation of QT interval and dispersion, but not yet part of the risk stratification in SSC [69]. Furthermore, cardiovascular magnetic resonance offers considerable additional utility in SSc by revealing cardiac involvement in early, asymptomatic diffuse SSc with normal routine cardiac evaluation and may allow the identification of myocardial edema and fibrosis [70, 71]. To date, cardiovascular magnetic resonance is considered the non-invasive gold standard for imaging macroscopic myocardial fibrosis. Just recently, Scleroderma Arrhythmia Clinical Utility Study (SAnCtUS), a multicentre, longitudinal study including 150 SSc patients prospectively recruited between 2010 and 2016, formed a score for the identification of patients at high risk of ventricular rhythm disturbances [72]. The SAnCtUS score is a four-category scoring system based on T2 ratio in cardiovascular magnetic resonance and late gadolinium enhancement, and increasing scores are associated with a greater prevalence of most types of rhythm disturbances at baseline [72]. Surrogate biochemical markers such as troponin and natriuretic peptides may also assist in the identification of patients requiring diligent surveillance and/or cardioprotective treatment [73]. Last but not least, electrophysiological studies with 24 h recording and implantable loop recorder may constitute an additional prognostic tool for the identification of patients at higher risk for arrhythmias [74]. Taken all together, a composite of non-invasive electrophysiological studies, serum cardiac biomarkers, and cardiac magnetic resonance could be utilized for the diagnostic workout of SHI in this population.
Pulmonary arterial hypertension
SSc-PAH is one of the most devastating complications of SSc affecting about 8 to 15% of patients [75, 76], while SSc is the most frequent cause of PAH associated with connective tissue disease [77]. According to the 6th World Symposium on Pulmonary Hypertension Task Force, the new hemodynamic definition of PAH includes a mean pulmonary arterial pressure (mPAP) > 20 mmHg, a pulmonary artery wedge pressure (PAWP) < 15 mmHg, and a pulmonary vascular resistance (PVR) ≥ 3 Wood Units [78]. Common pathophysiologic mechanisms encompass cell hyperproliferation and vasoconstriction, smooth muscle cell hypertrophy, intimal fibrosis, and microthrombosis, all of which lead to a progressive remodeling of small- and medium-sized pulmonary arterioles that results in obstructive pulmonary arteriopathy typically occurred in PAH [71, 79].
PAH is one of the leading causes of death in SSc patients and an independent predictor of mortality [80]. Despite recent therapeutic advances, survival remains poor and is worse compared to idiopathic PAH or PAH associated with congenital heart disease [81,82,83]. The REVEAL Registry reported 3-year survival rates among 344 prevalent and 166 incident SSc-PAH patients to be 61.4% ± 2.7% and 51.2% ± 4.0%, respectively [84]. The PHAROS Registry reported better survival in 160 incident SSc-PAH patients with 1-, 3-, 5-, and 8-year cumulative survival rates of 95%, 75%, 63%, and 49%, respectively [85]. Male gender, older age at diagnosis of SSc, diffuse SSc, increased PVR and mean right atrial pressure, short 6-min walk distance, and low diffusion lung capacity are some independent predictors of mortality in SSc-PAH [80, 84, 85]. The coexistence of pulmonary fibrosis and PAH results in dramatic deterioration of outcomes and survival [86].
Early diagnosis of PAH in SSc patients is challenging but also essential as early targeted PAH pharmacotherapy results in better long-term outcomes. Non-specific clinical manifestations such as dyspnea, fatigue, and exercise intolerance often lead to a delayed diagnosis for more than 2 years from symptom onset. In addition, pulmonary hypertension in SSc may be attributed to other causes, such as left heart disease, interstitial lung disease, or chronic thromboembolic pulmonary hypertension, which should be ruled out following a diagnostic algorithm proposed by the European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines [87]. A high index of clinical suspicion is required for further diagnostic work up in these patients. Right heart catheterization is the gold standard procedure for the diagnosis of PAH. According to ESC/ERS guidelines, symptomatic SSc patients must undergo right heart catheterization if transthoracic echocardiography reveals a tricuspid regurgitation velocity more than 2.8 m/s, or in case of a lower tricuspid regurgitation, velocity along with other echocardiographic signs that suggest intermediate or high probability for PAH [87].
Several screening tests have been also proposed for the early detection of PAH considering the high prevalence and mortality among SSc patients [88]. For asymptomatic patients with uncorrected diffusion lung capacity < 80% of predicted value, annual screening should be considered. Both the ESC/ERS and American Heart Association (AHA) guidelines recommend initial screening upon SSc diagnosis by echocardiography [87, 89]. The DETECT algorithm uses a combination of clinical, laboratory, electrocardiographic, and pulmonary function parameters for initial screening, with subsequent transthoracic echocardiogram if the patients are considered as high risk [90]. The Australian Scleroderma Interest Group (ASIG) algorithm incorporates NT-proBNP and pulmonary function tests for initial screening with subsequent transthoracic echocardiogram if initial screening is positive [91]. If any of these tests are positive, then right heart catheterization should be performed to confirm or rule out PAH. In addition, the progression from an early to an active or late scleroderma pattern on NVC is also a predictor for the development of PAH in SSc [91, 92].
Pulmonary arterial vasodilator therapies have dramatically reduced morbidity and mortality and improved quality of life among patients with PAH [93]. PAH-specific treatments include four classes of medications which are endothelin-receptor antagonists, prostacyclin analogs, phosphodiesterase-5 inhibitors, and guanylate cyclase stimulators, which are largely extrapolated from idiopathic PAH clinical trials due to the smaller number of patients with SSc-PAH. Current proposed management is treating newly diagnosed, therapy-naive SSc-PAH patients with an upfront combination of two oral medications [94]. The application of the available risk stratification algorithms both at diagnosis and during follow-up is mandatory for the estimation of 1-year mortality risk in SSc-PAH patients in order to guide possible intensification of targeted treatment using a triple combination therapy for patients at high risk [94,95,96].
Management of CVD risk in SSc
The management of CVD risk in SSc patients is demanding and ideally needs to be based on principles and practical steps that clinicians should follow. As in other systemic disorders, the interplay between systemic inflammation, autoimmunity, classical CVD factors enhanced by disease-specific vasculopathy and fibrosis of visceral organs poses a number of difficulties in both assessment and management of CVD risk. The optimal control of the various aspects of the disease with vasodilators, immunosuppressants, and more recently antifibrotic drugs may contribute to the improvement of myocardial function and the reduction of atherosclerotic disease burden [97]. For example, EUSTAR database analysis concluded that treatment with calcium channels blockers—routinely administrated for Raynaud’s phenomenon—may also play a protective role against the development of left ventricular dysfunction [98]. In addition, vasoactive agents prescribed for the treatment of SSc-PAH may also improve overall myocardial performance in SSc with beneficial effects on cardiac function [99]. On the other hand, intensive immunosuppressive treatment such as intravenous steroids and/or cyclophosphamide could halt inflammatory process in cardiac tissue defined as myocarditis and/or myocardial edema in cardiac magnetic resonance–based studies [100, 101] (Table 3).
Regular screening for CVD risk factors is essential and initiation of antihypertensives or lipid lowering agents should follow guidelines for general population. Last but not least, the complexity of disease mandates the cooperation across a wide spectrum of medical specialties including not only rheumatologists and cardiologists but also a number of experts in CVD imaging as well as allied health professionals as necessary for each patient needs.
Conclusions
CVD is a frequent complication of autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis. In the last few years, a number of studies suggest that patients with SSc may develop atherosclerotic alterations more often compared to the healthy individuals. The mechanisms promoting macrovascular disease in SSc are not fully understood. There are insufficient data to support the contribution of traditional risk factors to the onset and progression of atherosclerosis per se, and it is postulated that macrovascular involvement in SSc is rather secondary to disease-related factors, such as inflammation, endothelial wall damage, and autoimmunity. Based on small-scale studies, there is a correlation between progressive microvasculopathy and parameters of atherosclerotic macrovascular disease, indicating that micro- and macrocirculation may constitute different sides of the same coin. Thus, the management of CVD risk in SSc is highly essential. Ideally, the use of vasodilators, immunosuppressants, and more recently antifibrotic drugs may contribute to the improvement of myocardial function and the reduction of atherosclerotic disease burden. Last but not least, large-scale and well-organized studies are mandatory in order to have a better understanding of the underlying mechanisms leading to large-vessel involvement and CVD in SSc.
Abbreviations
- CVD:
-
cardiovascular disease
- SSc:
-
systemic sclerosis
- ESC/ERS:
-
European Society of Cardiology/European Respiratory Society
- PAH:
-
pulmonary arterial hypertension
- SHI:
-
scleroderma heart involvement
- FMD:
-
flow-mediated dilatation
- cIMT:
-
carotid intima-media thickness
- CAD:
-
coronary artery disease
References and Recommended Reading
Katsumoto TR, Whitfield ML, Connolly MK. The pathogenesis of systemic sclerosis. Annu Rev. Pathol Mech Dis. 2011;6(1):509–37. https://doi.org/10.1146/annurev-pathol-011110-130,312.
Van Den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an american college of rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. 2013;65(11):2737–47. https://doi.org/10.1002/art.38098.
Butt SA, Jeppesen JL, Fuchs C, Mogensen M, Engelhart M, Torp-Pedersen C, et al. Trends in incidence, mortality, and causes of death associated with systemic sclerosis in Denmark between 1995 and 2015: a nationwide cohort study. BMC Rheumatol. 2018;2:36. https://doi.org/10.1186/s41927-018-0043-6.
Nussinovitch U, Shoenfeld Y. Atherosclerosis and macrovascular involvement in systemic sclerosis: myth or reality. Autoimmun Rev. 2011;10(5):259–66. https://doi.org/10.1016/j.autrev.2010.09.014.
Cannarile F, Valentini V, Mirabelli G, et al. Cardiovascular disease in systemic sclerosis. Ann Transl Med. 2015;3(1):8. https://doi.org/10.3978/j.issn.2305-5839.2014.12.12.
Sherer Y, Shoenfeld Y. Mechanisms of disease: atherosclerosis in autoimmune diseases. Nat Clin Pract Rheumatol. 2006:99–106. https://doi.org/10.1038/ncprheum0092.
Psarras A, Soulaidopoulos S, Garyfallos A. A critical view on cardiovascular risk in systemic sclerosis. Rheumatol Int. 2016. https://doi.org/10.1007/s00296-016-3530-3.
Pokeerbux MR, Giovannelli J, Dauchet L, Mouthon L, Agard C, Lega JC, et al. Survival and prognosis factors in systemic sclerosis: data of a French multicenter cohort, systematic review, and meta-analysis of the literature. Arthritis Res Ther. 2019 Apr 3;21(1):86. https://doi.org/10.1186/s13075-019-1867-1.
Tyndall AJ, Bannert B, Vonk M, Airo P, Cozzi F, Carreira PE, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69:1809–15. https://doi.org/10.1136/ard.2009.114264.
Soriano A, Afeltra A, Shoenfeld Y. Is atherosclerosis accelerated in systemic sclerosis? Novel insights. Curr Opin Rheumatol. 2014;26:653–7. https://doi.org/10.1097/BOR.0000000000000115.
Hettema ME, Bootsma H, Kallenberg CG. Macrovascular disease and atherosclerosis in SSc. Rheumatology (Oxford). 2008;47:578–83. https://doi.org/10.1093/rheumatology/ken078.
Oreska S, Tomcik M. Atherosclerosis and cardiovascular risk in systemic sclerosis, systemic sclerosis, Mislav Radic, editors. IntechOpen. 2017.
Gikas A, Lambadiari V, Sotiropoulos A, Panagiotakos D, Pappas S. Prevalence of major cardiovascular risk factors and coronary heart disease in a sample of Greek adults: The Saronikos Study. Open Cardiovasc Med J. 2016;10:69–80. https://doi.org/10.2174/1874192401610010069.
Zegkos T, Kitas G, Dimitroulas T. Cardiovascular risk in rheumatoid arthritis: assessment, management and next steps. Ther Adv Musculoskelet Dis. 2016;8(3):86–101. https://doi.org/10.1177/1759720X16643340.
Simeón-Aznar CP, Fonollosa-Plá V, Tolosa-Vilella C, Espinosa-Garriga G, Campillo-Grau M, Ramos-Casals M, et al. Registry of the Spanish Network for Systemic Sclerosis: survival, prognostic factors, and causes of death. Medicine (Baltimore). 2015;94:e1728. https://doi.org/10.1097/MD.0000000000001728.
Ngian GS, Sahhar J, Proudman SM, Stevens W, Wicks IP, Van Doornum S. Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis. 2012;71:1980–3. https://doi.org/10.1136/annrheumdis-2011-201,176.
Man A, Zhu Y, Zhang Y, Dubreuil M, Rho YH, Peloquin C, et al. The risk of cardiovascular disease in systemic sclerosis: a population- based cohort study. Ann Rheum Dis. 2013;72:1188–93. https://doi.org/10.1136/annrheumdis-2012-202,007.
Chu SY, Chen YJ, Liu CJ, Tseng WC, Lin MW, Hwang CY, et al. Increased risk of acute myocardial infarction in systemic sclerosis: a nationwide population-based study. Am J Med. 2013;126:982–8. https://doi.org/10.1016/j.amjmed.2013.06.025.
Ali H, Ng KR, Low AH. A qualitative systematic review of the prevalence of coronary artery disease in systemic sclerosis. Int J Rheum Dis. 2015;18:276–86. https://doi.org/10.1111/1756-185X.12566.
Zeng Y, Li M, Xu D, Hou Y, Wang Q, Fang Q, et al. Macrovascular involvement in systemic sclerosis: Evidence of correlation with disease activity. Clin Exp Rheumatol. 2012;30:S76–80.
Butt SA, Jeppesen JL, Torp-Pedersen C, Sam F, Gislason GH, Jacobsen S, et al. Cardiovascular Manifestations of Systemic Sclerosis: a Danish Nationwide Cohort Study. J Am Heart Assoc. 2019;8:e013405. https://doi.org/10.1161/JAHA.119.013405.
Lippi G, Caramaschi P, Montagnana M, Salvagno GL, Volpe A, Guidi G, et al. Lipoprotein[a] and the lipid profile in patients with systemic sclerosis. Clin Chim Acta. 2006;364:345–8. https://doi.org/10.1016/j.cca.2005.07.015.
Mok MY, Lau CS, Chiu SS, Tso AW, Lo Y, Law LS, et al. Systemic sclerosis is an independent risk factor for increased coronary artery calcium deposition. Arthritis Rheum. 2011;63:1387–95. https://doi.org/10.1002/art.30283.
Ho M, Veale D, Eastmond C, Nuki G, Belch J. Macrovascular disease and systemic sclerosis. Ann Rheum Dis. 2000;59:39–43. https://doi.org/10.1136/ard.59.1.39.
Avina-Zubieta JA, Man A, Yurkovich M, Huang K, Sayre EC, Choi HK. Early cardiovascular disease after the diagnosis of systemic sclerosis. Am J Med. 2016;129:324–31. https://doi.org/10.1016/j.amjmed.2015.10.037.
Abraham D, Distler O. How does endothelial cell injury start? The role of endothelin in systemic sclerosis. Arthritis Res Ther. 2007;9. https://doi.org/10.1186/ar2186.
Abraham DJ, Krieg T, Distler J, Distler O. Overview of pathogenesis of systemic sclerosis. Rheumatology (Oxford). 2009;48(Suppl 3):iii3–7. https://doi.org/10.1093/rheumatology/ken481.
Zakopoulos NA, Kotsis VT, Gialafos EJ, Papamichael CM, Pitiriga VC, Mitsibounas DN, et al. Systemic sclerosis is not associated with clinical or ambulatory blood pressure. Clin Exp Rheumatol. 2003;21:199–204.
Sgonc R, Gruschwitz MS, Boeck G, Sepp N, Gruber J, Wick G, et al. Endothelial cell apoptosis in systemic sclerosis is induced by antibody-dependent cell-mediated cytotoxicity via CD95. Arthritis Rheum. 2000;43:2550–62. https://doi.org/10.1002/1529-0131(200011)43:11<2550::AID-ANR24>3.0.CO;2-H.
Nordin A, Jensen-Urstad K, Björnådal L, Pettersson S, Larsson A, Svenungsson E, et al. Ischemic arterial events and atherosclerosis in patients with systemic sclerosis: a population-based case-control study. Arthritis Res Ther. 2013;15:R87. https://doi.org/10.1186/ar4267.
Khurma V, Meyer C, Park GS, McMahon M, Lin J, Singh RR, et al. A pilot study of subclinical coronary atherosclerosis in systemic sclerosis: coronary artery calcification in cases and controls. Arthritis Rheum. 2008;59:591–7. https://doi.org/10.1002/art.23540.
Palinski W, Hörkkö S, Miller E, Steinbrecher UP, Powell HC, Curtiss LK, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest. 1996;98:800–14. https://doi.org/10.1172/JCI118853.
Ungprasert P, Sanguankeo A, Upala S. Risk of ischemic stroke in patients with systemic sclerosis: a systematic review and meta-analysis. Mod Rheumatol. 2016;26:128–31. https://doi.org/10.3109/14397595.2015.1056931.
Dave AJ, Fiorentino D, Lingala B, Krishnan E, Chung L. Atherosclerotic cardiovascular disease in hospitalized patients with systemic sclerosis: higher mortality than patients with lupus and rheumatoid arthritis. Arthritis Care Res (Hoboken). 2014;66:323–7. https://doi.org/10.1002/acr.22152.
D’Angelo WA, Fries JF, Masi AT, Shulman LE. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med. 1969;46:428–40. https://doi.org/10.1016/0002-9343(69)90044-8.
Hesselstrand R, Scheja A, Akesson A. Mortality and causes of death in a Swedish series of systemic sclerosis patients. Ann Rheum Dis. 1998;57(11):682–6. https://doi.org/10.1136/ard.57.11.682.
Hesselvig JH, Kofoed K, Wu JJ, Dreyer L, Gislason G, Ahlehoff O. Localized scleroderma, systemic sclerosis and cardiovascular risk: a danish nationwide cohort study. Acta Derm Venereol. 2018;98:361–5. https://doi.org/10.2340/00015555-2842.
Youssef P, Brama T, Englert H, Bertouch J. Limited scleroderma is associated with increased prevalence of macrovascular disease. J Rheumatol. 1995;22:469–72.
Soulaidopoulos S, Triantafyllidou E, Garyfallos A, Kitas GD, Dimitroulas T. The role of nailfold capillaroscopy in the assessment of internal organ involvement in systemic sclerosis: a critical review. Autoimmun Rev. 2017;16(8):787–95. https://doi.org/10.1016/j.autrev.2017.05.019.
Repa A, Avgoustidis N, Kougkas N, Bertsias G, Zafiriou M, Sidiropoulos P. Nailfold Videocapillaroscopy as a candidate biomarker for organ involvement and prognosis in patients with systemic sclerosis. Mediterr J Rheumatol. 2019;30(1):48–50. https://doi.org/10.31138/mjr.30.1.48.
Sandoo A. Important considerations for examining endothelial dysfunction in rheumatoid arthritis. Mediterr J Rheumatol. 2017;28(3):112–5. https://doi.org/10.31138/mjr.28.3.112.
Jung KH, Lim MJ, Kwon SR, Kim D, Joo K, Park W. Nailfold capillary microscopic changes and arterial stiffness in Korean systemic sclerosis patients. Modern rheumatology. 2015;25(2):328–31. https://doi.org/10.3109/14397595.2014.881955.
Rollando D, Bezante GP, Sulli A, Balbi M, Panico N, Pizzorni C, et al. Brachial artery endothelial-dependent flow-mediated dilation identifies early-stage endothelial dysfunction in systemic sclerosis and correlates with nailfold microvascular impairment. J Rheumatol. 2010;37(6):1168–73. https://doi.org/10.3899/jrheum.091116.
Alegre Sancho JJ, Robustillo Villarino M, Albert Espí G, Vergara Dangond C, Vicens Bernabeu E, Valls Pascual È, et al. SAT0197 Capillaroscopy and macrovascular disease in patients with systemic sclerosis. Annals of the Rheumatic Diseases. 2016;75(Suppl 2):739. https://doi.org/10.1136/annrheumdis-2016-eular.4652.
Soulaidopoulos S, Pagkopoulou E, Katsiki N, Triantafyllidou E, Karagiannis A, Garyfallos A, et al. Arterial stiffness correlates with progressive nailfold capillary microscopic changes in systemic sclerosis: results from a cross-sectional study. Arthritis research & therapy. 2019;21(1):253. https://doi.org/10.1186/s13075-019-2051-3.
Clements PJ, Lachenbruch PA, Furst DE, Paulus HE, Sterz MG. Cardiac score. A semiquantitative measure of cardiac involvement that improves prediction of prognosis in systemic sclerosis. Arthritis and rheumatism. 1991;34(11):1371–80. https://doi.org/10.1002/art.1780341105.
Kahan A, Allanore Y. Primary myocardial involvement in systemic sclerosis. Rheumatology (Oxford, England). 2006;45(Suppl 4):iv14–7. https://doi.org/10.1093/rheumatology/kel312.
Lambova S. Cardiac manifestations in systemic sclerosis. World journal of cardiology. 2014;6(9):993–1005. https://doi.org/10.4330/wjc.v6.i9.993.
Rodriguez-Reyna TS, Hinojosa-Azaola A, Martinez-Reyes C, Nunez-Alvarez CA, Torrico-Lavayen R, Garcia-Hernandez JL, et al. Distinctive autoantibody profile in Mexican Mestizo systemic sclerosis patients. Autoimmunity. 2011;44(7):576–84. https://doi.org/10.3109/08916934.2011.592886.
Hesselstrand R, Scheja A, Shen GQ, Wiik A, Akesson A. The association of antinuclear antibodies with organ involvement and survival in systemic sclerosis. Rheumatology (Oxford, England). 2003;42(4):534–40. https://doi.org/10.1093/rheumatology/keg170.
Kuwana M, Kaburaki J, Okano Y, Tojo T, Homma M. Clinical and prognostic associations based on serum antinuclear antibodies in Japanese patients with systemic sclerosis. Arthritis and rheumatism. 1994;37(1):75–83. https://doi.org/10.1002/art.1780370111.
Steen VD. Autoantibodies in systemic sclerosis. Seminars in arthritis and rheumatism. 2005;35(1):35–42. https://doi.org/10.1016/j.semarthrit.2005.03.005.
Kahan A, Devaux JY, Amor B, Menkes CJ, Weber S, Nitenberg A, et al. Nifedipine and thallium-201 myocardial perfusion in progressive systemic sclerosis. The New England journal of medicine. 1986;314(22):1397–402. https://doi.org/10.1056/nejm198605293142201.
Kahan A, Devaux JY, Amor B, Menkes CJ, Weber S, Foult JM, et al. Pharmacodynamic effect of dipyridamole on thallium-201 myocardial perfusion in progressive systemic sclerosis with diffuse scleroderma. Ann Rheum Dis. 1986;45(9):718–25. https://doi.org/10.1136/ard.45.9.718.
Kahan A, Devaux JY, Amor B, Menkes CJ, Weber S, Venot A, et al. Nicardipine improves myocardial perfusion in systemic sclerosis. The Journal of rheumatology. 1988;15(9):1395–400.
Kahan A, Devaux JY, Amor B, Menkes CJ, Weber S, Venot A, et al. The effect of captopril on thallium 201 myocardial perfusion in systemic sclerosis. Clinical pharmacology and therapeutics. 1990;47(4):483–9. https://doi.org/10.1038/clpt.1990.61.
Ferri C, Giuggioli D, Sebastiani M, Colaci M, Emdin M. Heart involvement and systemic sclerosis. Lupus. 2005;14(9):702–7. https://doi.org/10.1191/0961203305lu2204oa.
Bulkley BH, Ridolfi RL, Salyer WR, Hutchins GM. Myocardial lesions of progressive systemic sclerosis. A cause of cardiac dysfunction. Circulation. 1976;53(3):483–90. https://doi.org/10.1161/01.cir.53.3.483.
Tzelepis GE, Kelekis NL, Plastiras SC, Mitseas P, Economopoulos N, Kampolis C, et al. Pattern and distribution of myocardial fibrosis in systemic sclerosis: a delayed enhanced magnetic resonance imaging study. Arthritis and rheumatism. 2007;56(11):3827–36. https://doi.org/10.1002/art.22971.
Deswal A, Follansbee WP. Cardiac involvement in scleroderma. Rheumatic diseases clinics of North America. 1996;22(4):841–60. https://doi.org/10.1016/s0889-857x(05)70304-5.
Ferri C, Emdin M, Nielsen H, Bruhlmann P. Assessment of heart involvement. Clinical and experimental rheumatology. 2003;21(3 Suppl 29):S24–8.
Bulkley BH, Klacsmann PG, Hutchins GM. Angina pectoris, myocardial infarction and sudden cardiac death with normal coronary arteries: a clinicopathologic study of 9 patients with progressive systemic sclerosis. American heart journal. 1978;95(5):563–9. https://doi.org/10.1016/0002-8703(78)90297-1.
Konstantopoulou P, Gialafos E, Moyssakis I, Tountas C, Konsta M, Vaiopoulos G, et al. Evolution and management of late onset cardiac involvement in a contemporary systemic sclerosis cohort. Mediterr J Rheumatol. 2016;27(3):102–7.
Becker M, Graf N, Sauter R, Allanore Y, Curram J, Denton CP, et al. EUSTAR Collaborators; EUSTAR Collaborators (numerical order of centres). Predictors of disease worsening defined by progression of organ damage in diffuse systemic sclerosis: a European Scleroderma Trials and Research (EUSTAR) analysis. Ann Rheum Dis. 2019 Sep;78(9):1242–8. https://doi.org/10.1136/annrheumdis-2019-215,145.
Amarnani A, Wengrofsky P, Tsui CL, Kariyanna PT, Kabani N, Salciccioli L, et al. Acute heart failure in scleroderma renal crisis: a case study for review of cardiac disease in systemic sclerosis. Am J Med Case Rep. 2020;8(1):1–7. https://doi.org/10.12691/ajmcr-8-1-1.
Gottdiener JS, Moutsopoulos HM, Decker JL. Echocardiographic identification of cardiac abnormality in scleroderma and related disorders. The American journal of medicine. 1979;66(3):391–8. https://doi.org/10.1016/0002-9343(79)91057-x.
Vacca A, Meune C, Gordon J, Chung L, Proudman S, Assassi S, et al. Cardiac arrhythmias and conduction defects in systemic sclerosis. Rheumatology (Oxford, England). 2014;53(7):1172–7. https://doi.org/10.1093/rheumatology/ket377.
Assassi S, Del Junco D, Sutter K, McNearney TA, Reveille JD, Karnavas A, et al. Clinical and genetic factors predictive of mortality in early systemic sclerosis. Arthritis and rheumatism. 2009;61(10):1403–11. https://doi.org/10.1002/art.24734.
Sebestyen V, Szucs G, Pall D, Ujvarosy D, Otvos T, Csige I, et al. Electrocardiographic markers for the prediction of ventricular arrhythmias in patients with systemic sclerosis. Rheumatology (Oxford, England). 2020;59(3):478–86. https://doi.org/10.1093/rheumatology/kez644.
Markousis-Mavrogenis G, Bournia VK, Panopoulos S, Koutsogeorgopoulou L, Kanoupakis G, et al. Cardiovascular magnetic resonance identifies high-risk systemic sclerosis patients with normal echocardiograms and provides incremental prognostic value. Diagnostics (Basel). 2019;9(4):pii: E220. https://doi.org/10.3390/diagnostics9040220.
Dimitroulas T, Giannakoulas G, Karvounis H, Settas L, Kitas GD. Systemic sclerosis-related pulmonary hypertension: unique characteristics and future treatment targets. Curr Pharm Des. 2012;18(11):1457–64. https://doi.org/10.2174/138161212799504704.
Mavrogeni S, Gargani L, Pepe A, Monti L, Markousis-Mavrogenis G, Santis M, et al. Cardiac magnetic resonance predicts ventricular arrhythmias in scleroderma: the Scleroderma Arrhythmia Clinical Utility Study (SAnCtUS). Rheumatology (Oxford, England). 2019. https://doi.org/10.1093/rheumatology/kez494.
Nordin A, Svenungsson E, Björnådal L, Elvin K, Larsson A, Jensen-Urstad K. Troponin I and echocardiography in patients with systemic sclerosis and matched population controls. Scand J Rheumatol. 2017 May;46(3):226–35. https://doi.org/10.1080/03009742.2016.1192217.
Bissell LA, Dumitru RB, Erhayiem B, Abignano G, Fent G, Kidambi A, et al. Abnormal electrophysiological testing associates with future incidental significant arrhythmia in scleroderma. Rheumatology (Oxford). 2020 Apr 1;59(4):899–900. https://doi.org/10.1093/rheumatology/kez434.
Morrisroe K, Stevens W, Proudman S, Nikpour M. A systematic review of the epidemiology, disease characteristics and management of systemic sclerosis in Australian adults. Int J Rheum Dis. 2017;20(11):1728–50. https://doi.org/10.1111/1756-185X.13203.
Hachulla E, Gressin V, Guillevin L, Carpentier P, Diot E, Sibilia J, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum. 2005;52(12):3792–800.
Arvanitaki A, Boutsikou M, Anthi A, Apostolopoulou S, Avgeropoulou A, Demerouti E, et al. Epidemiology and initial management of pulmonary arterial hypertension: real-world data from the Hellenic pulmOnary hyPertension rEgistry (HOPE). Pulm Circ. 2019;9(3):2045894019877157. https://doi.org/10.1177/2045894019877157.
Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1). https://doi.org/10.1183/13993003.01913-2018.
Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, et al. Inflammation, growth fators, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54(1 Suppl):S10–9.
Morrisroe K, Stevens W, Huq M, Prior D, Sahhar J, Ngian GS, et al. Survival and quality of life in incident systemic sclerosis-related pulmonary arterial hypertension. Arthritis Res Ther. 2017;19(1):122. https://doi.org/10.1186/s13075-017-1341-x.
Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173(9):1023–30. https://doi.org/10.1164/rccm.200510-1668OC.
McGoon MD, Miller DP. REVEAL: a contemporary US pulmonary arterial hypertension registry. Eur Respir Rev. 2012;21(123):8–18.
Escribano-Subias P, Blanco I, Lopez-Meseguer M, Lopez-Guarch CJ, Roman A, Morales P, et al. Survival in pulmonary hypertension in Spain: insights from the Spanish registry. Eur Respir J. 2012;40(3):596–603. https://doi.org/10.1183/09031936.00101211.
Chung L, Domsic RT, Lingala B, Alkassab F, Bolster M, Csuka ME, et al. Survival and predictors of mortality in systemic sclerosis-associated pulmonary arterial hypertension: outcomes from the pulmonary hypertension assessment and recognition of outcomes in scleroderma registry. Arthritis Care Res (Hoboken). 2014;66(3):489–95.
Kolstad KD, Li S, Steen V, Chung L, Investigators P. Long-term outcomes in systemic sclerosis-associated pulmonary arterial hypertension From the Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma Registry (PHAROS). Chest. 2018;154(4):862–71. https://doi.org/10.1016/j.chest.2018.05.002.
Michelfelder M, Becker M, Riedlinger A, Siegert E, Drömann D, Yu X, et al. Interstitial lung disease increases mortality in systemic sclerosis patients with pulmonary arterial hypertension without affecting hemodynamics and exercise capacity. Clin Rheumatol. 2017 Feb;36(2):381–90. https://doi.org/10.1007/s10067-016-3504-6.
Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46(4):903–75. https://doi.org/10.1093/eurheartj/ehv317.
Demerouti ETI, Dimitroulas T, Giannakoulas G, Katsimpri P, Mitrouska I, Orfanos S, et al. Pulmonary arterial hypertension in connective tissue disorders. The emerging role of screening and early diagnosis. A position paper for Greek Rheumatologists. Mediterr J Rheumatol. 2019;30(2):90–3. https://doi.org/10.31138/mjr.30.2.90.
McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53(17):1573–619. https://doi.org/10.1161/CIRCULATIONAHA.109.192230.
Coghlan JG, Denton CP, Grunig E, Bonderman D, Distler O, Khanna D, et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis. 2014;73(7):1340–9. https://doi.org/10.1136/annrheumdis-2013-203,301.
Thakkar V, Stevens WM, Prior D, Moore OA, Byron J, Liew D, et al. N-terminal pro-brain natriuretic peptide in a novel screening algorithm for pulmonary arterial hypertension in systemic sclerosis: a case-control study. Arthritis Res Ther. 2012;14(3):R143. https://doi.org/10.1186/ar3876.
Avouac J, Lepri G, Smith V, Toniolo E, Hurabielle C, Vallet A, et al. Sequential nailfold videocapillaroscopy examinations have responsiveness to detect organ progression in systemic sclerosis. Semin Arthritis Rheum. 2017;47(1):86–94. https://doi.org/10.1016/j.semarthrit.2017.02.006.
Argula RG, Ward C, Feghali-Bostwick C. Therapeutic challenges and advances in the management of systemic sclerosis-related pulmonary arterial hypertension (SSc-PAH). Ther Clin Risk Manag. 2019;15:1427–42.
Galie N, Channick RN, Frantz RP, Grunig E, Jing ZC, Moiseeva O, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53(1). https://doi.org/10.1183/13993003.01889-2018.
Kylhammar D, Kjellstrom B, Hjalmarsson C, Jansson K, Nisell M, Soderberg S, et al. A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur Heart J. 2018;39(47):4175–81.
Benza RL, Gomberg-Maitland M, Elliott CG, Farber HW, Foreman AJ, Frost AE, et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL Risk Score Calculator 2.0 and Comparison With ESC/ERS-Based Risk Assessment Strategies. Chest. 2019;156(2):323–37.
Ahmed S, Pattanaik SS, Rai MK, Nath A, Agarwal V. Interstitial lung disease in systemic sclerosis: insights into pathogenesis and evolving therapies. Mediterr J Rheumatol. 2018;29(3):140–7. https://doi.org/10.31138/mjr.29.3.140.
Allanore Y, Meune C, Vonk MC, Airo P, Hachulla E, Caramaschi P, et al. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis. 2010 Jan;69(1):218–21. https://doi.org/10.1136/ard.2008.103382.
Sato T, Ambale-Venkatesh B, Lima JAC, Zimmerman SL, Tedford RJ, Fujii T, et al. The impact of ambrisentan and tadalafil upfront combination therapy on cardiac function in scleroderma associated pulmonary arterial hypertension patients: cardiac magnetic resonance feature tracking study. Pulm Circ. 2018 Jan-Mar;8(1):2045893217748307. https://doi.org/10.1177/2045893217748307.
Mavrogeni S, Markousis-Mavrogenis G, Koutsogeorgopoulou L, Dimitroulas T, Bratis K, Kitas GD, et al. Cardiovascular magnetic resonance imaging pattern at the time of diagnosis of treatment naïve patients with connective tissue diseases. Int J Cardiol. 2017 Jun 1;236:151–6. https://doi.org/10.1016/j.ijcard.2017.01.104.
Gargani L, Todiere G, Guiducci S, Bruni C, Pingitore A, De Marchi D, et al. Early detection of cardiac involvement in systemic sclerosis: the added value of magnetic resonance imaging. JACC Cardiovascular imaging. 2019;12(5):927–8. https://doi.org/10.1016/j.jcmg.2018.09.025.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Eleni Angeloudi declares that she has no conflict of interest. Eleni Pagkopoulou declares that she has no conflict of interest. Alexandra Arvanitaki declares that she has no conflict of interest. Stergios Soulaidopoulos declares that he has no conflict of interest. Alexandros Garyfallos declares that he has no conflict of interest. George Kitas declares that he has no conflict of interest. Theodoros Dimitroulas declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Scleroderma
Rights and permissions
About this article
Cite this article
Angeloudi, E., Pagkopoulou, E., Arvanitaki, A. et al. Cardiovascular Risk in Systemic Sclerosis. Curr Treat Options in Rheum 6, 282–298 (2020). https://doi.org/10.1007/s40674-020-00152-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40674-020-00152-z