Abstract
Systemic Sclerosis (SSc) is an autoimmune disorder characterized by microvascular injury and diffuse fibrosis of the skin and internal organs. While macrovascular disease and higher risk for cardiovascular events are well documented in other systemic rheumatic diseases such as rheumatoid arthritis and systemic lupus erythematosus, the presence and extent of atherosclerosis among patients with SSc is yet to be established. Primary cardiac involvement, due to impairment of coronary microvascular circulation and myocardial fibrosis, considerably affects prognosis and life expectancy of individuals with SSc, representing one of the leading causes of death in this population. On the other hand the existence and prevalence of atherosclerotic coronary disease remains an issue of debate as studies comparing structural and morphological markers of atherosclerosis and cardiovascular events between SSc patients and the general population have yielded controversial results. The aim of this review is to summarize recent literature about the prevalence of cardiovascular disease in SSc, review the surrogate markers of CVD that have been evaluated and examine whether common pathogenic mechanisms exist between SSc and macrovascular disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic Sclerosis (SSc) is a rare connective tissue disease characterized by endothelial dysfunction, dysregulation of innate and adaptive immunity and extensive fibrosis. The prevalence of SSc ranges from 50 to 300 cases per million depending on the geographical region, with a higher incidence observed in North America, Southern Europe, and Australia [1, 2]. Women are affected more frequently than men (3:1–14:1) and the average age of onset and diagnosis is in the fifth decade of life [3]. SSc is a heterogeneous disorder affecting many internal organs and with a wide range of prognosis. It is commonly classified in two subtypes: diffuse cutaneous and limited cutaneous SSc. In the former, fibrosis is mainly restricted to distal extremities and face, while Raynaud’s phenomenon is typically present before skin and visceral involvement. In contrast, diffuse SSc presents with a more aggressive progression characterized by severe manifestations such as extensive skin fibrosis, tendon contractures, interstitial lung disease and renal crisis. Pulmonary arterial hypertension (PAH), which imparts a high risk of mortality, occurs equally in both disease subsets [4].
Small vessel disease dominates the pathophysiology and clinical manifestations of SSc but there is increasing interest in large-vessel involvement, particularly atherosclerotic cardiovascular disease (CVD). It is well established that other autoimmune rheumatic diseases such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) associate with accelerated atherosclerosis and higher risk for CVD events such as stroke and myocardial infarction [5]. The relative risk for patients with SSc remains unclear.
In this review, we summarize recent epidemiological studies about the prevalence of CVD in SSc in comparison with the general population and/or other rheumatic diseases, and address the question whether common pathogenic mechanisms exist between SSc and macrovascular disease.
Search strategy
The online databases MEDLINE and EMBASE were searched until January 2016 for either research papers or review articles concerning the micro- and macro-vascular involvement in SSc. The following terms were used as keywords to search for relevant publications: systemic sclerosis, scleroderma, myocardial fibrosis in combination with micro- and macro-vascular disease, cardiac involvement, atherosclerosis, cardiovascular disease and coronary arteries. Articles that had been published as full journal articles in English were included in our review. Poster presentations, conference proceedings, not accessible abstracts, data from ongoing pharmaceutical research and not translated in English reports were excluded [6].
Atherosclerosis and CVD in autoimmune rheumatic diseases
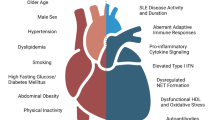
Several systemic autoimmune disorders are associated with premature atherosclerosis and a higher risk for developing CVD. For example patients suffering from RA—the most extensively studied systemic inflammatory disease in this field—have considerably higher CVD morbidity and mortality compared to general population to the point that RA is now considered an independent risk factor for early atherosclerosis and CVD [7, 8]. Similarly higher rates of vascular disease and CVD events have been demonstrated in seronegative arthropathies, SLE and systemic vasculitis [9, 10]. Although traditional CVD risk factors, such as smoking, arterial hypertension and hyperlipidaemia appear to be more prevalent, underdiagnosed and undertreated in aforementioned conditions, they are not sufficient on their own to explain the higher rates of CVD events and deaths observed in these patients [11]. It is now well-documented that the aetiology of vascular injury in systemic inflammatory diseases is multifactorial with several distinct mechanisms affecting each other on several levels. Interrelations between systemic inflammation, autoimmune activation, coagulation disturbances and potential cardiotoxic effects of anti-rheumatic medications constitute an important contributor to excess CV risk common in all systemic disorders [12]. Coronary microvascular dysfunction is another one player recently acknowledged and studied in the majority of systemic inflammatory diseases [13]. In addition disease specific mechanisms may have a further input in heightening CVD risk in different disease settings. For example psoriatic arthritisis is associated with a worse metabolic profile compared with other inflammatory arthritides [14] whistle the presence of anti-endothelial cell and anti-phospholipid antibodies represent an additional mechanism promoting vascular disease and atherosclerosis in SLE [15]. The links between classic CVD risk factors, systemic inflammatory load and autoimmune dysregulation are not fully understood but these pathways appear to be interdependent suggesting that potential therapeutic benefits of targeting systemic inflammation is likely to reduce CVD risk. To lend more support substantial amount of evidence has demonstrated that conventional and biologic disease modifying drugs not only control systemic inflammatory procedure but also exert beneficial effects on the vascular function [16, 17] and metabolic parameters such as insulin resistance [18, 19]. On the other hand anti-inflammatory treatment may exhibit detrimental effects on CVD risk profile by increasing blood pressure, worsening established heart failure and increasing lipid levels [20]. All the above underline the complexity of the mechanisms involved in vascular mortality associated with systemic inflammatory diseases and outline potential areas of interest and research in Ssc where different pathophysiologic pathways such as dysregulation of connective tissue remodelling, fibrosis of internal organs and endothelial cell activation interfere with systemic inflammation leading to cardiac and vascular disease and high CVD mortality [21].
Atherosclerosis and CVD in SS
Epidemiological and clinical data
In the past, the main cause of mortality in patients with SSc was scleroderma renal crisis, but the introduction of angiotensin-converting enzyme (ACE) inhibitors in the 1980s drastically improved the prognosis of renal complications [22]. In the present era, cardiopulmonary complications such as heart failure, pulmonary fibrosis and hypertension represent the leading causes of death in SSc [23].
Impairment of coronary microvascular circulation and cardiac tissue fibrosis are the hallmark of SSc-related myocardial dysfunction. Macrovascular involvement was thought to have a lesser effect on CVD and overall morbidity. However, recent data and case reports suggest that coronary and cerebral vascular disease might be at least partially responsible for high morbidity and mortality rates associated with cardiac disease among SSc subjects [24–28]. A meta-analysis by Au et al. confirmed that patients with SSc are indeed at a higher risk of atherosclerosis, but its pattern appears to be less aggressive compared with other rheumatic diseases, such as SLE and RA [29].
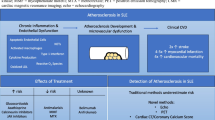
A large cohort study investigating mortality and causes of death among 344 Danish SSc patients demonstrated that 25 % of deaths were due to cardiovascular causes including acute myocardial infarction, heart failure and cerebrovascular event, in comparison with 26 % which were directly attributable to the disease, mainly caused by SSc renal crisis and pulmonary artery hypertension [27]. Another study by Hesselstrand et al. in 1998 showed approximately 20 % of deaths to be related to CVD [28]. An extended study from the European League Against Rheumatism (EULAR) Scleroderma Trials and Research (EUSTAR) database in 2010 showed that 55 % of deaths were directly SSc-related. Interestingly, 26 % of SSc-related deaths were attributable to cardiac causes, mainly heart failure due to PAH and pulmonary fibrosis and arrhythmias without existing cardiomyopathy. Among non-SSc-related fatalities though, almost one-third of patients’ deaths (29 %) were related to cardiovascular events, which were defined as myocardial infarction, non ischemic left heart failure, stroke and arrhythmias [29]. A recent study by Dave et al. investigated the atherosclerotic CVD in hospitalized patients with systemic sclerosis by utilizing US Nationwide Inpatient Sample [30]. A total number of 308,452 hospitalizations of SSc were included 5.4 % of which, were related to cardiovascular causes. Intriguingly, CVD-related hospitalizations of SSc patients presented a significantly higher ratio of mortality than similar hospitalization of other rheumatic diseases such as SLE and RA. This increased mortality ratio in SSc patients might be explained by primary heart involvement, calcification of valvular structures and both microvascular and macrovascular damage which increases the total risk of CVD (Fig. 1).
Vascular disease in SSc. Endothelial cell activation due to various reasons, for example hypoxic stimuli, is the main event in the initiation and perturbation of vascular dysfunction leading to the pathological changes and clinical manifestations of the disease. Endothelial cell injury and the subsequent overexpression of adhesion molecules intravascularly results in the recruitment of immune cells and the derangement of vascular homeostasis. Local tissue ischemia accompanied by the production of such mediators induces fibrosis of internal organs including cardiac tissue. Primary cardiac disease due to patchy myocardial fibrosis is the major contributor to heart involvement associated with progressively advanced functional and structural impairment of the myocardium. The presence of pulmonary hypertension irrespective of the underlying mechanism—arterial pulmonary hypertension, hypoxia-induced in case of interstitial lung fibrosis or venoo-occlusive disease—adversely affects myocardial performance in direct or indirect way. On the other hand inflammatory vascular injury and atherosclerosis share several common pathogennetic mechanisms and vascular abnormalities related to SSc may promote the formation and development of atherosclerotic plaque in this population. However macrovascular atherosclerotic disease resulting in CVD events such as myocardial infraction, stroke and peripheral vascular disease remains an issue of debate; despite conflicting data it seems that atherosclerosis have an impact to the increased CVD mortality rates in SSc patients
Regarding macrovascular atherosclerotic disease Man et al. [31] carried out a large cohort study utilizing a UK primary care database from 1986 to 2011. The study included 865 patients and demonstrated that the incidence rate for myocardial infarction and stroke was 4.4 and 4.8 per 1000 person/year in SSc respectively compared with 2.5 and 2.5 in 8643 controls. Furthermore, the Australian Scleroderma Cohort Study confirmed an approximately threefold increased prevalence of coronary heart disease in 850 SSc patients compared with controls, including a history of myocardial infarction, percutaneous coronary intervention and coronary artery bypass, taking also into account conventional risk factors [32, 33]. More recently Zubieta et al. confirmed these findings by demonstrating in a cohort of 1239 SSc patients a significant increased risk for myocardial infarction and stroke (HR 3.49 and 2.35 respectively) in a well-characterised cohort of 1239 SSc patients compared with age and sex matched controls [34].
In conclusion rapidly increasing amount of evidence suggest that macrovascular coronary and cerebral atherosclerotic disease might have a crucial—unrecognised and underestimated until recently—impact on cardiovascular morbidity and mortality. It appears that cardiovascular risk is increased in SSc patients compared to the general population however the heterogeneity of the studies regarding methodology, definition of CVD events, cohorts size, and outcomes does not allow definite conclusions to be drawn. Clearly further research is warranted to determine the prevalence of accelerated atherosclerosis in this population. In that respect the presence of SSc-related mechanisms of cardiac damage—namely vasospasm and structural alterations of coronary microvasculature—as well as the effects of immunosuppressive treatment on total cardiovascular risk need to be taken into account in the design of future prospective studies in large cohorts with hard pre-defined cardiac end points and accurate characterisation of cardiovascular events.
Coronary arteries
The first autopsy study to assess the coronary arteries in SSc patients was carried out in 1969. D’Angelo et al. [35] compared 58 patients with SSc with 58 matched controls and the results suggested a significant increase in atherosclerotic lesions in small coronary arteries and arterioles in patients with SSc. However, no difference was reported in medium-size coronary arteries between patients and controls. A later retrospective cohort study investigated the macrovascular involvement in females with limited SSc assessing the distribution of vascular disease in the peripheral, coronary and cerebral arterial territories, noted a higher relative risk for peripheral macrovascular disease but no significant difference in the prevalence of coronary artery disease compared to controls [36]. Notably, the presence of coronary heart disease was correlated with PAH. Komocsi et al. [37] reported the strong relation between PAH and coronary heart disease by using right heart catheterization and coronary angiography in 30 SSc patients.
A retrospective review of 1009 hospital admissions of SSc patients showed that coronary artery disease was not a major reason for hospitalization and mortality; only 11 of them (1.09 %) were admitted for acute myocardial infarction [38]. The diagnosis of acute myocardial infarction was made by measuring serum myocardial creatinine kinase and assessing electrocardiographic changes. Three of these patients had normal coronary arteries on cardiac catheterization and left ventricular hypertrophy was the predominant electrocardiographic finding. Seven of these patients had significant renal impairment though, which might complicate the results by affecting the levels of serum myocardial creatinine kinase.
Data from SSc patients with no clinical history of CVD disease also showed interesting results. A small study in 17 patients with SSc by Khurma et al. [39] demonstrated by calculating coronary calcium score that the prevalence of subclinical coronary atherosclerosis is greater in SSc patients compared to 17 matched healthy controls. Tarek et al. [40] also performed a small angiographic study in 14 asymptomatic SSc (5 limited, 9 diffuse) female patients. Coronary catheterization showed a total of 19 angiographic abnormalities, while three out of five patients with limited SSc presented stenosis, but none from the diffuse group. Even though both studies included a small number of patients, their findings suggest that atherosclerosis might affect coronary arteries quite early in asymptomatic SSc patients. In summary, studies aiming to assess the presence of macrovascular coronary lesions atherosclerosis in SSc patients have provided conflicting data (Table 1). The fact that the majority of these studies included a small number of patients does not allow safe estimations for the prevalence of coronary artery disease in SSc indicating for one more time the need for further well-designed studies.
Carotid arteries
Several studies have evaluated the prevalence of atherosclerosis in carotid arteries in patients with SSc by ultrasonography. Increased carotid intima-media thickness (CIMT) is well recognized to be associated with conventional CVD risk factors and future CVD events such as stroke and coronary heart disease [5]. In the context of SSc a few studies demonstrated significantly increased CIMT in SSc patients compared with controls [40–47]. Subclinical atherosclerosis was correlated in some studies with different parameters including age, lipid profile, anti-topoisomerase-I (anti-Scl-70) antibodies, or even genetic features such as ACE polymorphisms. On the other hand, multiple other studies failed to demonstrate a difference in CIMT values between SSc patients and controls [48–51]. Regarding the prevalence of atherosclerotic plaque in carotid arteries, Ho et al. [52] showed that 64 % of a total of 52 SSc patients had carotid artery disease compared with only 35 % of the controls; no significant differences in traditional cardiovascular risk factors were reported. In addition, Schiopu et al. [53] showed that SSc patients had a higher prevalence of carotid plaque than matched controls and patients with plaque had increased levels of angiogenic and fibrotic factors; no differences in CIMT were reported though. Interestingly a recently published study [54] found that subclinical atherosclerosis assessed by carotid arteries ultrasonography was equally prevalent among 110 SSc and 110 age and sex-matched RA individuals providing further support to the notion that atherosclerosis should be carefully evaluated in SSc. Today it remains unknown whether morphological or structural markers of accelerated atherosclerosis are increased in patients with SSc as a number of controlled studies have yielded inconclusive results (Table 2). However the small numbers of patients and controls groups as well as the variation in the methological and sonographic assessment underline for one more time the necessity for large prospective clinical trials with pre-defined assessment protocols and outcomes.
Peripheral arteries
Several techniques have been used to evaluate the prevalence of peripheral artery disease in SSc patients: ankle brachial pressure index (ABPI) for lower extremities, blood pressure inter arm difference for proximal arterial disease of the upper extremities, pulse wave velocity and pulse wave analysis for arterial stiffness [5]. Youssef et al. [36] found a significantly higher prevalence of peripheral vascular disease in SSc patients compared with controls (58 vs. 10 %). Seven studies assessed macrovascular endothelial function with brachial artery flow-mediated vasodilation but the results were inconclusive. Some of them showed impairment of vascular function in SSc patients compared to healthy controls [40, 41, 55, 56]. However, these findings were not confirmed in other studies [57, 58]. Moreover, in a small study by Stucker et al. [57], 27 out of 29 SSc patients had a stenosis of the arteries of the upper extremities. The severity of Raynaud’s phenomenon was not correlated with the severity of the angiographic findings, but anti-Scl-70 antibodies were more frequent among patients with arterial occlusions and stenosis refractory to vasodilator drugs, particularly tolazoline hydrochloride.
Evaluation of vascular morphology in other anatomical areas of the arterial tree is also of great interest. An autopsy case–control study of 35 SSc patients showed a prominent increase in intimal thickening of medium and large renal vessels of patients with diffuse cutaneous SSc without a history of scleroderma renal crisis [58]. Another retrospective cohort study of 20 SSc patients examined arteries from the limps, neck, and abdomen by using Doppler [59]. The lunar arteries were significantly narrower in SSc than of those of 20 matched for age, sex, and the presence/absence of hypertension, hyperlipidaemia, smoking, and diabetes statuscontrols. However these small studies are not sufficient to conclude whether peripheral macrovascular disease is more prominent in SSc patients and more importantly whether it does contribute to CVD mortality in this condition.
Pathogenic mechanisms for CVD in systemic sclerosis
Traditional risk factors
The prevalence of traditional CVD risk factors in SSc has not been assessed in large prospective studies. However, the current amount of evidence suggest that traditional CVD risk factors do not seem to differ considerably in SSc patients [33, 60] compared to general population. Zakopoulos et al. examined 40 SSc patients and 45 controls, but it demonstrated no difference in blood pressure on 24-h ambulatory blood pressure monitoring between the two groups [61]. Another study examining 48 SSc patients and 46 healthy controls demonstrated a slight increase in blood pressure and fasting glucose as well as a lower body mass index in SSc patients [62].
Regarding cholesterol levels in SSc patients, the results are also contradictory. Lippi et al. [63] examined lipid profile of 31 female SSc patients and 33 matched healthy controls to investigate the potential contribution of lipid abnormalities to vascular complications. SSc patients presented significant differences in median and 25-75th percentile distribution of lipoprotein[a] compared with the control group. Nevertheless, all other parameters (e.g., total cholesterol, low density lipoproteins, high density lipoproteins, triglycerides, atherogenic index of plasma) presented no adverse alterations. In contrast, another study by Borba et al. [64] having examined fasting lipids in 24 female SSc patients and 24 matched controls demonstrated lower levels of low density lipoproteins and total cholesterol. Interestingly, these findings were clinically correlated with PAH and immunologically with anti-centromere antibodies. Szucs et al. [55] reported that SSc patients might have elevated levels of low density lipoproteins, homocysteine, and CRP, all of which are associated with an elevated risk for atherosclerosis.
In conclusion there is no robust data to support that traditional CVD risk factors are more prevalent in SSc patients and are linked with increased rates of cardiac complications and mortality in this condition.
Endothelial dysfunction
Endothelial cell activation is the central immunologic and inflammatory disturbance resulting in the characteristic widespread vasculopathy, typical for SSc. It appears that endothelial injury occurs early in the course of the disease as SSc individuals exhibit early signs of vasculopathy such as Raynaud’s phenomenon associated with morphological changes in capillaries before or at disease onset. Although the identity of the initial stimuli remains unclear endothelial cell insult is considered the main driver of vascular changes, excess matrix production and connective tissue accumulation characterizing SSc [65]. Activated endothelial cells upregulate the expression of adhesion molecules and promote inflammatory cell migration, intimal fibrosis and fibroblast proliferation leading to disruption of the balance between turnover and deposition of extracellular proteins and to the derangement of endothelium homeostasis by inducing the production of vasoconstriction mediators—mainly endothelin-1—and by downregulating the synthesis of vasodilators such as nitric oxide and prostacyclin [64, 66].
Dysregulation of apoptosis can also be involved in impaired vascular function in SSc. Environmental stimuli can promote endothelial cells apoptosis, which have been shown to be associated with CVD pathologies including atherosclerosis, thrombosis and connective tissue diseases [67]. Particularly in SSc, endothelial cells apoptosis is an important component of vascular damage and it is mediated by anti-endothelial cell antibodies via Fas-dependent pathway [67–69]. Whether such changes in endothelial cells function associated with inflammatory and fibrotic processes are involved in the development of atherosclerotic vascular disease and to which extent remains to be determined in SSc.
Primary heart involvement in SSc
Primary heart involvement in SSc is difficult to be estimated accurately for multiple reasons including the long asymptomatic course of the disease, the variety of clinical manifestations, the diagnostic method, and the definition used to characterize the disease. For example, endomyocardial biopsy studies have revealed myocardial involvement in the vast majority of SSc patients, while echocardiography based studies showed that only 2–5 % of SSc patients have abnormal left ventricular injection fraction. Cardiac disease can potentially affect all structures of the heart; more frequently, it presents as myocardial hypertrophy, arrhythmias, pericardial effusion, abnormal coronary vasoreactivity, valvular impairment, and heart failure [60, 70, 71]. Unfortunately, when heart involvement manifests clinically, it is usually associated with advanced signs of heart failure and has an ominous prognosis.
Irrespective of clinical phenotype, the hallmark of heart disease in this population is cardiac tissue fibrosis with patchy fibrotic deposits, equally distributed throughout left and right ventricular myocardium [72, 73]. The abnormal vasoreactivity leading to cardiac ischemia might play an important role in the initiation and evolution of myocardial injury to myocardial fibrosis [74]. Notably, reversible ischemia confirmed by transient myocardial perfusion abnormalities is a unique pattern of SSc, not observed in other rheumatic diseases [75, 76]. As the disease progresses, small perfusion defects can culminate in irreversible tissue damage, myocardial fibrosis and dysfunction.
Besides functional abnormalities, structural defects of coronary microcirculation can importantly contribute to heart disease in SSc; this is suggested by myocardial perfusion abnormalities found in SSc patients by using cardiac catheterization, echocardiography and radionucleotide imaging [77–79]. Follansbee et al. [79] showed that 20 out of 26 patients with SSc had profound cardiac abnormalities by using thallium-perfusion scanning, but notably only six of them had clinically overt heart involvement.
Diffuse fibrosis of right and left ventricles can lead to depressed myocardial contractility, which can be undetected for many years. Left ventricular ejection fraction is not sensitive enough to evaluate subclinical myocardial dysfunction [78]. However, tissue Doppler echocardiography and cardiac magnetic resonance imaging are able to detect these defects in myocardial function at asymptomatic stages [80, 81]. Schattke et al. indicated that tissue Doppler is a useful tool to detect early right ventricular systolic impairment in SSc patients with or without pulmonary hypertension, while it correlates isolated diastolic dysfunction with skin involvement [60, 82]. Interestingly, tissue Doppler echocardiographic abnormalities, mainly right and left ventricular diastolic dysfunction, were associated with plasma levels of N-terminal pro-brain natriuretic peptide and asymmetric dimethylarginine in SSc patients compared to controls [83]. These findings could suggest possible links for cardiac and endothelial dysfunction to neuroendocrine derangement in SSc.
Cardiac magnetic resonance imaging studies have also indicated that the prevalence of clinically silent cardiac involvement is significantly high among SSc [84, 85]. The main patterns of cardiac involvement revealed by using cardiac magnetic resonance are left ventricular diastolic dysfunction and kinetic abnormalities, thinning of left ventricle myocardium, alterations in left and right ventricle ejection fractions as well as right ventricle dilatation related to the presence of PAH. The results of several studies have demonstrated that is a sensitive technique for diagnosing heart involvement in SSc even in asymptomatic patients with normal echocardiographic evaluation [85, 86]. For example Mavrogeni et al. [87] examined 46 newly diagnosed SSc patients without clinical evidence of heart involvement by using inflammation and stress perfusion-fibrosis cardiac magnetic resonance. Two patients had acute myocardial inflammation, while all the rest presented with significant myocardial perfusion defects compared to matched controls. More importantly, eleven out of these patients showed further asymptomatic deterioration of myocardial circulation in 2 years of follow-up. All the above data suggest that CMR can be a useful additional tool complementing the physician’s clinical skills along with echocardiography to assess cardiac involvement in asymptomatic patients with SSc [88].
Pulmonary arterial hypertension and right heart involvement
PAH is among the most serious complications of SSc and one of the leading causes of death in this population. The incidence of SScPAH is 5–13 % based on right catheterization studies and is associated with worse prognosis compared to other forms of PAH [89–92]. Coexistence of primary and secondary heart disease may accounts for the worst outcome and higher mortality and morbidity rates in this group. Right ventricular dysfunction as a consequence of the increasing afterload is common in SSc patients with chronic PAH. Interestingly, there is evidence that SScPAH leads to worse right ventricular performance compared to idiopathic PAH attributable to grater pulmonary arterial load due to the stiffening of the vessels or the primary heart involvement [93]. In addition, patients with SScPAH more often present with left heart abnormalities, such as left ventricular hypertrophy, left ventricular diastolic dysfunction and left atrial dilation than those with idiopathic PAH [94]. In this regard echocardiographic data support that left atrial volume—an indicator of diastolic left ventricular dysfunction—is a predictor of elevated systolic pulmonary artery pressure [95]. Other studies have reported a higher frequency of connective tissue disease related arrhythmias in patients with SScPAH than in idiopathic PAH [96]. A study by Mathai et al. [97] demonstrated significantly higher levels of B-type natriuretic peptide in patients with SScPAH compared to those with idiopathic PAH, supporting the systemic character of heart involvement by the activation of cardiac neurohormonal system. The aforementioned data reveal the global myocardial involvement in SScPAH but more research is needed to elucidate the interaction between pulmonary vascular and parenchymal disease, and the impaired myocardial function.
Therapeutic intervention
Although no specific guidelines have been established for treatment of cardiac manifestations in SSc, standard therapies should be considered. According to the EUSTAR though, immunosuppressive therapy does not affect the course of heart involvement [98]. In contrast, early treatment with calcium channel blockers, angiotensin-converting enzyme inhibitors, and endothelin receptor antagonists, can be beneficial for some patients on myocardial perfusion and contractility, as they improve cardiac microcirculation [72]. In particular, calcium channel blockers showed a confirmed long-term benefit on left ventricular dysfunction [98]. It is worth noting that treatment with beta-blockers is generally avoided in SSc patient due to exacerbation of Raynauds.
It is worth noting that novel vasodilator agents such as phosphodiesterase-5 (PDE5) inhibitors improve endothelial function in patients with endothelial damage as demonstrated by changes in serum markers of endothelial activation [99]. Data from animal experimental models suggest that PDE5 inhibitors reduce circulating cytokines and chemokines, improve metabolic parameters and suppress oxidative stress, providing powerful cardioprotective effects [100, 101]. In addition to their major role as a leading therapy for pulmonary hypertension and other vascular complication such as digital ulcers, PDE5 inhibitors may provide further benefit to SSc patients, by modifying coronary microcirculation and alleviating cardiac ischemic injury.
Evaluation of the overall CVD risk is of paramount importance and recommendations applied to general population for minimizing CVD risk should be considered. Even though traditional risk factors (arterial hypertension, obesity, dyslipidemia) do not seem to be increased among patients with SSc, CVD risk prevention and monitoring guidelines applied to general population are highly recommended. For example blood pressure should be closely monitored and treatment with antihypertensive drugs should be considered—when necessary—to minimize the risk of heart failure. Finally yet importantly, patients can benefit the most by educational programmes and lifestyle change such as increasing physical activity or adopting a better diet, since it will considerably improve their quality of life [102, 103].
Conclusions
Although SSc has been long seen as a microvascular disorder, recent data have emerged macrovascular complications and accelerated atherosclerosis as significant contributors to cardiac morbidity on the top of small coronary vessels vasculopathy. Taking into consideration that inflammation and endothelial dysfunction are prominent players in disease progression, common pathogenic mechanisms may participate in the pathogenesis of vascular abnormalities in SSc and atherosclerosis albeit it in different way or to a lesser extend compared with other systemic inflammatory disorders. Large population-based studies are essential to evaluate the prevalence and the outcome of CVD as well as to identify the subgroups of patients at higher risk for CVD events. Recommendations for cardiac assessment, CVD risk stratification and prevention strategies in this particular population are currently lacking but are obviously important not only for the management of the patients but also for the acknowledgment of the higher CVD risk among rheumatologists and other physicians dealing with this challenging disease. Last but not least further research in the field may delineate the exact mechanisms involved in both micro- and macrovascular injury in SSc and provide the rationale for targeted treatment interventions early in the course of the disease and before CVD complications progress to advanced stages.
References
Barnes J, Mayes MD (2012) Epidemiology of systemic sclerosis: incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Curr Opin Rheumatol 24:165–170
Chifflot H et al (2008) Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum 37:223–235
Gabrielli A et al (2009) Scleroderma. N Engl J Med 360:1989–2003
Dimitroulas T, Giannakoulas G, Karvounis H, Settas L, Kitas GD (2012) Systemic sclerosis-related pulmonary hypertension: unique characteristics and future treatment targets. Curr Pharm Des 18:1457–1464
Cannarile F et al (2015) Cardiovascular disease in systemic sclerosis. Ann Transl Med 3:8
Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD (2011) Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int 31(11):1409–1417
Sherer Y, Shoenfeld Y (2006) Mechanisms of disease: atherosclerosis in autoimmune diseases. Nat Clin Pract Rheumatol 2:99–106
Kitas GD, Gabriel SE (2011) Cardiovascular disease in rheumatoid arthritis: state of the art and future perspectives. Ann Rheum Dis 70:8–14
Hollan I et al (2013) Cardiovascular disease in autoimmune rheumatic diseases. Autoimmun Rev 12:1004–1015
Nurmohamed MT, Heslinga M, Kitas GD (2015) Cardiovascular comorbidity in rheumatic diseases. Nat Rev Rheumatol 11:693–704
Gonzalez A, Maradit Kremers H, Crowson CS, Ballman KV, Roger VL, Jacobsen SJ et al (2008) Cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non-rheumatoid arthritis patients? Ann Rheum Dis 67:64–69
Zegos T, Kitas G, Dimitroulas T (2016) Cardiovascular risk in rheumatoid arthritis: assessment, management and next steps. Ther Adv Musculoskelet Dis 8:86–101
Faccini A, Kaski JC, Camici PG (2016) Coronary microvascular dysfunction in chronic nflammatory rheumatoid diseases. Eur Heart J 37:1799–1806
Sharma A, Gopalakrishnan D, Kumar R, Vijayvergiya R, Dogra S (2013) Metabolic syndrome in psoriatic arthritis patients: a cross-sectional study. Int J Rheum Dis 16:667–673
Medina G et al (2003) Increased carotid artery intima-media thickness may be associated with stroke in primary antiphospholipid syndrome. Ann Rheum Dis 62:607–610
Westlake SL, Colebatch AN, Baird J, Kiely P, Quinn M, Choy E et al (2010) The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology (Oxford) 49:295–307
Sandoo A, van Zanten JJ, Toms TE, Carroll D, Kitas GD (2012) Anti-TNFα therapy transiently improves high density lipoprotein cholesterol levels and microvascular endothelial function in patients with rheumatoid arthritis: a pilot study. BMC Musculoskelet Disord 13:127
Kiortsis DN, Mavridis AK, Vasakos S, Nikas SN, Drosos AA (2005) Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann Rheum Dis 64:765–766
Stavropoulos-Kalinoglou A, Metsios GS, Panoulas VF, Nightingale P, Koutedakis Y, Kitas GD (2012) Anti-tumour necrosis factor alpha therapy improves insulin sensitivity in normal-weight but not in obese patients with rheumatoid arthritis. Arthritis Res Ther 14:R160
Gasparyan AY, Ayvazyan L, Cocco G, Kitas GD (2012) Adverse cardiovascular effects of antirheumatic drugs: implications for clinical practice and research. Curr Pharm Des 18:1543–1555
Nurmohamed MT, Kitas G (2011) Cardiovascular risk in rheumatoid arthritis and diabetes: how does it compare and when does it start? Annals of the rheumatic diseases. Ann Rheum Dis 70:881–883
Dimitroulas T, Sarafidis P, Roma V, Karagiannopoulou G, Kapoulas S, Dimitroula H et al (2010) Scleroderma renal crisis accompanied by new-onsetpulmonary arterial hypertension: an acute systemic endothelial injury? Casereport and literature. Inflamm Allergy Drug Targets 9:313–318
Simeón-Aznar CP, Fonollosa-Plá V, Tolosa-Vilella C, Espinosa-Garriga G, Campillo-Grau M, Ramos-Casals M et al Spanish Scleroderma Study Group (SSSG); Autoimmune Diseases Study Group (GEAS); Spanish Society of Internal Medicine (SEMI) (2015) Registry of the Spanish Network for systemic sclerosis: survival, prognostic factors, and causes of death. Medicine (Baltimore) 94:e1728
Nussinovitch U, Shoenfeld Y (2011) Atherosclerosis and macrovascular involvement in systemic sclerosis: myth or reality. Autoimmun Rev 10:259–266
Psarras A et al (2014) Giant cell arteritis and systemic sclerosis: a rare overlap syndrome. Rheumatol Rep 6:1
Hupp SL (1989) Giant cell arteritis associated with progressive systemic sclerosis. J Clin Neuroophthalmol 9:126–130
Jacobsen S et al (1998) Mortality and causes of death of 344 Danish patients with systemic sclerosis (scleroderma). Br J Rheumatol 37:750–755
Hesselstrand R et al (1998) Mortality and causes of death in a Swedish series of systemic sclerosis patients. Ann Rheum Dis 57:682–686
Tyndall AJ et al (2010) Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 69:1809–1815
Dave AJ et al (2014) Atherosclerotic cardiovascular disease in hospitalized patients with systemic sclerosis: higher mortality than patients with lupus and rheumatoid arthritis. Arthritis Care Res (Hoboken) 66:323–327
Man A et al (2013) The risk of cardiovascular disease in systemic sclerosis: a population-based cohort study. Ann Rheum Dis 72:1188–1193
Ngian GS et al (2012) Prevalence of coronary heart disease and cardiovascular risk factors in a national cross-sectional cohort study of systemic sclerosis. Ann Rheum Dis 71:1980–1983
Ngian GS et al (2011) Cardiovascular disease in systemic sclerosis–an emerging association? Arthritis Res Ther 13:237
Aviña-Zubieta JA, Man A, Yurkovich M, Huang K, Sayre EC, Choi HK (2016) Early cardiovascular disease after the diagnosis of systemic sclerosis. Am J Med 129:324–331
D’Angelo WA et al (1969) Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med 46:428–440
Youssef P et al (1995) Limited scleroderma is associated with increased prevalence of macrovascular disease. J Rheumatol 22:469–472
Komocsi A et al (2010) Overlap of coronary disease and pulmonary arterial hypertension in systemic sclerosis. Ann Rheum Dis 69:202–205
Derk CT, Jimenez SA (2007) Acute myocardial infarction in systemic sclerosis patients: a case series. Clin Rheumatol 26:965–968
Khurma V et al (2008) A pilot study of subclinical coronary atherosclerosis in systemic sclerosis: coronary artery calcification in cases and controls. Arthritis Rheum 59:591–597
Tarek G et al (2006) Coronary angiographic findings in asymptomatic systemic sclerosis. Clin Rheumatol 25:487–490
Lekakis J et al (1998) Short-term estrogen administration improves abnormal endothelial function in women with systemic sclerosis and Raynaud’s phenomenon. Am Heart J 136:905–912
Sherer Y et al (2007) Early atherosclerosis and autoantibodies to heat-shock proteins and oxidized LDL in systemic sclerosis. Ann N Y Acad Sci 1108:259–267
Bartoli F et al (2007) Angiotensin-converting enzyme I/D polymorphism and macrovascular disease in systemic sclerosis. Rheumatology (Oxford) 46:772–775
Bartoli F et al (2007) Flow-mediated vasodilation and carotid intima-media thickness in systemic sclerosis. Ann N Y Acad Sci 1108:283–290
Kaloudi O et al (2007) Circulating levels of Nepsilon-(carboxymethyl)lysine are increased in systemic sclerosis. Rheumatology (Oxford) 46:412–416
Kumar U, Verma N, Kumar AK, Hari S, Yadav R, Sreenivas V et al (2010) Endothelial dysfunction in Indian patients with systemic sclerosis[abstract]. Ann Rheum Dis 69(Suppl 3):692
Tsifetaki N et al (2010) Subclinical atherosclerosis in scleroderma patients. Scand J Rheumatol 39:326–329
Hettema ME, Zhang D, de Leeuw K, Stienstra Y, Smit AJ, Kallenberg CG, Bootsma H (2008) Early atherosclerosis in systemic sclerosis and its relation to disease or traditional risk factors. Arthritis Res Ther 10:R49
Roustit M et al (2008) Discrepancy between simultaneous digital skin microvascular and brachial artery macrovascular post-occlusive hyperemia in systemic sclerosis. J Rheumatol 35:1576–1583
Piccione MC et al (2011) Early identification of vascular damage in patients with systemic sclerosis. Angiology 62:338–343
Liu J et al (2011) Preferential macrovasculopathy in systemic sclerosis detected by regional pulse wave velocity from wave intensity analysis: comparisons of local and regional arterial stiffness parameters in cases and controls. Arthritis Care Res (Hoboken) 63:579–587
Ho M et al (2000) Macrovascular disease and systemic sclerosis. Ann Rheum Dis 59:39–43
Schiopu E et al (2014) Prevalence of subclinical atherosclerosis is increased in systemic sclerosis and is associated with serum proteins: a cross-sectional, controlled study of carotid ultrasound. Rheumatology (Oxford) 53:704–713
Ozen G, Inanc N, Unal AU, Korkmaz F, Sunbul M, Ozmen M et al (2016) Subclinical Atherosclerosis in systemic sclerosis: not less frequent than rheumatoid arthritis and not detected with cardiovascular risk indices. Arthritis Care Res (Hoboken) 2016 Feb 11 [Epub ahead of print]
Szucs G et al (2007) Endothelial dysfunction precedes atherosclerosis in systemic sclerosis–relevance for prevention of vascular complications. Rheumatology (Oxford) 46:759–762
Rollando D et al (2010) Brachial artery endothelial-dependent flow-mediated dilation identifies early-stage endothelial dysfunction in systemic sclerosis and correlates with nailfold microvascular impairment. J Rheumatol 37:1168–1173
Stucker M et al (2000) Macroangiopathy of the upper extremities in progressive systemic sclerosis. Eur J Med Res 5:295–302
Trostle DC et al (1988) Renal vascular histology and morphometry in systemic sclerosis. A case-control autopsy study. Arthritis Rheum 31:393–400
Stafford L et al (1998) Distribution of macrovascular disease in scleroderma. Ann Rheum Dis 57:476–479
Dimitroulas T, Giannakoulas G, Karvounis H, Garyfallos A, Settas L, Kitas GD (2014) Micro- and macro-vascular treatment targets in scleroderma heart disease. Curr Pharm 20:536–544
Zakopoulos NA et al (2003) Systemic sclerosis is not associated with clinical or ambulatory blood pressure. Clin Exp Rheumatol 21:199–204
Zeng Y et al (2012) Macrovascular involvement in systemic sclerosis: evidence of correlation with disease activity. Clin Exp Rheumatol 30(2 Suppl 71):S76–S80
Lippi G et al (2006) Lipoprotein[a] and the lipid profile in patients with systemic sclerosis. Clin ChimActa 364(1–2):345–348
Borba EF et al (2005) Lipoprotein profile in limited systemic sclerosis. Rheumatol Int 25:379–383
Abraham DJ, Distler O (2007) How does endothelial cell injury start? The role of endothelin in systemic sclerosis. Arthritis Res Ther 9(Suppl 2):S2
Abraham DJ, Krieg T, Distler O (2009) Overview of pathogenesis of systemic sclerosis. Rheumatology (Oxford)48(Suppl 3):iii3-7
Assaly R et al (2001) Initial evidence of endothelial cell apoptosis as a mechanism of systemic capillary leak syndrome. Chest 120:1301–1308
Muro Y et al (2009) An evaluation of the efficacy of the toe brachial index measuring vascular involvement in systemic sclerosis and other connective tissue diseases. Clin Exp Rheumatol 27(3 Suppl 54):26–31
Sgonc R et al (2000) Endothelial cell apoptosis in systemic sclerosis is induced by antibody-dependent cell-mediated cytotoxicity via CD95. Arthritis Rheum 43:2550–2562
Fernández-Codina A, Simeón-Aznar CP, Pinal-Fernandez I, Rodríguez-Palomares J, Pizzi MN, Hidalgo CE (2015) Cardiac involvement in systemic sclerosis: differences between clinical subsets and influence on survival. Rheumatol Int 2015 Oct 25 [Epub ahead of print]
Muresan L, Petcu A, Pamfil C, Muresan C, Rinzis M, Mada RO (2016) Cardiovascular profiles of scleroderma patients arrhythmias and conduction disorders. Acta Reumatol Port 41:26–39
Cannarile F, Valentini V, Mirabelli G, Alunno A, Terenzi R, Luccioli F (2015) Cardiovascular disease in systemic sclerosis. Ann Transl Med 3:8
Fernandes F et al (2003) Cardiac remodeling in patients with systemic sclerosis with no signs or symptoms of heart failure: an endomyocardial biopsy study. J Card Fail 9:311–317
Lekakis J, Mavrikakis M, Emmanuel M, Prassopoulos V, Papazoglou S, Papamichael C et al (1997) Cold-induced coronary Raynaud’s phenomenon in patients with systemic sclerosis. Clin Exp Rheumatol 16:135–140
Gustafsson R, Kazzam E, Mannting F, Waldenström A, Hällgren R (1989) Cold-induced reversible myocardial ischaemia in systemic sclerosis. The Lancet 334:475–479
Long A, Duffy G, Bresnihan B (1986) Reversible myocardial perfusion defects during cold challenge in scleroderma. Rheumatology 25:158–161
Mueller KA, Mueller II, Eppler D, Zuern CS, Seizer P, Kramer U (2015) Clinical and histopathological features of patients with systemic sclerosis undergoing endomyocardial biopsy. PLoS ONE 10:e0126707
Kahan A, Nitenberg A, Foult JM, Amor B, Menkes CJ, Devaux JY et al (1985) Decreased coronary reserve in primary scleroderma myocardial disease. Arthritis Rheum 28:637–646
Follansbee WP, Curtiss EI, Medsger TA Jr, Steen VD, Uretsky BF, Owens GR (1984) Rodnan GP (1984) Physiologic abnormalities of cardiac function in progressive systemic sclerosis with diffuse scleroderma. NEJM 310:142–148
Desai CS, Lee DC, Shah SJ (2011) Systemic sclerosis and the heart: current diagnosis and management. Curr Opin Rheumatol 23:545–554
Can I, Onat AM, Aytemir K, Akdogan A, Ureten K, Kiraz S et al (2009) Detecting subclinical biventricular impairment in scleroderma patients by use of pulsed-wave tissue Doppler imaging. Tex Heart Inst J 1:36
Schattke S, Knebel F, Grohmann A, Dreger H, Kmezik F, Riemekasten G et al (2010) Early right ventricular systolic dysfunction in patients with systemic sclerosis without pulmonary hypertension: a Doppler Tissue and Speckle Tracking echocardiography study. Cardiovasc Ultrasound 8:1
Dimitroulas T, Giannakoulas G, Papadopoulou K, Karvounis H, Dimitroula H, Koliakos G et al (2010) Early detection of cardiac involvement in systemic sclerosis assessed by tissue-Doppler echocardiography: relationship with neurohormonal activation and endothelial dysfunction. J Rheumatol 37:993–999
Mavrogeni S, Sfikakis PP, Dimitroulas T, Koutsogeorgopoulou L, Karabela G, Katsifis G et al (2015) Imaging patterns of cardiovascular involvement in mixed connective tissue disease evaluated by cardiovascular magnetic resonance. Inflamm Allergy Drug Targets 4:111–116
Barison A et al (2015) Early myocardial and skeletal muscle interstitial remodelling in systemic sclerosis: insights from extracellular volume quantification using cardiovascular magnetic resonance. Eur Heart J Cardiovasc Imaging 16:74–80
Ntusi NA, Piechnik SK, Francis JM, Ferreira VM, Rai AB, Matthews PM et al (2014) Subclinical myocardial inflammation and diffuse fibrosis are common in systemic sclerosis—a clinical study using myocardial T1-mapping and extracellular volume quantification. J Cardiovasc Magn Reson 16:21
Mavrogeni S, Bratis K, Karabela G et al (2015) Cardiovascular Magnetic Resonance Imaging clarifies cardiac pathophysiology in early, asymptomatic diffuse systemic sclerosis. Inflamm Allergy Drug Targets 14:29–36
Mavrogeni SI, Kitas GD, Dimitroulas T, Sfikakis PP, Seo P, Gabriel S et al (2016) Cardiovascular magnetic resonance in rheumatology: current status and recommendations for use. Int J Cardiol 217:135–148
Hachulla E, Gressin V, Guillevin L et al (2005) Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum 52:3792–3800
Hunzelmann N, Genth E, Krieg T et al (2008) The registry of the German Network for Systemic Scleroderma: frequency of disease subsets and patterns of organ involvement. Rheumatology (Oxford) 47:1185–1192
Avouac J, Airò P, Meune C et al (2010) Prevalence of pulmonary hyper- tension in systemic sclerosis in European Caucasians and metaana- lysis of 5 studies. J Rheumatol 37:2290–2298
Phung S, Strange G, Chung LP et al (2009) Prevalence of pulmonary arterial hypertension in an Australian scleroderma population: screening allows for earlier diagnosis. Intern Med J 39:682–691
Sanz J, Kariisa M, Dellegrottaglie S, Prat-Gonzalez S, Garcia MJ, Fuster V, Rajagopalan S (2009) Evaluation of pulmonary artery stiffness in pulmonary hypertension with cardiac magnetic resonance. J Am Coll Cardiol Img 2:286–295
Fisher MR, Mathai SC, Champion HC et al (2006) Clinical differences between idiopathic and scleroderma-related pulmonary hypertension. Arthritis Rheum 54:3043–3050
Dimitroulas T, Giannakoulas G, Papadopoulou K et al (2010) Left atrial volume and N-terminal pro-B type natriuretic peptide are associated with elevated pulmonary artery pressure in patients with systemic sclerosis. Clin Rheumatol 29:957–964
Tongers J, Schwerdtfeger B, Klein G et al (2007) Incidence and clinical relevance of supraventricular tachyarrhythmias in pulmonary hypertension. Am Heart J 153:127–132
Mathai SC, Bueso M, Hummers LK et al (2010) Disproportionate elevation of N-terminal pro-brain natriuretic peptide in scleroderma-related pulmonary hypertension. Eur Respir J 35:95–104
Allanore Y, Meune C, Vonk MC et al (2010) Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of systemic sclerosis patients. Ann Rheum Dis 69:218–221
Aversa A, Greco E, Bruzziches R, Pili M, Rosano G, Spera G (2007) Relation-ship between chronic tadalafil administration and improvement of endothelial function in men with erectile dysfunction: a pilot study. Int J Impot Res 19:200–207
Koka S, Das A, Salloum FN, Kukreja RC (2013) Phosphodiesterase-5 inhibitor tadalafil attenuates oxidative stress and protects against myocardial ischemia/reperfusion injury in type 2 diabetic mice. Free Radic Biol Med 60:80–88
Varma A, Das A, Hoke NN, Durrant DE, Salloum FN, Kukreja RC (2012) Anti-inflammatory and cardioprotective effects of tadalafil in diabetic mice. PLoS One7:e45243
Metsios G, Lahart IM (2015) Exercise as medicine in rheumatoid arthritis. Mediterr J Rheumatol 26:54–61
John H, Carroll D, Kitas GD (2011) Cardiovascular education for people with rheumatoid arthritis: what can existing patient education programmes teach us? Rheumatology (Oxford) 50:1751–1759
Domsic RT, Dezfulian C, Shoushtari A, Ivanco D, Kenny E, Kwoh CK et al (2014) Endothelial dysfunction is present only in the microvasculature and microcirculation of early diffuse systemics clerosis patients. Clin Exp Rheumatol 32(6 Suppl 86):S-154-60
Turiel M, Gianturco L, Ricci C, Sarzi-Puttini P, Tomasoni L, Colonna Vde G et al (2013) Silent cardiovascular involvement in patients with diffuse systemic sclerosis: a controlled cross-sectional study. Arthritis Care Res (Hoboken) 2013(65):274–280
Authors contributions
Review concept and design: Antonios Psarras, Theodoros Dimitroulas. Drafting of the manuscript: Antonios Psarras, Stergios Soulaidopoulos. Critical revision of the manuscript for important intellectual content: George Kitas, Alexandros Garyfallos, Theodoros Dimitroulas.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Psarras, A., Soulaidopoulos, S., Garyfallos, A. et al. A critical view on cardiovascular risk in systemic sclerosis. Rheumatol Int 37, 85–95 (2017). https://doi.org/10.1007/s00296-016-3530-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-016-3530-3