Abstract
Purpose of review
Report of currently available medical strategies for treatment of childhood chronic uveitis in the biologic era.
Recent Findings
The management of non-infectious chronic uveitis in children is based on immunomodulatory treatment. In case of failure to conventional disease-modifying anti-rheumatic drugs (cDMARDs) and/or frequent flares, tumor necrosis factor-alpha (TNF-α) blocking agents represent the first biologic choice. Adalimumab is the TNF-α inhibitor more frequently adopted. Two multicenter, double blind, randomized, placebo-controlled trials stated its efficacy and safety in this clinical setting.
For refractory disease not responsive to TNF-α inhibitors, emerging biologic therapies have been reported. Most of the current literature refers to expert opinion and remains non-standardized. However, retrospective studies and short case series report tocilizumab, abatacept, and rituximab as promising biologic alternatives in patients with refractory, sight-threatening uveitis even in children.
Summary
The role of anti-TNF-α inhibitor in chronic uveitis therapy met unanimous level of agreement. Rescue therapy approach still remains controversial. Randomized controlled trials and large series with long-term follow-up are mandatory to assess efficacy and cost effectiveness in this challenging disease.
Trial registration
ClinicalTrials.gov ID: NCT01279954. ClinicalTrials.gov ID: NCT04088409
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uveitis is an inflammatory disorder involving the uveal tract. [1]. In children, non-infectious, chronic uveitis is a relatively uncommon but serious disease, with the potential for significant complications and blindness. Ocular inflammation can be associated with an underlying systemic condition or not related to an identifiable origin; in this case, it is termed idiopathic. Juvenile idiopathic arthritis (JIA) is the most commonly associated disease, and uveitis is typically anterior and bilateral [1]. Compared to adults, childhood uveitis is characterized by poor prognosis and higher risk of secondary complications in up to 80% of patients after 3 years and in almost 100% after 20 years of disease [2]. The most common complications include cataract, glaucoma, hand-shaped keratopathy, synechiae, macular edema, ocular hypotony, retinal detachment, and optic atrophy. Up to 30% of patients shows reduced visual acuity and up to 10% develops blindness [3]. Evidence suggests that an environmental trigger in a genetically susceptible individual leads to a release of pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and interleukins (IL) [4]. Therefore, an immunomodulatory approach appears a useful strategy for the management of non-infectious uveitis. However, few pediatric randomized controlled trials have been conducted [5••, 6••, 7••, 8••]. Despite the lack of evidences, immunomodulatory therapy remains the most effective approach to control ocular inflammation, reduce exposure to systemic corticosteroids, and decrease the incidence of complications. The aim of this review is to report currently available medical strategies for assessment and treatment of childhood chronic uveitis.
Assessment
A close collaboration between pediatric rheumatologist and ophthalmologist is pivotal for a proper diagnostic work-up and therapeutic pathway.
The diagnosis requires complete ocular evaluation with slit-lamp examination. Uveitis is classified according to the location of inflammatory process as anterior, intermediate, posterior, and panuveitis [9]. It can be acute with resolution within 3 months, or chronic, characterized by the persistence of disease with prompt (within 3 months) relapses after discontinuation of therapy [10].
The level of intraocular inflammation is classified with the Standardized Uveitis Nomenclature (SUN) Working Group criteria, based on Tyndall (anterior chamber cells). It reflects the number of cells in a field that is the size of a 1 mm × 1 mm slit-lamp [9].
Etiological diagnosis is investigated through the collection of clinical history, complete physical examination and laboratory, and imaging work-up [10]. Nearly half of total patients remain without a detectable origin. Table 1 resumes the most common causes of childhood uveitis.
JIA is the most frequent cause of anterior chronic uveitis in childhood. Considering these patients, according to the last American College of Rheumatology guidelines, an ophthalmologic evaluation should be performed at the time of diagnosis and periodically repeated regardless of the absence of symptoms. The frequency of ocular examination is defined on the basis of the subtype of arthritis, the age at onset, the duration of the disease, and the presence of ANA [11••]. Moreover, the Multinational Interdisciplinary Working Group for Uveitis in Childhood (MIWGUC) has defined outcome measures to define response to treatment, inactive disease, and damage for JIA-U patients. These measures are based on three distinct groups: arthritis activity according to the rheumatologist’s clinical evaluation, level of ocular inflammation detected by ophthalmological evaluation and patient reported outcomes including disruption to school attendance [12].
Treatment
Pharmacologic treatment
Corticosteroids (CS) represent the first-line treatment. In case of lack of response or relapsing disease course, conventional disease-modifying anti-rheumatic drugs (cDMARDs) are the second-line treatment. Methotrexate (MTX) remains the first choice and other classes of cDMARDS, i.e., azathioprine (AZT), mycophenolate mofetil (MMF), and cyclosporine A (CsA) are considered alternative drugs [3].
The third line of treatment, biological disease-modifying anti-rheumatic drugs (bDMARDs), acts against specific cytokines or their receptors. TNF-α blocking agents are the main bDMARDs employed in the treatment of chronic childhood uveitis in case of failure of cDMARDs or in case of frequent flares (more than one episode/month for at least 3 months) despite therapy. Among TNF-α inhibitors, adalimumab (ADA), infliximab (IFX), and golimumab (GLM) are more frequently adopted. Etanercept (ETA) is not recommended for uveitis [1, 13••, 14].
However, a subset of patients fails to respond to TNF-α blockers or is intolerant and may benefit from switching to another class of biologic drugs, i.e., tocilizumab (TCZ), abatacept (ABA), rituximab (RTX), and canakinumab (CAM). These children have severe recalcitrant disease. Most evidence about treatment is based on expert opinion or clinical experience and remains non-standardized [15, 16].
Main available options of treatment are summarized in Table 2 and Table 3.
Surgery
Some complications might require surgical treatment. If a cataract substantially impairs visual acuity, the standard surgical treatment is removal of the lens by phacoemulsification [3, 11••, 13••]. Glaucoma might also require management by goniotomy, insertion of a drainage device, or trabeculectomy. Improved outcomes are associated with the control of intraocular inflammation, both perioperatively and postoperatively [13••].
Discussion
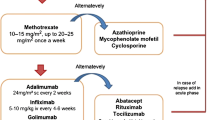
According to the recent consensus-based recommendations from the SHARE (Single Hub and Access point for pediatric Rheumatology in Europe) initiative group, a step-by-step approach is currently considered the gold standard in the management of uveitis [11, 13, 24, 25]. Figure 1 proposes a step-up therapeutic algorithm for non-infectious uveitis.
Immediate systemic immunosuppression is recommended if poor prognostic factors are present at the first visit [13••].
The first step, for mild forms, is represented by topical application of CS [3]. In the presence of complications and poor prognosis factors or in case of lack of response, the recourse to additional systemic CS is usually required [3, 25].
A persistent ocular inflammation, the occurrence of frequent flares, or further complications outline a second step to cDMARDs in order to obtain remission and taper steroid agents [3, 25]. MTX represents the first choice in children with refractory uveitis [11••]. In a meta-analysis, MTX induced a reduction of ocular inflammation in up to 73% of children affected by autoimmune chronic uveitis refractory to steroid therapy [26]. If MTX appears ineffective or the patient is intolerant to the drug, it is recommended to add or switch to a bDMARDs; among biologics, anti-TNFs are the first line [11••].
AZT, CsA, and MMF have been considered as an alternative second immunosuppressive agent in MTX-refractory uveitis or in MTX-intolerant patients. AZT use in treating childhood uveitis is not routine, limited data are available, and currently the introduction of other antimetabolites with fewer side effects makes physicians less prone to prescribe it. In the same way, CsA benefits are limited by its adverse effects, and even though its efficacy in adult uveitis has been shown in numerous studies, data about its application in children with uveitis are limited [3].
ADA represents the first choice among TNF-α inhibitors [27,28,29,30]. Available prospective and retrospective studies report a remission rate ≥ 75% in JIA-U patients after 4–12 weeks of treatment [25]. ADA efficacy is higher when it is used as first dDMARDs [31,32,33,34] and when it is given earlier during the course of the disease [34, 35]. It seems that ADA has the potential to improve the further course of uveitis and prevent relapses [32, 36, 37]. Finally, it reduces steroid requirement and the rate of subsequent ocular surgery [6, 31, 38]. The effect of ADA on achieving inactivity and decreasing the complication rate is greater than IFX [1, 39, 40].
IFX is the other option, and even evidence is not supported by randomized controlled trials (RCT) in childhood. It should be considered in patients’ refractory to MTX combined with another monoclonal TNF-α antibody [25].
An update of the evidences about JIA-U treatment described that IFX determined a remission in 43–94% of cases during a follow-up period of 2–12 months with a concurrent tapering of MTX and topical corticosteroids [25].
Based on the current evidence, ETA should not be considered for JIA-U [13, 18]. Favorable outcomes have been reported for articular involvement, whereas the effectiveness in ocular inflammation seems to be limited [41,42,43,44]. A randomized controlled trial involving pediatric patients did not show significant difference between ETA and placebo [45].
The efficacy of these three TNF-α inhibitors (ADA, IFX, and ETA) was compared in a systematic review. Simonini et al. analyzed 229 children: 31 receiving ADA; 54 ETA, and 144 IFX. ADA and IFX showed superior efficacy than ETA, with improvement of intraocular inflammation of 87, 72, and 33%, respectively [1].
The efficacy and safety of ADA was confirmed in two recent multicenter, double-blind, RCTs in children with JIA-U: SYCAMORE and ADJUVITE trials [5••, 6••].
The SYCAMORE trial evaluated the efficacy and safety of ADA in combination with MTX versus MTX alone to control disease activity in JIA-U non-responsive to MTX. Patients were randomized in two groups to receive ADA (60 children) or placebo (30 children) during a 2-year follow-up. After the second interim analysis, the study was discontinued, due to the significantly lower risk of treatment failure in the ADA group (16/60) than the placebo group (18/30; HR 0.25; 95% CI = 0.12–0.49; p < 0.0001) [6••].
ADJUVITE trial assessed the efficacy and safety of ADA in the treatment of refractory JIA-U or idiopathic anterior uveitis. Thirty-one patients were randomized, 16 to ADA treatment, and 15 to placebo group. At the end of double-blind period, primary outcome analysis showed 56% (9/16) responders in the ADA arm compared with the 20% (3/15) in the placebo group. In the subsequent open-label phase, all patients received ADA reporting no more inflammation or less inflammation in most of cases. In addition, most patients stopped or decreased oral and topical steroids [5••].
Therefore, ADA is currently considered the most efficacious TNF-α-blocker for childhood uveitis. In case of failure and loss of efficacy, it can be useful to consider testing for anti-drug antibodies (Abs) and drug trough level [13••]. If the patient has no anti-drug Abs but low trough levels, a valuable attempt consists in increasing the dose or shortening the interval of administration [13••]. If ocular inflammation persists despite this measure, a switch to a second anti TNF-α should be considered [46].
At this regard, GLM may represent an effective treatment option. Case reports and retrospective case series have shown a positive effect of GLM in patients with JIA-U refractory to cDMARDs and another TNF-α inhibitor [17, 47].
A small case series described 3 patients affected by refractory JIA-U, treated with GLM: 2 out 3 have achieved quiescence for a period of 6 and 18 months, respectively [17]. Cordero-Coma et al. reported that 12 out of 13 adult patients (92.3%) with different types of uveitis, refractory to combined administration of cDMARDs and another TNF-α inhibitor, achieved complete control of inflammation after 6 months of treatment with GLM. A visual improvement associated with decrease of mean values of central retinal thickness was observed [48].
Finally, a systematic review supported that a switch to a second anti-TNFα agent results in improvement of ocular activity when a first anti-TNF-α agent fails. Study cohort included 40 children (34 ADA and 6 IFX); 75% responded to second anti-TNF-α treatment [46].
Unfortunately, some patients do not respond properly to TNF-α inhibitors, other patients have to withdraw therapy due to adverse effect or specific contraindications. These cases represent a major clinical challenge for the need of alternative options.
Given the lack of RCT and prospective studies, most of the available literature refers to retrospective studies and short case series.
The detection of disease biomarkers of active ocular inflammation has suggested the recourse to new potential targeted therapy. High levels of inflammatory cytokines in the vitreous fluid, in the aqueous humor, and also in the sera of patient affected by non-infectious uveitis have been reported [49, 50]. Accordingly, the relevant role of IL-6 in the pathogenesis of uveitis has justified the employment of TCZ (3–7).
The first report about TCZ efficacy regarded 3 adult patients suffering from chronic refractory JIA-U. In these case series, 2 out of 3 patients achieved suppression of uveitis after TCZ [51]. Subsequent case series reported a similar favorable clinical response [52,53,54].
Calvo-Rio et al. conducted the first multicenter retrospective study involving the largest cohort of pediatric patients with JIA-U (25 patients) administered with TCZ. After a median follow-up of 12 months, complete remission was observed in 19 of 25 patients. Significant reduction in the prednisone dosage and significant improvement of best corrected visual acuity (BCVA) were achieved. Nine patients with macular edema obtained a statistically significant decrease in central foveal thickness (CFT) at the 12-month evaluation [55].
In particular, IL-6 was considered having a pivotal role in the development of cystoid macular edema (CME) as uveitis complication since elevated intraocular IL-6 levels correlated with the presence and severity of CME. CME is a swelling of the macula with fluid collection within the intracellular spaces of the retina, and it represents the leading cause of blindness in patients with uveitis [56]. IL-6 may be correlated with CME, directly, by increasing endothelial permeability or, indirectly, by inducing vascular endothelial growth factor (VEGF) [8, 56]. A retrospective single-center study reported a highly significant CME improvement in 12 patients after 24 months of TCZ therapy [56]. STOP-Uveitis, the first RCT conducted confirmed the TCZ efficacy in adult subjects. [8••].
A third retrospective multicenter study including 25 Spanish pediatric and adult patients reported a significant decrease in CME in all patients and remission in 14 of them [57].
At the moment, TCZ is still an off-label indication for uveitis with or without CME; however, IL-6 inhibition appears a promising and safe option. In this regard, the first pediatric RCT to assess the clinical effectiveness and safety of TCZ with MTX in JIA-U, the APTITUDE trial has been recently completed [7••] .
Experimental models of autoimmune uveitis have demonstrated that the. block between T cells and antigen-presenting cells might reduce ocular inflammation [58]. Hence, in 2008, Angeles et al. described the first case of a patient suffering refractory JIA-U that obtained a sustained recovery after ABA [59]. Further attempts have reported analog good results in terms of clinical response and steroid sparing effect. These data regarded a limited number of patients, and most of them were affected by JIA-U [60,61,62]. Only a single case series by Marrani et al. described a favorable effect of ABA also in case of idiopathic non-infectious uveitis [62].
The largest retrospective study about rescue therapy with ABA was performed by Tappeneir et al. and involved 21 children with JIA-U. Compared with previous reports, this study showed a different outcome. A sustained response was seen in less than 15% of patients with severe uveitis, inactivity in 11 patients, but 8 of them recurred [63].
A second multinational retrospective study compared ABA as first biologic therapy (ABA-1) and as third-line treatment (ABA-2) in severe JIA-U. Thirty-one patients, 14 in the ABA-1 group, and 17 in the ABA-2 group were enrolled. After 12 months, 17 subjects (54.8%) showed clinical remission, and no significative differences between the two groups were found [64].
Although definite conclusions cannot be drawn because of the small number of patients and their retrospective nature, it is reasonable to consider ABA as a treatment option for refractory uveitis, particularly for anterior type with recurrent course. Recently, a clinical trial on ABA safety profile and efficacy in non-infectious uveitis has been completed (ClinicalTrials.gov ID: NCT01279954).
Moreover, a growing amount of literature suggests that RTX may be useful for inflammatory ocular diseases. In this perspective, RTX may be considered as treatment for sight-threatening forms of uveitis [19, 65,66,67,68,69].
A retrospective multicenter case series evaluated the clinical outcome of 10 patients with severe JIA-U and active arthritis treated with two infusions of RTX (375 mg/m2). Uveitis improvement and tapering of immunosuppressant medications was reported in 7 patients during a 7 to 18-month follow-up. New ocular flares occurred in 4 out of 7 responders after 6–9 months concurrently to CD20 cells restoration. However, a recall infusion suppressed inflammation in 3 out of 4 patients [18].
Similar results have been obtained by the Italian group that reported a complete control of uveitis in 7 out 8 patients with JIA-U with RTX [69]. A subsequent study of the same group published comparable data about RTX efficacy in maintaining inactivity for a longer period (26–62 months) [67].
Further studies are needed to assess the efficacy and the exact dosing regimen of RTX. However, it may be considered as a treatment alternative in patients with the most aggressive forms of uveitis.
The use of IL-1 blockers in the treatment of pediatric uveitis is limited to a few of case series [20, 70, 71]. Brambilla et al. described ocular remission in 2 girls suffering from non-infectious uveitis [20].
A rising interest in the pathogenetic role of the IL-23 in ocular disorders has encouraged the employment of ustekinumab in uveitis therapy [72]. At the moment, only a single case reported a control of ocular inflammation in a child affected by uveitis related to HLA-B27+ juvenile psoriatic arthritis [73].
Recently, a single case report described an improvement in refractory JIA-U and CME when treated with the Janus kinase (JAK) inhibitor tofacitinib (5 mg twice daily) [74]. Miserocchi et al. reported an improvement of ocular inflammation in 4 patients with long-term history of JIA-U treated with JAK inhibitors, namely, baricitinib (3 cases) and tofacitinib (1 case) [75]. An international, multicenter open-label, active-controlled study of the safety and efficacy of oral baricitinib for patients with JIA-U is planned starting (ClinicalTrials.gov ID: NCT04088409).
If the role of anti TNF-α inhibitors as first-line bDMARDs in case of failure of conventional treatment for uveitis has been widely demonstrated and has reached an unanimous level of agreement, not-standardized data are reported about the timeline of tapering and discontinuation of immunosuppressive therapies in case of long-lasting inactivity. Most authors share the recommendations to wait at least 2 years of inactive disease off topical steroids, before reducing systemic immunosuppression [13••]. Once disease remission is reached and therapy is discontinued, Simonini et al. have identified three clinical predictors of relapse in childhood autoimmune chronic uveitis after stopping systemic treatment: type of disease, time, and type of systemic therapy to achieve inactivity. A higher probability of maintaining remission after discontinuing treatment was shown in idiopathic uveitis compared with JIA-U, if inactivity had been obtained within 6 months from starting systemic treatment and if the treatment adopted was an TNF-α inhibitor compared with MTX [76].
On the opposite side, the management of rescue therapy in case of lack of response to anti TNF-α blockers is still controversial.
Several challenges exist in the timely and optimal treatment of uveitis. In this regard, the recourse to RCTs and large series with long-term follow-up is essential to assess efficacy and cost-effectiveness.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Simonini G, Druce K, Cimaz R, Macfarlane GJ, Jones GT. Current evidence of anti-tumor necrosis factor α treatment efficacy in childhood chronic uveitis: a systematic review and meta-analysis approach of individual drugs: anti-TNFα treatment in childhood chronic uveitis. Arthritis Care Res. 2014;66:1073–84. https://doi.org/10.1002/acr.22214.
Gamalero L, Simonini G, Ferrara G, Polizzi S, Giani T, Cimaz R. Evidence-based treatment for uveitis. Isr Med Assoc J IMAJ. 2019;21:475–9.
Simonini G, Cantarini L, Bresci C, Lorusso M, Galeazzi M, Cimaz R. Current therapeutic approaches to autoimmune chronic uveitis in children. Autoimmun Rev. 2010;9:674–83. https://doi.org/10.1016/j.autrev.2010.05.017.
van Kooij B, Rothova A, Rijkers GT, de Groot-Mijnes JDF. Distinct cytokine and chemokine profiles in the aqueous of patients with uveitis and cystoid macular edema. Am J Ophthalmol. 2006;142:192–4. https://doi.org/10.1016/j.ajo.2006.02.052.
•• Quartier P, Baptiste A, Despert V, Allain-Launay E, Koné-Paut I, Belot A, et al. ADJUVITE: a double-blind, randomised, placebo-controlled trial of adalimumab in early onset, chronic, juvenile idiopathic arthritis-associated anterior uveitis. Ann Rheum Dis. 2018;77:1003–11. https://doi.org/10.1136/annrheumdis-2017-212089. ADJUVITE trial was one of the two available double blind randomised, placebo-control trials assessing the efficacy and safety of ADA in the treatment of refractory JIA-U or idiopathic anterior uveitis.
•• Ramanan AV, Dick AD, Jones AP, McKay A, Williamson PR, Compeyrot-Lacassagne S, et al. Adalimumab plus methotrexate for uveitis in juvenile idiopathic arthritis. N Engl J Med. 2017;376:1637–46. https://doi.org/10.1056/NEJMoa1614160. The SYCAMORE trial demonstrated the efficacy and safety of ADA in combination with MTX versus MTX alone, with regard to controlling disease activity in refractory JIA-U non- responsive to MTX.
Pubmeddev, RA et. al A phase II trial protocol of Tocilizumab in anti-TNF refractory patients with JIA-associated uveitis (the APTITUDE trial). - PubMed - NCBI n.d. APTITUDE trial is the first pediatric clinical trial designed to assess the clinical effectiveness and safety of TCZ with MTX in JIA-U, it has been recently completed.
•• Sepah YJ, Sadiq MA, Chu DS, Dacey M, Gallemore R, Dayani P, et al. Primary (Month-6) Outcomes of the STOP-Uveitis study: evaluating the safety, tolerability, and efficacy of tocilizumab in patients with noninfectious uveitis. Am J Ophthalmol. 2017;183:71–80. https://doi.org/10.1016/j.ajo.2017.08.019. STOP-Uveitis was the first randomized clinical trial conducted to evaluate the effect of TCZ in adult subjects with active non-infectious uveitis. TCZ was effective in improving visual acuity and reducing vitreous haze and central macular thickness.
Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–16. https://doi.org/10.1016/j.ajo.2005.03.057.
Biswas J, Majumder P. Pediatric uveitis: an update. Oman J Ophthalmol. 2013;6:140. https://doi.org/10.4103/0974-620X.122267.
•• Angeles-Han ST, Ringold S, Beukelman T, Lovell D, Cuello CA, Becker ML, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the screening, monitoring, and treatment of juvenile idiopathic arthritis–associated uveitis. Arthritis Care Res. 2019;71:703–16. https://doi.org/10.1002/acr.23871. This guideline provides direction for clinicians and patients/parents making decisions on the screening, monitoring, and management of children with JIA and uveitis, using GRADE methodology and informed by a consensus process with input from rheumatology and ophthalmology experts, current literature, and patient/parent preferences and.
Foeldvari I, Klotsche J, Simonini G, Edelsten C, Angeles-Han ST, Bangsgaard R, et al. Proposal for a definition for response to treatment, inactive disease and damage for JIA associated uveitis based on the validation of a uveitis related JIA outcome measures from the Multinational Interdisciplinary Working Group for Uveitis in Childhood (MIWGUC). Pediatr Rheumatol. 2019;17:66. https://doi.org/10.1186/s12969-019-0345-2.
Constantin T, Foeldvari I, Anton J, de Boer J, Czitrom Guillaume S, Edelsten C, et al. Consensus-based recommendations for the management of uveitis associated with juvenile idiopathic arthritis: the SHARE initiative. Ann Rheum Dis 2018:annrheumdis-2018-213131. https://doi.org/10.1136/annrheumdis-2018-213131. The SHARE initiative identified recommendations for the diagnosis and treatment of JIA-associated uveitis f to suggest a standard of care for JIA-associated uveitis patients throughout Europe.
Valesini G, Iannuccelli C, Marocchi E, Pascoli L, Scalzi V, Di Franco M. Biological and clinical effects of anti-TNFα treatment. Autoimmun Rev. 2007;7:35–41. https://doi.org/10.1016/j.autrev.2007.03.003.
Simonini G, Cimaz R, Jones GT, Macfarlane GJ. Non-anti-TNF biologic modifier drugs in non-infectious refractory chronic uveitis: the current evidence from a systematic review. Semin Arthritis Rheum. 2015;45:238–50. https://doi.org/10.1016/j.semarthrit.2015.05.006.
Gaggiano C, Rigante D, Tosi GM, Vitale A, Frediani B, Grosso S, et al. Treating juvenile idiopathic arthritis (JIA)-related uveitis beyond TNF-α inhibition: a narrative review. Clin Rheumatol. 2020;39:327–37. https://doi.org/10.1007/s10067-019-04763-3.
William M, Faez S, Papaliodis GN, Lobo A-M. Golimumab for the treatment of refractory juvenile idiopathic arthritis-associated uveitis. J Ophthalmic Inflamm Infect. 2012;2:231–3. https://doi.org/10.1007/s12348-012-0081-y.
Heiligenhaus A, Miserocchi E, Heinz C, Gerloni V, Kotaniemi K. Treatment of severe uveitis associated with juvenile idiopathic arthritis with anti-CD20 monoclonal antibody (rituximab). Rheumatology. 2011;50:1390–4. https://doi.org/10.1093/rheumatology/ker107.
Miserocchi E, Modorati G. Rituximab for noninfectious uveitis. In: Miserocchi E, Modorati G, Foster CS, editors. Dev. Ophthalmol., vol. 51, Basel: S. KARGER AG; 2012, p. 98–109. https://doi.org/10.1159/000336188.
Brambilla A, Caputo R, Cimaz R, Simonini G. Canakinumab for childhood sight-threatening refractory uveitis: a case series. J Rheumatol. 2016;43:1445–7. https://doi.org/10.3899/jrheum.160064.
Sen HN, Levy-Clarke G, Faia LJ, Li Z, Yeh S, Barron KS, et al. High-dose daclizumab for the treatment of juvenile idiopathic arthritis-associated active anterior uveitis. Am J Ophthalmol. 2009;148:696–703.e1. https://doi.org/10.1016/j.ajo.2009.06.003.
Nussenblatt RB, Peterson JS, Foster CS, Rao NA, See RF, Letko E, et al. Initial evaluation of subcutaneous daclizumab treatments for noninfectious uveitis: a multicenter noncomparative interventional case series. Ophthalmology. 2005;112:764–70. https://doi.org/10.1016/j.ophtha.2004.12.034.
Nussenblatt RB, Thompson DJS, Li Z, Chan CC, Peterson JS, Robinson RR, et al. Humanized anti-interleukin-2 (IL-2) receptor alpha therapy: long-term results in uveitis patients and preliminary safety and activity data for establishing parameters for subcutaneous administration. J Autoimmun. 2003;21:283–93. https://doi.org/10.1016/s0896-8411(03)00113-6.
Jari M, Shiari R, Salehpour O, Rahmani K. Epidemiological and advanced therapeutic approaches to treatment of uveitis in pediatric rheumatic diseases: a systematic review and meta-analysis. Orphanet J Rare Dis. 2020;15:41. https://doi.org/10.1186/s13023-020-1324-x.
Heiligenhaus A, Minden K, Tappeiner C, Baus H, Bertram B, Deuter C, et al. Update of the evidence based, interdisciplinary guideline for anti-inflammatory treatment of uveitis associated with juvenile idiopathic arthritis. Semin Arthritis Rheum. 2019;49:43–55. https://doi.org/10.1016/j.semarthrit.2018.11.004.
Simonini G, Paudyal P, Jones GT, Cimaz R, Macfarlane GJ. Current evidence of methotrexate efficacy in childhood chronic uveitis: a systematic review and meta-analysis approach. Rheumatology. 2013;52:825–31. https://doi.org/10.1093/rheumatology/kes186.
Anink J, Otten MH, Gorter SL, Prince FHM, van Rossum MAJ, van den Berg JM, et al. Treatment choices of paediatric rheumatologists for juvenile idiopathic arthritis: etanercept or adalimumab? Rheumatol Oxf Engl. 2013;52:1674–9. https://doi.org/10.1093/rheumatology/ket170.
Beukelman T, Ringold S, Davis TE, DeWitt EM, Pelajo CF, Weiss PF, et al. Disease-modifying antirheumatic drug use in the treatment of juvenile idiopathic arthritis: a cross-sectional analysis of the CARRA registry. J Rheumatol. 2012;39:1867–74. https://doi.org/10.3899/jrheum.120110.
Foeldvari I, Becker I, Horneff G. Uveitis events during adalimumab, etanercept, and methotrexate therapy in juvenile idiopathic arthritis: data from the biologics in pediatric rheumatology registry. Arthritis Care Res. 2015;67:1529–35. https://doi.org/10.1002/acr.22613.
Ramanan AV, Dick AD, Benton D, Compeyrot-Lacassagne S, Dawoud D, Hardwick B, et al. A randomised controlled trial of the clinical effectiveness, safety and cost-effectiveness of adalimumab in combination with methotrexate for the treatment of juvenile idiopathic arthritis associated uveitis (SYCAMORE trial). Trials. 2014;15:14. https://doi.org/10.1186/1745-6215-15-14.
García-De-Vicuña C, Díaz-Llopis M, Salom D, Bou R, Díaz-Cascajosa J, Cordero-Coma M, et al. Usefulness of adalimumab in the treatment of refractory uveitis associated with juvenile idiopathic arthritis. Mediat Inflamm. 2013;2013:560632–6. https://doi.org/10.1155/2013/560632.
Schmeling H, Minden K, Foeldvari I, Ganser G, Hospach T, Horneff G. Efficacy and safety of adalimumab as the first and second biologic agent in juvenile idiopathic arthritis: the German Biologics JIA Registry. Arthritis Rheumatol Hoboken NJ. 2014;66:2580–9. https://doi.org/10.1002/art.38741.
Simonini G, Taddio A, Cattalini M, Caputo R, de Libero C, Parentin F, et al. Superior efficacy of adalimumab in treating childhood refractory chronic uveitis when used as first biologic modifier drug: adalimumab as starting anti-TNF-α therapy in childhood chronic uveitis. Pediatr Rheumatol Online J. 2013;11:16. https://doi.org/10.1186/1546-0096-11-16.
Tynjälä P, Kotaniemi K, Lindahl P, Latva K, Aalto K, Honkanen V, et al. Adalimumab in juvenile idiopathic arthritis-associated chronic anterior uveitis. Rheumatol Oxf Engl. 2008;47:339–44. https://doi.org/10.1093/rheumatology/kem356.
Magli A, Forte R, Navarro P, Russo G, Orlando F, Latanza L, et al. Adalimumab for juvenile idiopathic arthritis-associated uveitis. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2013;251:1601–6. https://doi.org/10.1007/s00417-013-2275-x.
Schmeling H, Horneff G. Etanercept and uveitis in patients with juvenile idiopathic arthritis. Rheumatol Oxf Engl. 2005;44:1008–11. https://doi.org/10.1093/rheumatology/keh658.
Trachana M, Pratsidou-Gertsi P, Pardalos G, Kozeis N, Badouraki M, Kanakoudi-Tsakalidou F. Safety and efficacy of adalimumab treatment in Greek children with juvenile idiopathic arthritis. Scand J Rheumatol. 2011;40:101–7. https://doi.org/10.3109/03009742.2010.517546.
Tappeiner C, Schenck S, Niewerth M, Heiligenhaus A, Minden K, Klotsche J. Impact of antiinflammatory treatment on the onset of uveitis in juvenile idiopathic arthritis: longitudinal analysis from a Nationwide pediatric rheumatology database. Arthritis Care Res. 2016;68:46–54. https://doi.org/10.1002/acr.22649.
Zannin ME, Birolo C, Gerloni VM, Miserocchi E, Pontikaki I, Paroli MP, et al. Safety and efficacy of infliximab and adalimumab for refractory uveitis in juvenile idiopathic arthritis: 1-year follow-up data from the Italian registry. J Rheumatol. 2013;40:74–9. https://doi.org/10.3899/jrheum.120583.
Simonini G, Taddio A, Cattalini M, Caputo R, De Libero C, Naviglio S, et al. Prevention of flare recurrences in childhood-refractory chronic uveitis: an open-label comparative study of adalimumab versus infliximab. Arthritis Care Res. 2011;63:612–8. https://doi.org/10.1002/acr.20404.
Tynjälä P, Lindahl P, Honkanen V, Lahdenne P, Kotaniemi K. Infliximab and etanercept in the treatment of chronic uveitis associated with refractory juvenile idiopathic arthritis. Ann Rheum Dis. 2007;66:548–50. https://doi.org/10.1136/ard.2006.058248.
Biester S, Deuter C, Michels H, Haefner R, Kuemmerle-Deschner J, Doycheva D, et al. Adalimumab in the therapy of uveitis in childhood. Br J Ophthalmol. 2007;91:319–24. https://doi.org/10.1136/bjo.2006.103721.
Vazquez-Cobian LB, Flynn T, Lehman TJA. Adalimumab therapy for childhood uveitis. J Pediatr. 2006;149:572–5. https://doi.org/10.1016/j.jpeds.2006.04.058.
Borchers AT, Selmi C, Cheema G, Keen CL, Shoenfeld Y, Gershwin ME. Juvenile idiopathic arthritis. Autoimmun Rev. 2006;5:279–98. https://doi.org/10.1016/j.autrev.2005.09.011.
Smith JA, Thompson DJS, Whitcup SM, Suhler E, Clarke G, Smith S, et al. A randomized, placebo-controlled, double-masked clinical trial of etanercept for the treatment of uveitis associated with juvenile idiopathic arthritis. Arthritis Rheum. 2005;53:18–23. https://doi.org/10.1002/art.20904.
Simonini G, Katie D, Cimaz R, Macfarlane GJ, Jones GT. Does switching anti-TNFα biologic agents represent an effective option in childhood chronic uveitis: the evidence from a systematic review and meta-analysis approach. Semin Arthritis Rheum. 2014;44:39–46. https://doi.org/10.1016/j.semarthrit.2014.03.001.
Miserocchi E, Modorati G, Pontikaki I, Meroni P, Gerloni V. Golimumab treatment for complicated uveitis. Clin Exp Rheumatol. 2013;31:320–1.
Cordero-Coma M, Calvo-Río V, Adán A, Blanco R, Álvarez-Castro C, Mesquida M, et al. Golimumab as rescue therapy for refractory immune-mediated uveitis: a three-center experience. Mediat Inflamm. 2014;2014:717598–5. https://doi.org/10.1155/2014/717598.
Noma H, Funatsu H, Mimura T, Harino S, Hori S. Vitreous levels of interleukin-6 and vascular endothelial growth factor in macular edema with central retinal vein occlusion. Ophthalmology. 2009;116:87–93. https://doi.org/10.1016/j.ophtha.2008.09.034.
Perez VL, Papaliodis GN, Chu D, Anzaar F, Christen W, Foster CS. Elevated levels of interleukin 6 in the vitreous fluid of patients with pars planitis and posterior uveitis: the Massachusetts eye & ear experience and review of previous studies. Ocul Immunol Inflamm. 2004;12:193–201. https://doi.org/10.1080/092739490500282.
Tappeiner C, Heinz C, Ganser G, Heiligenhaus A. Is tocilizumab an effective option for treatment of refractory uveitis associated with juvenile idiopathic arthritis? J Rheumatol. 2012;39:1294–5. https://doi.org/10.3899/jrheum.120010.
Adán A, Mesquida M, Llorenç V, Espinosa G, Molins B, Hernández MV, et al. Tocilizumab treatment for refractory uveitis-related cystoid macular edema. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2013;251:2627–32. https://doi.org/10.1007/s00417-013-2436-y.
Tappeiner C, Mesquida M, Adán A, Anton J, Ramanan AV, Carreno E, et al. Evidence for Tocilizumab as a treatment option in refractory uveitis associated with juvenile idiopathic arthritis. J Rheumatol. 2016;43:2183–8. https://doi.org/10.3899/jrheum.160231.
Papo M, Bielefeld P, Vallet H, Seve P, Wechsler B, Cacoub P, et al. Tocilizumab in severe and refractory non-infectious uveitis. Clin Exp Rheumatol. 2014;32:S75–9.
Calvo-Río V, Santos-Gómez M, Calvo I, González-Fernández MI, López-Montesinos B, Mesquida M, et al. Anti-Interleukin-6 receptor Tocilizumab for severe juvenile idiopathic arthritis-associated uveitis refractory to anti-tumor necrosis factor therapy: a multicenter study of twenty-five patients. Arthritis Rheumatol Hoboken NJ. 2017;69:668–75. https://doi.org/10.1002/art.39940.
Mesquida M, Molins B, Llorenç V, Hernández MV, Espinosa G, Sainz de la Maza M, et al. Twenty-four month follow-up of tocilizumab therapy for refractory uveitis-related macular edema. Retina Phila Pa. 2018;38:1361–70. https://doi.org/10.1097/IAE.0000000000001690.
Vegas-Revenga N, Calvo-Río V, Mesquida M, Adán A, Hernández MV, Beltrán E, et al. Anti-IL6-receptor tocilizumab in refractory and noninfectious uveitic cystoid macular edema: multicenter study of 25 patients. Am J Ophthalmol. 2019;200:85–94. https://doi.org/10.1016/j.ajo.2018.12.019.
Silver PB, Hathcock KS, Chan CC, Wiggert B, Caspi RR. Blockade of costimulation through B7/CD28 inhibits experimental autoimmune uveoretinitis, but does not induce long-term tolerance. J Immunol Baltim Md 1950. 2000;165:5041–7. https://doi.org/10.4049/jimmunol.165.9.5041.
Angeles-Han S, Flynn T, Lehman T. Abatacept for refractory juvenile idiopathic arthritis-associated uveitis- a case report. J Rheumatol. 2008;35:1897–8.
Elhai M, Deslandre CJ, Kahan A. Abatacept for refractory juvenile idiopathic arthritis-associated uveitis: two new cases. Comment on the article by Zulian et al. Arthritis Care Res. 2011;63:307–8; author reply 308. https://doi.org/10.1002/acr.20359.
Kenawy N, Cleary G, Mewar D, Beare N, Chandna A, Pearce I. Abatacept: a potential therapy in refractory cases of juvenile idiopathic arthritis-associated uveitis. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2011;249:297–300. https://doi.org/10.1007/s00417-010-1523-6.
Marrani E, Paganelli V, de Libero C, Cimaz R, Simonini G. Long-term efficacy of abatacept in pediatric patients with idiopathic uveitis: a case series. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2015;253:1813–6. https://doi.org/10.1007/s00417-015-3140-x.
Tappeiner C, Miserocchi E, Bodaghi B, Kotaniemi K, Mackensen F, Gerloni V, et al. Abatacept in the treatment of severe, longstanding, and refractory uveitis associated with juvenile idiopathic arthritis. J Rheumatol. 2015;42:706–11. https://doi.org/10.3899/jrheum.140410.
Birolo C, Zannin ME, Arsenyeva S, Cimaz R, Miserocchi E, Dubko M, et al. Comparable efficacy of abatacept used as first-line or second-line biological agent for severe juvenile idiopathic arthritis-related uveitis. J Rheumatol. 2016;43:2068–73. https://doi.org/10.3899/jrheum.151389.
Tomkins-Netzer O, Taylor SRJ, Lightman S. Can rituximab induce long-term disease remission in patients with intra-ocular non-infectious inflammation? Ophthalmologica. 2013;230:109–15. https://doi.org/10.1159/000351426.
Miserocchi E, Pontikaki I, Modorati G, Gattinara M, Meroni PL, Gerloni V. Anti-CD 20 monoclonal antibody (rituximab) treatment for inflammatory ocular diseases. Autoimmun Rev. 2011;11:35–9. https://doi.org/10.1016/j.autrev.2011.07.001.
Miserocchi E, Modorati G, Berchicci L, Pontikaki I, Meroni P, Gerloni V. Long-term treatment with rituximab in severe juvenile idiopathic arthritis-associated uveitis. Br J Ophthalmol. 2016;100:782–6. https://doi.org/10.1136/bjophthalmol-2015-306790.
Pelegrin L, Jakob E, Schmidt-Bacher A, Schwenger V, Becker M, Max R, et al. Experiences with rituximab for the treatment of autoimmune diseases with ocular involvement. J Rheumatol. 2014;41:84–90. https://doi.org/10.3899/jrheum.130206.
Miserocchi E, Pontikaki I, Modorati G, Bandello F, Meroni PL, Gerloni V. Rituximab for Uveitis. Ophthalmology. 2011;118:223–4. https://doi.org/10.1016/j.ophtha.2010.07.031.
Fabiani C, Vitale A, Emmi G, Lopalco G, Vannozzi L, Guerriero S, et al. Interleukin (IL)-1 inhibition with anakinra and canakinumab in Behçet’s disease-related uveitis: a multicenter retrospective observational study. Clin Rheumatol. 2017;36:191–7. https://doi.org/10.1007/s10067-016-3506-4.
Simonini G, Xu Z, Caputo R, De Libero C, Pagnini I, Pascual V, et al. Clinical and transcriptional response to the long-acting interleukin-1 blocker canakinumab in Blau syndrome-related uveitis. Arthritis Rheum. 2013;65:513–8. https://doi.org/10.1002/art.37776.
Pepple KL, Lin P. Targeting interleukin-23 in the treatment of noninfectious uveitis. Ophthalmology. 2018;125:1977–83. https://doi.org/10.1016/j.ophtha.2018.05.014.
Salek SS, Pradeep A, Guly C, Ramanan AV, Rosenbaum JT. Uveitis and juvenile psoriatic arthritis or psoriasis. Am J Ophthalmol. 2018;185:68–74. https://doi.org/10.1016/j.ajo.2017.10.018.
Bauermann P, Heiligenhaus A, Heinz C. Effect of Janus kinase inhibitor treatment on anterior uveitis and associated Macular edema in an adult patient with juvenile idiopathic arthritis. Ocul Immunol Inflamm. 2019;27:1232–4. https://doi.org/10.1080/09273948.2019.1605453.
Miserocchi E, Giuffrè C, Cornalba M, Pontikaki I, Cimaz R. JAK inhibitors in refractory juvenile idiopathic arthritis-associated uveitis. Clin Rheumatol. 2020;39:847–51. https://doi.org/10.1007/s10067-019-04875-w.
Simonini G, Bracaglia C, Cattalini M, Taddio A, Brambilla A, De Libero C, et al. Predictors of relapse after discontinuing systemic treatment in childhood autoimmune chronic uveitis. J Rheumatol. 2017;44:822–6. https://doi.org/10.3899/jrheum.161336.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Roberta Ponti declares that she has no conflict of interest. Maria Vincenza Mastrolia declares that she has no conflict of interest. Gabriel Simonini declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pediatric Rheumatology
Rights and permissions
About this article
Cite this article
Ponti, R., Mastrolia, M.V. & Simonini, G. Non-Infectious Chronic Uveitis in Childhood: Assessment and Treatment in the Biological Era. Curr Treat Options in Rheum 6, 228–244 (2020). https://doi.org/10.1007/s40674-020-00151-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40674-020-00151-0