Abstract
Rationale and objective
Patients with atypical hemolytic uremic syndrome (aHUS) have long been considered ineligible for kidney transplantation (KTx) in several centers due to the high risk of disease recurrence, graft loss and life-threatening complications. The availability of Eculizumab (ECU) has now overcome this problem. However, the best approach towards timing, maintenance schedule, the possibility of discontinuation and patient monitoring has not yet been clearly established.
Study design
This is a single center case series presenting our experience with KTx in aHUS.
Setting and participants
This study included 26 patients (16 females) with a diagnosis of aHUS, who spent a median of 5.5 years on kidney replacement therapy before undergoing KTx. We compared the aHUS relapse rate in three groups of patients who underwent KTx: patients who received no prophylaxis, patients who underwent plasma exchange, those who received Eculizumab prophylaxis. Complement factor H-related disease was by far the most frequent etiology (n = 19 patients).
Results
Untreated patients and patients undergoing pre-KTx plasma exchange prophylaxis had a relapse rate of 0.81 (CI 0.30–1.76) and 3.1 (CI 0.64–9.16) events per 10 years cumulative observation, respectively, as opposed to 0 events among patients receiving Eculizumab prophylaxis. The time between Eculizumab doses was tailored based on classic complement pathway activity (target to < 30%). Using this strategy, 12 patients are currently receiving Eculizumab every 28 days, 5 every 24–25 days, and 3 every 21 days.
Conclusion
Our experience supports the prophylactic use of Eculizumab in patients with a previous history of aHUS undergoing KTx, especially when complement dysregulation is well documented by molecular biology.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atypical hemolytic uremic syndrome (aHUS) is a rare, progressive and life-threatening disease characterized by platelet consumption, microangiopathic, mechanical non-immune-mediated hemolysis, and acute kidney damage [1]. Organ damage may not be limited to the kidney, with a proportion of patients experiencing neurological involvement and other extra-renal manifestations. The disease, which is often triggered by infections, pregnancy, delivery and surgery, is mainly caused by the uncontrolled activation of the alternative complement pathway. Overactivation is related to mutations in genes encoding complement regulatory proteins i.e. complement factor H (CFH), complement factor I (CFI), membrane co-factor protein CD46 (MCP), complement factor B (CFB), and complement component 3 (C3) [2,3,4,5]. Auto-antibodies against CFH (anti-CFHAAs) have also been identified as pathogenic factors, mainly in patients with a CFHR1-CFHR3 deletion [6].
Atypical HUS accounts for at least 10–15% of all cases of HUS in children [7]. In adults it may be even more frequent and severe, as the case-fatality rate during the acute phase is 2–10% and disease progression to end-stage kidney disease (ESKD) occurs in at least 30% of cases [8].
Patients with complement alternative pathway dysregulation due to genetic abnormalities are at life-long risk of disease relapse. The disease has long been considered by many centers a contraindication to kidney transplantation (KTx) because of the very high risk of recurrence (80% within one year), graft loss and life-threatening complications [8,9,10].
The complement inhibitor Eculizumab (ECU) (Soliris, Alexion Pharmaceuticals Inc., Boston, MA, USA), is a humanized monoclonal antibody whose specific high affinity to C5 blocks its cleavage into pro-inflammatory C5a, preventing the formation of membrane attack complex C5b-9. ECU has led to excellent results in treating the disease during the acute phase and in preventing relapses once the active thrombotic microangiopathy (TMA) is in remission [11,12,13,14,15]. The availability of ECU, as the first and only approved treatment for aHUS since 2011, has made it possible for patients to safely undergo KTx. However, it is unclear whether ECU should be used prophylactically or just for the treatment of post-KTx relapses. Furthermore, regarding the prophylactic use of ECU, to date, the best maintenance schedule, the possibility of discontinuation and patient monitoring have not yet been clearly established.
The aim of the present paper is to describe our experience with KTx in a cohort of pediatric and adult patients with a documented diagnosis of aHUS, specifically focusing on the strategies used to prevent disease relapse and on the non-conventional use of prophylactic ECU tailored on global complement activity.
Methods
Study population
This retrospective analysis considered all pediatric and adult patients with a diagnosis of aHUS-related ESKD undergoing KTx (from both living and deceased donors) at our Center during the last 20 years. They were all screened for known acquired (antiCFHAAs) or genetic complement dysregulation at our Molecular Biology Laboratory. Demographic and clinical characteristics at disease onset, at KTx and during the follow up were collected from the patients’ charts.
All adult patients and, for minors, their legally authorized representatives, provided consent for the study and for its publication. The study was approved by our Institution’s Review Board.
Laboratory procedures
Genetic work out of patients was carried out by Sanger sequencing of the genes known to be involved in aHUS (CFH, CFB, CFI, C3, MCP and THBD) and by multiplex ligation-dependent probe amplification (MLPA) search for macro-rearrangements such as hybrid CFH-CFHR genes. In the last years, next-generation sequencing of a multiple gene custom panel extended the analysis to CFH-related and DGKE genes. The functional significance of any unpublished and uncommon variants was assessed by means of in silico analysis.
HLA class I and class II were typed as previously described [16]. Anti-HLA class I and class II IgG antibodies (Abs) were detected using a LABScreen Mixed kit and single-antigen bead (SAB) assays (One Lambda, Los Angeles, CA, USA) [17]; all results above a Mean Fluorescence Intensity (MFI) cut-off value of 1,000 were considered positive. Before testing, all of the serum samples were pre-treated with disodium EDTA (Sigma-Aldrich, Milan, Italy) [18].
Heat-inactivated patient serum samples were tested using C1qScreen™ (One Lambda) in order to identify complement-binding antibodies as previously described [19].
Definitions
Relapsing aHUS was defined as the concomitant presence of platelet consumption (platelet count < 150,000/mm3 or an acute reduction to < 50%), evidence of hemolysis (anemia or haptoglobin levels below the lower normal limit of 30 mg/dL or lactic dehydrogenase above the upper normal limit), and worsening kidney function (increased serum creatinine or hematuria or proteinuria).
Prophylaxis of aHUS relapse
Before 2007, very few aHUS patients had undergone KTx at our Center (n: 6) and no specific preventive measures were being taken at that time. Given the poor results, patients undergoing KTx between 2007 and 2011 received plasma prophylaxis (PP) to prevent disease relapse [20, 21]. Our protocol consisted of one plasma exchange (PEX) (exchanging 1.5 plasma volume) using fresh-frozen plasma (FFP) immediately before KTx, followed by several sessions after KTx (at least six within the first three weeks) and subsequent regular weekly FFP infusions (20 mL/kg) aimed at maintaining disease remission. After 2010, the availability and proven efficacy of ECU prompted us to change the procedure, initially administering ECU after pre-KTx PEX, and later administering ECU prophylaxis alone as a single administration of 900 mg (in adults) immediately before KTx, followed by a second and third dose in subsequent weeks.
Tailored Eculizumab maintenance treatment
Following the initial ECU doses the subsequent ones were based on the classic complement pathway activity (CCPA), with the aim of maintaining a CCPA of < 30% [22, 23]. CCPA was measured right before the third dose of ECU, and if ≤ 30%, the subsequent doses were delayed. Continual monitoring of the CCPA right before each other dose was performed with the aim of keeping it suppressed. This new approach to ECU dosing based on CCPA was first developed in patients with aHUS with a native kidney [23] and later applied to patients with a KTx.
Immunosuppression regimen
The immunosuppression protocol for KTx included steroids, basiliximab, a calcineurin inhibitor (cyclosporine or tacrolimus) and mycophenolate mofetil.
Results
Baseline data
Over the last 20 years, a total of 26 patients (16 females) with an established diagnosis of aHUS followed up at our Center have undergone 35 KTx, the vast majority of which have been performed since 2010, after ECU became available. Seventeen patients were at their first transplantation, whereas 9 had undergone a previous KTx (including some that had not been performed at our Center). Ten were children at the time of aHUS onset, 6 of whom were still of pediatric age at the time of KTx. Two patients had had pregnancy-related aHUS. Two patients received a graft from a living related donor: one (No. 18), with anti-CFH autoantibody-related disease without gene mutations, while the other (No. 25) had hematopoietic stem cell transplantation (HSCT)-related aHUS (her father was the donor of both the bone marrow and later of the kidney) [24].
Patients had spent a median of 5.5 years, (IQR 2.8–8.2) on dialysis before KTx and some (n: 5) had been on dialysis for more than ten years.
All of the patients except No. 23 had complement dysregulation, the most frequent of which was CFH abnormality (19 patients, 2 of whom also had another complement regulatory gene mutation) (Table 1). Altogether, 9 of the 26 patients experienced a relapse of aHUS after KTx, all without C5-inhibition treatment. The relapses occurred in 7 of the 19 subjects with a complement abnormality involving CFH, the 2 remaining relapsing patients had CFI gene abnormalities.
Prevention of relapses
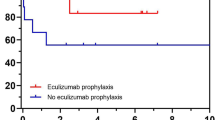
Our experience with KTx has moved from using no prophylaxis against relapses (until 2007) to the routine use of PP (2007–2010) and, since 2010, prophylaxis with ECU. Some of the patients prescribed with plasma or ECU prophylaxis subsequently discontinued it, therefore the preventive strategies were not mutually exclusive (Fig. 1).
Ten patients underwent KTx without any prophylaxis against relapse. This was due to various factors including unclear pathophysiology, the specific genetic etiology had not yet been identified or the risk of relapse was considered very low (CFI mutations) or unlikely (MCP mutations). The relapse rate in this group was 0.81 events/10 years of cumulative observation (CI 0.30–1.76), over a total of 74.3 patient-years, and a median of 6.2 years (IQR 7.0–10.8).
From 2007, six patients entered an intensive program of FFP supplementation (see “Methods”) before and after KTx. The total cumulative observation in plasma prophylaxis was 9.6 patient-years (median of 0.9 years, IQR 0.2–3.5), and the relapse rate was 3.1 events/10 years (CI 0.64–9.16).
From 2010, the use of ECU as a prophylactic strategy became the standard of care at our Center (see “Methods”). A total of 23 patients were transplanted with the use of ECU and the relapse rate was zero, over a cumulative observation period of 78.5 patient-years (median 3 years, IQR 1–5).
Eculizumab discontinuation and tailored treatment
Two patients discontinued ECU prophylaxis: No. 18 because the anti-CFHAAbs responsible for aHUS were no longer detectable a few months after KTx, and No. 23 because she had no documented complement abnormality. Neither of them relapsed during an observation period of 6.9 and 2 years, respectively [24, 25].
Twenty patients remained on ECU maintenance prophylaxis, with the time between ECU doses being extended on the basis of a target CCPA of < 30% (see “Methods”). Twelve are currently receiving infusions every 28 days, 5 every 24–25 days, and 3 every 21 days.
The CCPA obtained using the ECU tailoring strategy is shown in Fig. 2: of the 439 determinations made during the entire cumulative observation period of 602 months, complement activity was completely blocked (CCPA ≤ 10%) in 358 (81%), in 68 (15.5%) it was impaired (CCPA 10–70%), and only 13 (3.0%) had a normal CCPA (> 70%). None of the patients relapsed during the entire cumulative observation period on tailored ECU treatment. As shown in Fig. 3, the platelet count never dropped below 100,000/mm3 and haptoglobin always remained within the normal range. The comparison of platelet count and haptoglobin concentration between blocked and only impaired CCPA did not reveal any difference (Fig. 4).
Complications
One patient on ECU developed severe meningococcal B sepsis before immunization against this serotype was available. The treatment was not discontinued and the patient fully recovered without sequelae.
No increase in incidence or severity of the infections frequently associated with KTx (UTI, upper respiratory tract infection) was observed in the other ECU-treated patients.
Discussion
In the past, patients with ESKD secondary to aHUS were exposed to long periods of dialysis because many centers discouraged KTx given the very high risk of disease relapse. On the other hand, although the estimated risk of relapse was as high as > 80%, some centers (including ours) occasionally did perform KTx without taking any preventive measures.
In the present study, we retrospectively compared three different approaches to patients with ESKD, secondary to aHUS undergoing KTx at our Center. Patients were divided into those who underwent KTx without any preventive measures, patients who received preventive treatment with large volumes of FFP and patients who underwent a prophylactic program with ECU starting right before KTx.
The comparison among the three groups shows how ECU prophylaxis was highly effective in preventing relapses compared to either FFP or no treatment at all. In our case series, ECU eliminated the risk of relapse over a cumulative observation period lasting as long as > 70 patient-years.
A similar finding was also recently reported by Siedlecki [26], who highlighted how patients from the Global aHUS Registry treated with Eculizumab during and after KTx had much better kidney outcomes in terms of GFR than patients treated only after a relapse.
Likewise, we do not support the use of ECU only for treating relapses, but also for prophylactic use, for two reasons; firstly, we are aware that an aHUS relapse on kidney grafts can have disrupting consequences, and because of the overall intrinsic risk of triggering a rejection that an aHUS relapse would have.
Our previous experience of KTx in aHUS patients without preventive measures to avoid relapses was associated with relatively poor outcomes. It may be important to underline that even subjects with CFI gene mutations exhibited a risk of relapse with 1 patient experiencing a relapse as late as 10 years after KTx (further complicated by acute rejection and ultimately by graft loss).
The high prevalence of complement abnormalities in the present series compared to that reported in the general population of aHUS warrants a comment. Patients reaching ESKD represent a subgroup and are not necessarily representative of the general population of patients with aHUS. Moreover, the relatively higher risk of relapse among patients who received plasma prophylaxis in comparison with those who underwent transplantation without any specific prophylaxis (3.1 vs 0.81 events/10 year) can be explained by a selection bias: all of the former patients had severe underlying defects (CFH mutations in 5/6) as against only 5/11 of the latter group.
Based on our experience, we think that all patients with ESKD due to confirmed or suspected aHUS (regardless of their age at onset or disease severity) should be carefully screened for all the known (genetic and acquired) causes of aHUS in order to make the most appropriate decisions with regard to prevention of disease recurrence.
Our current protocol asserts that idiopathic cases and patients with MCP mutations receive a single 900 mg dose of ECU just before KTx in order to protect the peri-transplant period, the most critical as to risk of triggering disease relapses. Anti-C5 treatment is then discontinued because idiopathic and MCP patients have a low risk of relapse, but patients are carefully monitored for relapses (see below), with particular attention during invasive procedures, acute infections or rejection.
In patients with aHUS due to anti-CFHAAs, an attempt to reduce the antibody titer below the limit of non-relapse (< 1000 AUs) is made before KTx. This can be done in various ways such as using steroids, PEX, rituximab, azathioprine or cyclophosphamide [27]. Once anti-CFHAA levels are stably below the given threshold, thus with little or no risk of relapse, C5 inhibition is discontinued, although the patient and the auto-antibody titer are still carefully monitored.
All patients with aHUS due to CFH, CFI, CFB and C3 defects receive peri-KTx and post-KTx ECU prophylaxis since a relapse might trigger acute rejection with sometimes, devastating consequences. The combination of the two events (relapse and rejection) seems to be more than just a coincidental occurrence: out of 11 lost grafts, three failed because of the combination of both events (see Table 1).
As far as monitoring patients for relapses at our center, patients that are not (or are no longer) receiving anti-C5 treatment are required to test their urine for hemoglobinuria every week at home, using a urine dipstick for the early identification of relapses, especially during acute illnesses and when they feel unwell [28].
With regard to complications, ECU treatment is associated with an increased risk of invasive meningococcal disease [29]. This risk is estimated to be 7,000–10,000 times higher in subjects with C5-C8 deficiencies, and 1,400 times higher in subjects with C9 deficiencies. However, in these patients it has been observed that the case-fatality rate is markedly lower (< 3%) compared to subjects without complement deficiencies. Thus, it can be speculated that the difference in severity is due to the reduced release of cytokines in the absence of the membrane-attack complex [30].
Our findings support and strengthen the value of complement functional tests (CCP) when monitoring the adequacy of ECU administration used for relapse prevention [22, 23]. Complement activity was suppressed by ECU in all of our patients, even though the time between subsequent doses had been progressively increased, from the standard 14 days to 21or even 28 days. Despite the increased between-dose interval, none of the patients experienced a disease relapse during the very long cumulative observation period (> 70 patient-years). In addition, for many patients with a body weight of 40–60 kg, 900 mg of ECU often proved to be enough to obtain adequate complement inhibition. Our strategy is not necessarily aimed at completely blocking complement function (CCP activity < 10%), but at limiting it to < 30%. Once again, no relapse was recorded during the entire observation period, including periods characterized by incomplete complement inhibition with CCP activity well above the set target (Fig. 4).
We are well aware that other investigators had a different approach with regard to the management of relapse risk of aHUS in KTx [31]. In particular Duineveld et al. reported a considerably large series of patients with aHUS who underwent KTx without any specific preventive measures and their good results seem to suggest that C5 inhibition is unnecessary [32]. However, as time goes by, a longer observation period might bring different conclusions as shown by our own past experience with cases relapsing several years after KTx (Fig. 1).
Among the possible limitations of the present study, one is that the diagnosis of aHUS relapse was mostly based on clinical grounds alone given that the low platelet count did not allow for a biopsy-proven diagnosis. Another limitation is that the work is retrospective as it covers a 20-year span (during which the management of transplantation has changed a great deal) and is based on a relatively small number of patients. Finally, anti-HLA autoantibodies were not systematically tested in this cohort (especially in the past) to allow any insight into their possible role in triggering the disease. A well-designed prospective study is clearly needed to answer the key question of whether C5 inhibition is necessary in all patients with atypical HUS undergoing KTx, and how long the treatment should be continued for.
In the meantime, patients need to be treated, and this can only be done based on the evidence stemming from the experience accrued with case series.
Our experience leads to the conclusion that patients undergoing KTx due to aHUS should receive ECU prophylaxis. We also suggest that the maintenance treatment schedule should be individualized according to CCP activity. This tailored treatment approach leads to excellent outcomes with an improvement in the patients’ quality of life and a significant reduction in costs.
References
Ardissino G, Possenti I, Tel F et al (2014) Time to change the definition of hemolytic uremic syndrome. Eur J Intern Med 25(2):e29
Noris M, Remuzzi G (2009) Atypical hemolytic-uremic syndrome. N Engl J Med 361(17):1676–1687
Karpman D, Loos S, Tati R et al (2017) Haemolytic uraemic syndrome. J Intern Med 281(2):123–148
Campistol JM, Arias M, Ariceta G et al (2015) An update for atypical haemolytic uraemic syndrome. A consensus document. Nefrologia 35(5):421–447
Fakhouri F, Zuber J, Frémeaux-Bacchi V et al (2017) Haemolytic uraemic syndrome. Lancet 390(10095):681–696
Skerka C, Joszi M, Zipfel P et al (2009) Autoantibodies in hemolytic uremic syndrome (HUS). Thromb Haemost 101(2):227–232
Ardissino G, Salardi S, Colombo E et al (2016) Epidemiology of haemolytic uremic syndrome in children. Data from the North Italian HUS network. Eur J Pediatr 175(4):465–473
Loirat C, Frameux BV (2008) Hemolitc uremic syndrome recurrence after renal transplantation. Pediatr Transpl 12(6):619–629
Santos AH Jr, Casey MJ, Wen X et al (2014) Outcome of kidney transplants for adults with hemolytic uremic syndrome in the US: a ten year database analysis. Ann Transplant 19:953–961
Noris M, Remuzzi G (2010) Thrombotic microangiopathy after kidney transplantation. Am J Transpl 10(7):1517–1523
Al-Akash SI, Almond PS, Savell VH Jr et al (2011) Eculizumab induces long-term remission in recurrent post-transplant HUS associated with C3 gene mutation. Pediatr Nephrol 26(4):613–619
Chatelet V, Fremeaux-Bacchi V, Lobbedez T et al (2009) Safety and long-term efficacy of eculizumab in a renal transplant patient with recurrent atypical hemolytic-uremic syndrome. Am J Transpl 9(11):2644–2645
Larrea CF, Cofan F, Oppenheimer F et al (2010) Efficacy of eculizumab in the treatment of recurrent atypical hemolytic-uremic syndrome after renal transplantation. Transplantation 89(7):903–904
Mache CJ, Acham-Roschitz B, Frémeaux-Bacchi V et al (2009) Complement inhibitor eculizumab in atypical hemolytic uremic syndrome. Clin J Am Soc Nephrol 4(8):1312–1316
Legendre CM, Licht C, Muus P et al (2013) Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med 368(23):2169–2181
Tagliamacco A, Cioni M, Comoli P et al (2014) DQ molecules are the principal stimulators of de novo donor-specific antibodies in nonsensitized pediatric recipients receiving a first kidney transplant. Transpl Int 27(7):667–673
Comoli P, Cioni M, Tagliamacco A et al (2016) Acquisition of C3d-binding activity by de novo donor-specific HLA antibodies correlates with graft loss in nonsensitized pediatric kidney recipients. Am J Transpl 16(7):2106–2116
Loupy A, Lefaucheur C, Vernerey D et al (2013) Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med 369(13):1215–1226
Adamski J (2014) Thrombotic Microangiopathy and indications for therapeutic plasma exchange. Hematol Am Soc Ematol Educ Program 2014(1):444–449
Ponticelli C (2007) De novo thrombotic microangiopathy. An underrated complication of renal transplantation. Clin Nephrol 67(6):335–340
von Baeyer H (2002) Plasmapheresis in thrombotic microangiopathy-associated syndromes: review of outcome data derived from clinical trials and open studies. Ther Apher 6(4):320–328 ((review))
Cugno M, Gualtierotti R, Possenti I et al (2014) Complement functional test for monitoring eculizumab therapy in patients with atypical haemolytic uremic syndrome. J Throb Hemost 12(9):1440–1448
Ardissino G, Tel F, Sgarbanti M et al (2018) Complement functional tests for monitoring eculizumab treatment in patients with atypical hemolytic uremic syndrome: an update. Pediatr Nephrol 33(3):457-461.28
Ardissino G, Cresseri D, Giglio F et al (2019) Haploidentical hematopoietic stem cell transplant complicated by atypical hemolytic uremic syndrome and kidney transplant from the same donor with no immunosuppression but C5 inhibition. Transplantation 103(2):e48–e51
Ardissino G, Testa S, Possenti I et al (2014) Discontinuation of eculizumab maintenance treatment for atypical hemolytic uremic syndrome: a report of 10 cases. Am J Kidney Dis 64(4):633–637
Siedlecki AM, Isbel N, Vande Walle J, James Eggleston J, Cohen DJ, Global aHUS Registry (2018) Eculizumab use for kidney transplantation in patients with a diagnosis of atypical hemolytic uremic syndrome. Kidney Int Rep. 4(3):434–446
Ardissino G, Possenti I, Tel F et al (2015) Discontinuation of Eculizumab treatment in atypical hemolytic uremic syndrome: an update. Am J Kidney Dis 66(1):172–173
Brambilla M, Ardissino G, Paglialonga F, Testa S, Capone V, Montini G (2021) Haemoglobinuria for the early identification of aHUS relapse: data from the ItalKId-HUS Network. J Nephrol. https://doi.org/10.1007/s40620-021-00965-8(Epub ahead of print)
Loirat C, Fakhouri F, Ariceta G et al (2016) An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol 31(1):15–39
Benamu E, Montoya JG (2016) Infections associated with the use of eculizumab: recommendations for prevention and prophylaxis. Curr Opin Infect Dis 29(4):319–329
Van den Brand JA, Verhave JC, Adang EM, Wetzels JF (2017) Cost-effectiveness of eculizumab treatment after kidney transplantation in patients with atypical haemolytic uraemic syndrome. Nephrol Dial Transpl 32(suppl_1):i115–i122. https://doi.org/10.1093/ndt/gfw353
Duineveld C, Verhave JC, Berger SP, van de Kar NCAJ, Wetzels JFM (2017) Living donor kidney transplantation in atypical hemolytic uremic syndrome: a case series. Am J Kidney Dis 70(6):770–777
Acknowledgements
We would like to thank the following physicians for their dedication to our and their patients and for the precious collaboration and commitment which was essential for performing the present study: Amir K. (Cernusco SN), Catalano F. (Reggio Calabria), Davoli D. (Modena), Del Vecchio L. (Lecco), Iannuzzella F. (Reggio Emilia), Giglio F. (Milano), Marinelli R. (Iesi), Milocco C. (Monfalcone), Palladino G. (Salerno), Peccatori J. (Milano), Serbelloni P. (Vimercate), Somenzi D. (Reggio Emilia). We are also thankful to “PROGETTO ALICE ONLUS. ASSOCIAZIONE PER LA LOTTA ALLA SEU” for their continuous and precious support.
Funding
No support/funding are present in this paper.
Author information
Authors and Affiliations
Contributions
Drs. GA, DC and MC performed data collection, analyzed the data, drafted the initial manuscript and revised and approved the final version. Drs. FT, AG, ST, MB and LF designed the data collection instruments, performed data collection, revised the article and approved the final version. Drs. SS, MS, Strumbo, SG, EG and MP performed data collection, edited, revised and approved the final version. Drs. MC, GC, ST, EMR, MC, PM and CB performed data collection, analyzed the data, revised the article and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
D. Cresseri: National (Italy) Coordinator of the Global aHUS Registry supported by Alexion Pharmaceuticals, Inc. G. Ardissino: member of the scientific advisory board of the global aHUS Registry supported by Alexion Pharmaceuticals, Inc. The other authors state that they have no conflict of interest.
Ethical statement
The study has received the approval of our Institution Review Board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ardissino, G., Cresseri, D., Tel, F. et al. Kidney transplant in patients with atypical hemolytic uremic syndrome in the anti-C5 era: single-center experience with tailored Eculizumab. J Nephrol 34, 2027–2036 (2021). https://doi.org/10.1007/s40620-021-01045-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-021-01045-7