Abstract
Background
Phosphorus is associated with mortality in patients with chronic kidney disease (CKD) not on dialysis, possibly through phosphorus-dependent vascular calcification. Although a phosphorus-restricted diet reduces serum phosphorus, it is unlikely that it reduces vascular calcification progression in CKD. This study evaluated whether a combined strategy of phosphorus-restricted diet and phosphate-binding therapy can reduce the risk of all-cause mortality and/or dialysis initiation by attenuating coronary artery calcification (CAC) progression in non-dialysis CKD patients.
Methods
This was a post hoc analysis of a subgroup of patients from a study that evaluated the impact of two phosphorus binder regimens on hard outcomes in CKD. Patients (n = 113) with stage 3–4 CKD and evidence of CAC on a phosphorus-restricted diet were randomized to receive either calcium carbonate or sevelamer added to their phosphorus-restricted diet. End-points were death for any cause and initiation of dialysis. Patients were monitored to the first event or to conclusion of the 36-month follow-up.
Results
Overall, treatment with calcium carbonate was associated with increased CAC progression and occurrence of all-cause mortality, dialysis initiation, and the composite end-point. After adjustment for confounders, sevelamer use was the only independent predictive factor of reduced risk of each endpoint but only if CAC progression was either absent or moderate. Accelerated progression (annual CAC increase >75th percentile of the study cohort) increased the risk of all-cause mortality and composite end-point (p = 0.01) independently of the use of sevelamer.
Conclusions
A significant reduction in all-cause mortality, dialysis initiation, and composite end-point risk was achieved by combining phosphorus-restricted diet and sevelamer in non-dialysis CKD patients with absent or moderate but not accelerated CAC progression. Future studies should investigate the role of serum phosphorus, the usefulness of a phosphorus-restricted diet, and the appropriateness of current normal ranges of serum phosphorus concentration in relation to events in non-dialyzed CKD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High serum phosphorus has been regarded for a long time as a strong risk factor for morbidity and mortality only in patients on maintenance dialysis (ESRD) [1–5]. In recent years the association between serum phosphorus and morbidity and mortality has also been reported in patients with and without chronic kidney disease (CKD) not yet requiring dialysis [6–8]. For instance, in coronary artery diseased patients without CKD, a significant graded independent relation between phosphate and the risk of death or cardiovascular events was observed for serum phosphorus concentrations >3.1 mg/dl [9]. The association between serum phosphorus concentration and morbidity and mortality is also apparent and clinically relevant in CKD patients not on dialysis. In a large cohort of 6,730 non-dialysis CKD patients from the Veterans Affairs Medical Centers, serum phosphorus concentration >3.5 mg/dl was associated with a significant increase in the risk of death by any cause [10]. Notably, in this study cohort, serum phosphorus was almost linearly associated with outcome and mortality increased with each subsequent 0.5 mg/dl increase in serum phosphorus [10]. A post hoc analysis of the REIN Study found that patients with serum phosphorus greater than 3.5 mg/dl were more than two times likely to experience progression to ESRD or doubling of serum creatinine than patients with serum phosphorus below this threshold [11].
Amongst many potential mechanisms, phosphorus may increase morbidity and mortality by inducing vascular calcification [12–14]. This negative effect on vascular muscle cells is evident not only in patients with ESRD but also in those with CKD. In the latter population, serum phosphorus levels in the upper normal range of normal have been associated to a higher prevalence of coronary artery calcification (CAC) [15, 16] and a faster CAC progression [17, 18]. In turn, faster CAC progression has been associated to increased risk of hard outcomes in non-dialyzed CKD patients [19].
A phosphorus-restricted diet is the mainstay to control serum phosphorus concentration and other markers of mineral metabolism in non-dialysis CKD. However, although a phosphorus-restricted diet lowers serum phosphorus, it does not attenuate CAC [18] and cardiac valve calcification [20] progression in non-dialyzed CKD patients when administered alone. On the other hand, existing data suggest that a combination of phosphorus-restricted diet and phosphorus binder administration can halt CAC and cardiac valve calcification progression in non-dialysis CKD patients with mild hyperphosphatemia [18, 20].
The present study evaluated whether a combined therapeutic strategy of phosphorus-restricted diet and phosphorus binders could reduce the risk of all-cause mortality and dialysis initiation, as well as the composite end-point of all-cause mortality and dialysis initiation by influencing CAC progression in non-dialysis CKD patients. To the best of our knowledge, no study has yet addressed this issue.
Methods
The data of the present study were extrapolated from a larger multicenter randomized study that evaluated the impact of two phosphorus binder regimens (calcium based versus calcium free phosphate binders) on hard outcomes (all-cause mortality, dialysis initiation and the composite end-point of dialysis initiation and mortality) in non-dialysis CKD patients [21]. Recruitment initiated in November 2005. Patients were randomized to receive either open label calcium carbonate or sevelamer hydrochloride (HCl) as phosphate binder therapy. Phosphate binder therapy was randomly assigned in a 1:1 fashion based on a single randomization list, computer-generated at the coordinating center. The study was conducted in adherence to the Declaration of Helsinki and the ethical committee at Solofra Hospital (Avellino, Italy) approved the study protocol. All recruited patients provided a signed informed consent prior to study entry.
Patients selected for the present study were those who had good compliance to a phosphorus-restricted diet during the observation period of the parent study and had CAC at baseline. Further inclusion criteria were: serum concentration of phosphorus, calcium and parathyroid hormone (PTH) regularly measured during the 6 months preceding enrolment; no prior therapy with phosphorus binders, vitamin D sterols, or statins; and at least two good quality multi-slice computed tomography (CT) scans of the coronary tree. Exclusion criteria were: presence of heart failure, coronary artery disease, previous myocardial infarction, coronary by-pass, coronary angioplasty, stroke, arrhythmia, liver dysfunction, or nephrotic syndrome. Finally, evidence of fast kidney function decline (defined as 24-h measured creatinine clearance loss ≥12 ml/min/year) was regarded as an exclusion criterion since it might have precluded a prolonged follow-up on account of the need to accelerate dialysis initiation. No vegetarians were present in the study cohort.
During the 6-month period preceding enrolment, patients received written dietary advice from the nephrologist. At study entry, qualified dieticians prescribed a phosphorus-restricted diet and counseled patients as well as family members. Phosphorus intake was established according to the patient’s degree of renal impairment. All patients were prescribed at least 30 kcal/kg per day of energy and 600–700 mg/day of phosphorus intake. Compliance to the phosphorus-restricted diet was assessed every 4 months by dieticians on the basis of a repeated interview and routine blood biochemistry. Dieticians were free to re-evaluate the adherence to the dietary regimen in a shorter time frame (every month) if needed.
Either calcium carbonate or sevelamer was randomly added to the phosphorus-restricted diet. Patients were informed that sevelamer use in CKD had not been approved by the Italian Health Department at the time the study was carried out. Calcium carbonate and sevelamer were the only phosphate binders available when the study started. Equivalent doses of sevelamer and calcium containing phosphate binders were prescribed as previously reported [18], to avoid any bias due to the different phosphate-binding capacity of study binders. Accordingly [18], the initial dose of the binder was 1,600 and 2,000 mg/day for sevelamer and calcium carbonate, respectively. Adherence to binder therapy was assessed by means of pill count by either dieticians or nephrologists at follow-up visits. During the study period, investigators were free to adjust the phosphorus binder dose to achieve and maintain levels of phosphorus and mineral metabolism parameters according to the Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines (the only ones available at the time of study initiation) [1]. A serum level of phosphorus between 2.7 and 4.6 mg/dl in CKD stages 3–4 and between 3.5 and 5.5 mg/dl in CKD stage 5 was rigorously imposed [1]. Investigators were blind to the presence of CAC but not to binder assignment. Other therapies were prescribed based on patients’ medical conditions and on laboratory parameters according to everyday practice. Routine blood chemistry assessments at study enrolment and either immediately before an event of interest (death or dialysis initiation) or at study completion (36th month) were regarded as baseline and final study assessments, respectively. Renal function was assessed via the 24-h measured creatinine clearance. Intact PTH was assayed by chemiluminescence and high-sensitivity C-reactive protein (hsCRP) by the immunoturbidimetric method. CAC was assessed by CT scan at study entry and at months 6, 12, and 24. CT scans were performed and analyzed by a single center (Department of Radiology, Solofra Hospital). Two independent readers who were unaware of the patients undergoing phosphate binder treatment scored the scans. CAC score was reported in Agatston units (AU). Changes of CAC score were presented as annualized progression; the following formula was used: CAC(follow-up) − CAC(baseline)/days of follow-up × 365 [22]. The annualized progression of CAC score was also classified as absent (≤25th percentile of the study cohort distribution), moderate (25–75th percentile) or accelerated (>75th percentile) [19]. Alternatively, CAC progression was defined if the annualized change of CAC score was ≥15 %. On the other hand, regression was defined if the last follow-up CAC score was lower than the one recorded at study entry. Recorded events were death for any cause and initiation of dialysis. The composite end-point of death for any cause and initiation of dialysis was also taken into account. The follow-up continued until the occurrence of any event of interest or completion of 36 months of follow-up from study initiation.
Statistical analysis
Data are expressed as mean ± standard deviation (SD), median and interquartile range, or frequencies. The t test for continuous variables and Chi square test were used for comparison of baseline clinical characteristics of patients between the two treatment arms (calcium vs. sevelamer). Kaplan–Meier survival curves were created and compared via a log-rank test between the two study arms. Multivariable Cox’s regression analysis was performed to calculate hazard ratios (HR) and assess factors independently associated with the endpoints. Cox’s analysis was adjusted for covariates regarded as traditional predictors of mortality or dialysis initiation such as age, gender, diabetes, hypertension as well as variables specifically associated with outcome in CKD such as glomerular filtration rate (GFR), and serum concentrations of calcium, phosphorus, PTH, CRP. The largest possible meaningful model considered included clinical and biochemical variables on the basis of their plausible univariable relation to the outcome, considering the overall model fit and hazard proportionality. Variables to be dropped as non-confounders were eliminated manually, monitoring variations of the exposure regression coefficient and giving validity precedence over precision. The contribution of covariates to explain the dependent variable was assessed using a 2-tailed Wald test. Model re-estimation, formal and graphic tests based on residuals, as well as time-interaction testing of the variables in the model were all used to assess model specification, proportionality assumption, and overall fit. Data are expressed as HR and 95 % confidence interval (CI). A p < 0.05 was considered significant.
Results
From the original cohort, a total of 113 (47 and 66 patients assigned to calcium carbonate and sevelamer, respectively) non-dialyzed CKD patients with good compliance to phosphorus-restricted diet and with CAC at baseline were selected and evaluated for current analyses. Clinical characteristics and baseline routine biochemical analyses are reported in Table 1.
Mean daily dose of sevelamer and calcium carbonate was 2,184 ± 592 mg (range 1,600–3,200) and 2,948 ± 504 mg (range 2,000–4,000), respectively. At follow-up conclusion, mineral metabolism control among patients on the phosphorus-restricted diet and treated with sevelamer was well controlled: serum phosphorus (mean ± SD), 4.03 ± 1.0 mg/dl (median 4.0 mg/dl; interquartile range [IQR] 3.1–4.9); serum calcium, 8.5 ± 0.6 mg/dl (8.6; 8.8–8.9); serum PTH, 225 ± 143 pg/ml (200; 120–314). Similar trends were noted also among patients on phosphorus-restricted diet and treated with calcium carbonate: serum phosphorus, 4.1 ± 0.9 mg/dl (4.4 mg/dl; 3.6–4.8); serum PTH, 301 ± 174 pg/ml (250 pg/ml; 177–440); serum calcium, 9.1 ± 0.8 mg/dl (8.4; 8.4–8.9).
Table 2 reports baseline values and changes at end of follow-up of CAC scores as well as the proportion of patients experiencing absent, moderate and accelerated CAC progression. When compared to sevelamer use, treatment with calcium carbonate was associated with a significant increase in CAC progression. Notably, CAC regression was observed in 24 patients on the phosphorus-restricted diet and treated with sevelamer versus 1 patient on phosphorus-restricted diet and treated with calcium carbonate.
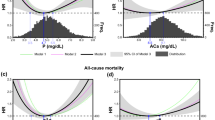
The event-free survival curves of patients on phosphorus-restricted diet and treated with either sevelamer or calcium carbonate are shown in Figs. 1, 2, 3. All-cause mortality, dialysis initiation, and composite end-point rates were less frequent among patients assigned to sevelamer. At study completion a total of 9 (13.6 %) vs. 15 (31.9 %) patients died, and 18 (27.3 %) vs. 23 (48.9 %) patients started dialysis in the sevelamer and calcium carbonate study arms, respectively.
All-cause mortality in patients on restricted phosphorus diet and treated either with sevelamer (continuous line) or calcium carbonate (dotted line). All-cause mortality rate was significantly higher (log-rank test: 5.18; p = 0.0229) among patients on a restricted phosphorus diet who were receiving calcium carbonate
Dialysis initiation in patients on restricted phosphorus diet treated either with sevelamer (continuous line) or calcium carbonate (dotted line). Dialysis initiation was significantly higher (log-rank test: 4.84; p < 0.0278) among patients on a restricted phosphorus diet who were receiving calcium carbonate
Event-free survival with respect to the composite end-point of all-cause mortality and dialysis initiation among patients treated with sevelamer (continuous line) or calcium carbonate (dotted line). Survival was significantly (log-rank test = 9.79; p < 0.0018) worse among patients on a restricted phosphorus diet who were receiving calcium carbonate
Results of the multivariable adjusted Cox’s regression models for all-cause mortality, dialysis initiation, and composite end-point are shown in Table 3A, B. After adjustment for confounders, absent CAC progression (defined as annualized change ≤25th percentile) and moderate (25–75th percentile) did not predict the risk of any outcome of interest. In these cases sevelamer was the only predictive factor of a reduced risk for all-cause mortality, initiation of dialysis and the composite end-point. In contrast, accelerated progression (>75th percentile of the study cohort distribution) significantly increased the risk of all-cause mortality (p = 0.01), composite end-point (p = 0.01) but not the risk of dialysis initiation occurrence. Notably, the effect of accelerated CAC progression was independent of sevelamer administration.
Finally it is worth noting that with the exception of all-cause mortality traditional risk factors such as age and presence of diabetes were not associated with outcome in this specific study cohort. Similarly, serum biomarkers of mineral metabolism such as phosphorus, calcium and PTH were not linked to outcomes.
Discussion
The present study addresses the question whether the association of a phosphorus-restricted diet and phosphorus binding therapy may reduce the rate of all-cause mortality, dialysis initiation, and composite end-point by influencing CAC progression in non-dialyzed CKD patients. The question arises from several findings both in CKD and ESRD. It has been demonstrated that in non-dialysis CKD patients high serum phosphorus concentration is associated with: (a) an excessive risk of cardiovascular mortality and CKD progression to ESRD [6–8, 11]; (b) cellular processes leading to vascular calcification [14]; (c) accelerated CAC progression [17] that in turn is linked to cardiovascular mortality [19]. In addition, observational data suggest that phosphate binder use as well as a low protein diet may reduce mortality in both CKD and ESRD patients [5]. Thus, a phosphorus-restricted diet is suggested as first-line therapy to target serum phosphorus concentration [1, 6, 23]. However, data have shown that the combination of phosphorus-restricted diet and phosphorus binders, but not a phosphorus-restricted diet alone, may reduce CAC and cardiac valve calcification progression in non-dialysis CKD patients [18, 20].
Our results confirm the existing data that suggest a differential impact of various phosphate binder regimens on CAC progression [18]. Indeed, patients allocated to a low-phosphate diet and sevelamer exhibited a significantly lower CAC progression compared to patients on low-phosphate diet and calcium-containing phosphate binders. Furthermore, a greater proportion of patients allocated to sevelamer also showed CAC regression.
This post hoc analysis expands the existing body of evidence on the relationship between CAC, phosphate lowering therapies, and outcomes in a distinctive population of mild hyperphosphatemic non-dialysis CKD patients on a phosphorus-restricted diet and with baseline CAC. In contrast to the combination of diet + calcium carbonate, the combination of sevelamer with a phosphorus restricted diet attenuated CAC progression and changed the risk of all-cause mortality, dialysis initiation as well as the risk of the composite end-point of all-cause mortality and dialysis initiation. Notably, renal and overall survival was only partially dependent on CAC progression. Indeed, sevelamer use was the only independent protective factor for all-cause mortality and dialysis initiation in patients with absent or moderate CAC progression, after accounting for confounders. However, sevelamer was no longer the only factor associated with outcome in patients with an accelerated CAC progression. All together, these findings suggest that renal and all-cause survival is strongly affected by phosphorus binder choice in some but not in all clinical circumstances. In light of the fact that accelerated CAC progression is a marker of advanced and active vasculopathy, it is conceivable that it may abolish any favourable effect of phosphorus-restricted diet plus sevelamer on outcomes.
Even though the initial study hypothesis that phosphate-binding therapy on top of a phosphorus-restricted diet could improve survivals by attenuating CAC progression in all clinical settings remains to be confirmed, our results are, in our opinion, stimulating. They are in sharp contrast with a recently published randomized placebo-controlled study that documented a significant vascular calcification increase in a cohort of non-dialyzed CKD patients treated with various phosphate binders (i.e. calcium acetate, lanthanum carbonate, and sevelamer carbonate) on a stable protein-restricted diet [24].
It is worth mentioning that in our study the effect of a combined therapy of phosphorus-restricted diet and phosphorus binders on CAC progression was not dependent on the serum concentrations of calcium, phosphorus or PTH. The absence of a significant association between CAC progression and markers of mineral metabolism is consistent with a previous report in a smaller cohort of non-dialysis CKD patients where survival was not assessed [17, 19].
All together, these findings could question whether phosphorus has an effect on the occurrence and progression of CAC and consequently they may challenge the usefulness of a phosphorus-restricted diet in reducing the vascular calcification process in non-dialyzed CKD patients. However, it should be taken into account that serum phosphorus represents less than 1 % of the total body phosphorus; it is conceivable that a positive phosphorus balance may be achieved without a significant change in its serum levels [25]. In addition, the vascular calcification process takes a long time, while serum phosphorus levels change rapidly in time due to the many compensatory mechanisms that counterbalance hyperphosphatemia until advanced stages of CKD. Finally, it is possible that a “high-normal” phosphorus level in the suggested range of normality by current guidelines could be already a sign of phosphorus metabolism imbalance, particularly in non-dialysis CKD patients with preserved compensatory mechanisms regulating mineral metabolism. In keeping with this hypothesis, recent data of a large study in non-dialysis CKD patients with mild hyperphosphatemia (defined as serum phosphorus >4.3 mg/dl) documented a 2- to 3-fold increase in the risk of dying of any cause or progressing to dialysis [26].
Limitations
Rather than phosphorus per se, the different chemical characteristics of the binders used in this study might have influenced the impact on outcomes through increased serum calcium concentration in patients assigned to receive calcium carbonate. However, final serum calcium concentration was not different between the two groups. In addition, multivariable analysis excluded any role of serum calcium concentration in CAC progression as well as hard outcomes. On the other hand, it also plausible that serum calcium like serum phosphorus is a poor marker of mineral balance and may not reflect an abnormal metabolism.
The adherence to medications could have been different between patients assigned to sevelamer or calcium carbonate due to the different mode of drug ingestion (i.e. swallowing vs. chewing). Nonetheless, substantial differences in the adherence to prescribed binder were excluded through pill count. The compliance with dietary phosphorus restriction in clinical practice is commonly poor even in non advanced stages of CKD [1]; however, the compliance may rise up to 90 % and be long-lasting when dieticians provide careful instruction and regular counselling and monitoring during the study period, on the one hand, and when patients are monitored closely and have regular contact with their kidney-care providers, on the other [1]. This was the case in the present study.
Bone parameters were not gathered in the present study, as this was not amongst the aims of the study. Finally, only a few patients experienced an accelerated CAC progression and this may have had an effect on results.
In conclusion, a significant reduction in the risk of all-cause mortality, initiation of dialysis, and composite end-point was achieved by combining a phosphorus-restricted diet with sevelamer treatment in non-dialysis CKD patients with absent and moderate CAC progression. In these clinical circumstances, sevelamer was the sole independent predictive factor of reduced risk. In contrast, sevelamer was no longer the sole independent predictive factor of reduced risk in cases where the CAC progression was accelerated.
Our data may stimulate a larger interventional study regarding the role of serum phosphorus, the usefulness of a phosphorus-restricted diet, and the appropriateness of current normal ranges of serum phosphorus concentration in relation to outcome in CKD patients not on dialysis.
References
National Kidney Foundation (2003) K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42:S1–S201
Block GA, Preston S, Klassen PS et al (2004) Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15:2208–2218
Young EW, Albert JM, Satayathum S, Goodkin DA et al (2005) Predictors and consequences of altered mineral metabolism: The Dialysis Outcomes and Practice Patterns Study. Kidney Int 67:1179–1187
Lacson E, Wang W, Hakim RM, Teng M, Lazarus MJ (2008) Associates of mortality and hospitalization in hemodialysis: potentially actionable laboratory variables and vascular access. Am J Kidney Dis 53:79–90
Isakova T, Gutierrez O, Chang Y et al (2009) Phosphorus binders and survival on hemodialysis. J Am Soc Nephrol 20:388–396
KDIGO Clinical Practice Guideline for the Diagnosis (2009) Evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int 76(S113):S1–S130
Bhuriya R, Li S, Chen S, McCullough PA, Bakris GL (2009) Plasma parathyroid hormone level and prevalent cardiovascular disease in CKD Stages 3 and 4: an Analysis From the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 53(S4):S3–S10
Covic A, Kothawala P, Bernal M, Robbins S, Chalian A, Goldsmith D (2009) Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transpl 24:1506–1523
Tonelli M, Sacks F, Pfeffer M, Gao Z, Curham G (2005) Relation between serum phosphorus level and cardiovascular event rate in people with coronary disease. Circulation 112:2627–2633
Kestenbaum B, Sampson JN, Rudser KD et al (2005) Serum phosphorus levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 16:520–528
Zoccali C, Ruggenenti P, Perna A et al (2011) REIN Study Group: phosphorus may promote CKD progression and attenuate renoprotective effect of ACE inhibition. Am Soc Nephrol 22:1923–1930
Mathew S, Tustison KS, Sugatani T, Chaudhary LR, Rifas L, Hruska KA (2008) The mechanism of phosphorus as a cardiovascular risk factor in CKD. J Am Soc Nephrol 19:1092–1105
Kestenbaum B (2007) Phosphorus metabolism in the setting of chronic kidney disease: significance and recommendations for treatment. Semin Dial 20:286–294
Giachelli C (2009) The emerging role of phosphorus in vascular calcification. Kidney Int 75:890–897
Ix JH, De Boer IH, Peralta CA et al (2009) Serum phosphorus concentrations and arterial stiffness among individuals with normal kidney function to moderate kidney disease in MESA. Clin J Am Soc Nephrol 4:609–615
Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA (2009) Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol 20:397–404
Russo D, Corrao S, Miranda I et al (2007) Progression of coronary artery calcification in predialysis patients. Am J Nephrol 27:152–158
Russo D, Miranda I, Ruocco C et al (2007) The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney Int 72:1255–1261
Russo D, Corrao S, Battaglia Y et al (2011) Progression of coronary artery calcification and cardiac events in patients with chronic renal disease not receiving dialysis. Kidney Int 80:112–118
Di Lullo L, Floccari F, Santoboni A et al (2013) Progression of cardiac valve calcification and decline of renal function in Non-dialysis CKD patients. J Nephrol 26:739–744
Di Iorio B, Bellasi A, Russo D (2012) Mortality in kidney disease patients treated with phosphorus binders: a randomized study. Clin J Am Soc Nephrol 7:487–493
Achenbach S, Ropers D, Pohle K et al (2002) Influence of lipid-lowering therapy on the progression of coronary artery calcification: a prospective evaluation. Circulation 106:1077–1082
Di Iorio B, Di Micco L, Torraca S et al (2012) Acute effects of very-low-protein diet on FGF23 Levels: a randomized study. Clin J Am Soc Nephrol 7:581–587
Block GA, Wheeler DC, Persky MS et al (2012) Effects of phosphorus binders in moderate CKD. J Am Soc Nephrol 23:1407–1415
Bellasi A, Cozzolino M, Adragao T, Di Iorio B, Russo D (2013) Phosphate binders in moderate chronic kidney disease: where do we stand? J Nephrol 26:993–1000. doi:10.5301/jn.5000258
Bellasi A, Mandreoli M, Baldrati L et al (2011) Chronic kidney disease progression and outcome according to serum phosphorus in mild-to-moderate kidney dysfunction. Clin J Am Soc Nephrol 6(4):883–891
Acknowledgments
Authors did not have any writing assistance. Authors state that they did not have sponsors in the study design, in the collection, analysis and interpretation of data, in the writing of the manuscript, and in the decision to submit the manuscript for publication.
Conflict of interest
D.R., A.P., L.R., and B.D.I. declare they do not have any conflict of interest; A.B. has received speaking honoraria from Sanifit, Sanofi, Genzyme, Amgen, and Abvie.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Russo, D., Bellasi, A., Pota, A. et al. Effects of phosphorus-restricted diet and phosphate-binding therapy on outcomes in patients with chronic kidney disease. J Nephrol 28, 73–80 (2015). https://doi.org/10.1007/s40620-014-0071-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-014-0071-2