Abstract
Purpose
Adversity in early life can induce metabolic defects in exposure to stress in adulthood. Therefore, the exploration of involving mechanisms can be helpful in the treatment of metabolic disorders. So, the present study was conducted in terms of exploring the effects of interaction between early postnatal stress and young adulthood psychological stress on insulin secretion and pancreatic GLUT-2 levels in male rats.
Methods
Footshock as a model of early life stress (at 2 weeks of age) and psychological stress induced by communication box as a model of young adulthood stress (at 8–10 weeks of age) were induced in male Wistar rats for five consecutive days (2 times/day). Blood samples were drawn to measure glucose, insulin, homeostatic model assessment of insulin resistance (HOMA-IR) and homeostasis model assessment of β-cell dysfunction (HOMA-B), before and after stress protocol in young adult rats. Corticosterone was measured on days 1 and 5 of stress induction. The day after the stress period, factors including glucose tolerance, TNF-alpha, isolated islets’ insulin output and levels of pancreatic GLUT-2 protein via western blotting were determined.
Results
The combination of early footshock exposure and psychological stress during adulthood did not affect plasma corticosterone, but increased plasma insulin, HOMA-IR, HOMA-B and TNF-alpha levels. Plasma TNF was not only increased by the combination of both stressors, but also after only E STR exposure. HOMA-IR was increased in both Psy STR and E + Psy-STR groups. Plasma glucose just increased in Psy STR group. The combination of these two life stressors further increased the in vitro insulin secretion from isolated islets in response to 16.7-mM glucose. The level of Glut2 was increased in Psy STR and decreased in both E STR and E + Psy STR groups. Finally, glucose tolerance was impaired and glucose-stimulated insulin secretion was increased in E + Psy STR group.
Conclusions
In conclusion, inducing stress in early life makes the organism more susceptible to metabolic defects in exposure to psychological stress later in life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic dysregulations are outcomes of stress being programmed by hyperactivity of the hypothalamic–pituitary–adrenal (HPA) axis induced by negative experiences in early life [1, 2]. Early postnatal stages of life are critical times to develop long-lasting alternations in health status throughout adulthood [1]. Furthermore, early life stress in combination with chronic psychological stress in adulthood induces the changes in neuroendocrine and metabolic responses [3,4,5].
Stress hormones such as glucocorticoids and catecholamines induce insulin resistance, produce hyperglycemia via increasing glucose release from the liver. Consequently, impaired insulin secretion from pancreatic islets and hyperinsulinemia are the most important outcomes of insulin resistance [6,7,8]. Many factors such as proinflammatory cytokines are involved in the development of reduced insulin sensitivity in peripheral tissues [9, 10]. Furthermore, it has been reported that early life stressors have an impact on the inflammatory factors such as tumor necrosis factor-alpha (TNF-alpha) that can be influenced by stress during adulthood [11]. To compensate for insulin resistance in the early stages, beta cells of Langerhans islets secrete more insulin to establish glucose homeostasis [7]. One of the key glucose sensors in insulin secretion from pancreatic beta cells is glucose transporter-2 (GLUT-2) [12] which can be affected by stress hormones [13].
Both human and animal studies showed that early life stress alters the metabolic responses to stressors later in life [14, 15]. Considering metabolic disorders as an important mortality factor in today’s world and the existence of many psychological stressors throughout today’s lifestyle (both early life and adulthood), few studies have been done so far in this context. So, further studies are needed to explore factors and mechanisms involving metabolic disorders induced by the combination of early life stress and adulthood psychological stress. So, the effect of interaction between early postnatal stress and young adulthood psychological stress on glucose metabolism aspects such as insulin resistance, glucose tolerance, insulin secretion from isolated islets, pancreatic GLUT-2 levels, and TNF-alpha and blood metabolic parameters was examined.
Materials and methods
Animals
Male (250 ± 10 g) and female (180 ± 20 g) Wistar rats (Pasture Institute, Tehran, Iran) were mated overnight and separated at 9 A.M. The pregnant rats were kept until delivery in a temperature-controlled room (22 ± 2 °C) with a 12-h light/dark cycle (light on at 07:00). Standard food produced by Pars Company (animal food producer, Iran) and tap water were provided on demand throughout the experimental period. For this experiment, male Wistar rat pups were randomly selected from eight litters; four male pups from each litter were randomly allocated into four different groups (n = 8/group): the N STR; non-stress (just placed inside the communication box at both level of age); E STR; early stress (received footshock in communication box at 2 weeks of age but not receiving psychological stress in adulthood); Psy STR; psychological stress (received psychological stress during young adulthood but without footshock at 2 weeks of age); E + Psy STR; early + pshychological stress (received both footshock stress at 2 weeks of age and psychological stress during young adulthood). All procedures were approved by the Animal Care and Use Committee of the Diabetic Research Center, Mazandaran University of Medical Sciences (IR.MAZUMS.IMAMHOSPITAL.REC.1397.3069).
Psychological stress induction protocol
A communication box (48 × 48 × 50 cm) [16] as the apparatus stressor, consisting of nine chambers (16 × 16 cm) was used to induce stress. The chambers are designed so that the rats can have visual, auditory, and olfactory communication with each other. The floor of five of the chambers was made from metal wire composed of stainless steel and connected to electricity, enabling the animals to receive electrical shocks. The floors of the other four chambers were covered by a plexiglas plate to avoid rats from sensing footshock for induction of psychological stress.
The rat pups at two weeks of age received electrical shocks (0.8 mA, 1 Hz) lasting five seconds, every 30 s for 30 min, twice daily, for five consecutive days [17]. During footshock induction, the pup rats screamed and exhibited an increased rate of urination and defecation. Moreover, the rats tried to escape from the shock by fore and hind paw withdrawal at postnatal age. The pups of all groups were kept alone in a cage for 5 min after removal from the communication box [17], and then they were returned to their home cages.
The young adult rats (at 8–10 weeks of age) received psychological stress twice daily for five consecutive days [18]. For induction of psychological stress, animals were placed in the chambers covered by a plexiglas plate at floors without receiving footshock and therefore, they were exposed to various emotional stimuli (jumping, struggling, vocalizing, defecating and urinating) arising from animals into the other five chambers receiving footshock which was applied only to induce psychological stress. During psychological stress exposure, the rats at a young adult age showed an increased rate of grooming, urination, and defecation. To omit the impact of a novel environment, the rats of all groups were placed in the communication box (2 times/day) for five consecutive days before the experiment commenced.
After removal from the communication box, the young adult rats were kept alone in a cage for 15 min before being returned to the animal facilities. Stress at both two levels of age was induced between 10:00–12:00 and 13:00–15:00, but the N STR group was kept in the communication box for the same period without receiving footshock or psychological stress.
Experimental design
The day before exposure to stress, glucose, insulin, homeostatic model assessment of insulin resistance (HOMA-IR), and homeostasis model assessment of β-cell dysfunction (HOMA-B) were measured (basal before). Corticosterone was determined immediately after second session exposure to stress (after 3 p.m.) on days 1 and 5. Moreover, 1 day after the termination of stress, TNF-alpha in the plasma was determined and the plasma glucose, insulin, HOMA-IR, and HOMA-B were measured after stress (basal after). Finally, after performing the intraperitoneal glucose tolerance test (IPGTT), the anesthetized young adult rats were decapitated and dissected to remove the pancreas tissue for measuring insulin secretion from isolated islets and detecting the GLUT-2 level (Fig. 1).
A timeline demonstrating the induction of early footshock stress during days after birth and psychological stress during young adulthood. The day before psychological stress glucose, insulin, HOMA-IR, and HOMA-B were measured. Corticosterone was measured on days 1 and 5 immediately after second-session exposure stress and one day after that. One day after last stress exposure glucose, insulin, HOMA-IR, HOMA-B, TNF-alpha, corticosterone, amount of GLUT2, insulin secretion, and glucose tolerance were measured
Blood sampling
After overnight fasting, drawing blood was done (at 8–8:30 a.m.) after pentobarbital (Sigma, USA) anesthesia 60 mg/kg; ip [19] by technic of tail cut. The blood sampling method was the same at all times. The plasma obtained by centrifuging (664×g) was separated and kept at − 70 °C to determine the blood parameters.
The plasma samples were analyzed for the insulin, corticosterone and TNF-alpha concentrations by the rat insulin Elisa kit (Mercodia, Sweden), corticosterone Elisa kit (DRG, Germany) and TNF-alpha Kit (Biolegend, CA, USA). The plasma glucose concentrations were determined using the glucose oxidase method (Pars Azmoon, Iran).
The IPGTT profile
For the assessment of glucose tolerance, the IPGTT was carried out. One day after the last exposure to the stress (day 6 immediately after blood drawing to measure the parameters of basal after) anesthetized young adult rats were injected with glucose intraperitoneally (20% solution in water, 2 g/kg BW) to detect the glucose and insulin at 10, 15, 30, 60, and 90 min after glucose injection [20].
The HOMA-IR index
To determine the HOMA-IR index, the values of the fasting plasma glucose and insulin levels were used; the formula is: HOMA-IR = (ci × cg)/22.5, where ci is fasting insulin level (μU/ml) and cg is fasting glucose level (mmol/L) [21].
The HOMA-B index
To calculate the HOMA-B as an index of the β-cell function, the values of fasting plasma glucose and insulin levels were used.
The formula is: HOMA-B = (20 × fasting insulin)/(fasting glucose –3.5) [22].
The islet isolation
The islet isolation was done using the collagenase technique suggested by Lacy and Kostianovsky [23] with slight modification. The entrance of common bile duct to duodenum was clamped, the duct was cannulated with a polyethylene catheter (Portex Intravenous Cannula 2.5 F, 0.75-mm OD) and 10-ml cold Hank’s buffer, in which collagenase P (Roche, Cat. # 11 213 865 001, Germany, 0.45 mg/ml) was diluted and then, it was gently injected into the duct. The inflated pancreas was removed and placed into a Petri dish and cleaned from non-pancreatic tissue. Then, the pancreas was placed into a 50-ml falcon tube and incubated in a 37 °C water bath for 17 min. Digestion was terminated by adding cold Hank’s solution up to 40 ml. The tube was shaken for 1 min and the suspension was dispensed into a glass container (7.5-cm diameter and 4.5-cm height). Cold Hank’s solution was added and aspirated after precipitation. The supernatant was removed, a process that was repeated three times. After the last aspiration, the islets were handpicked (Blue Light stereomicroscope, USA) (first-picking).
Glucose-stimulated insulin secretion
Glucose-stimulated insulin secretion was examined at different glucose concentrations (5.6 and 16.7 mM). From the isolated islets of each young adult rat, five groups of ten islets for each glucose concentrations were picked randomly (second-picking) and placed in the plastic cups (a total of 20 cups for each condition). All procedures for islets separation were performed on the ice tray. After removing the excess hank’s solution, 1 ml of Krebs Ringer Solution (pH 7.4) [19] containing 5.6- or 16.7-mM glucose was added to the cups and incubated for 90 min (at the beginning the cups were gassed with 95% O2/5% CO2 for 5 min) at 37 °C. Then, the supernatant part was removed and stored at − 70 °C for insulin assays.
Western blotting
The whole extract of rat’s pancreas tissue was lysed and the protein concentration was determined similar to previous study [24]. The proteins were electrophoresed in 12% SDS-PAGE gels, transferred to polyvinylidene fluoride (PVDF) membranes, and probed with rabbit polyclonal to GLUT-2 (ab104622, Abcam) at 1:1000 dilution, in TBST-Tween buffer overnight at 4 °C. After washing, membranes were incubated for 90 min at room temperature with secondary antibody (Anti-rabbit IgG, Cell signaling). Blots were revealed by ECL advanced kit (Amersham Biosciences, USA). Quantification of the obtained data was carried out by a densitometry scan of films. Data analysis was done by Image J software.
Statistical analysis
All data are expressed as mean ± SEM. Analysis of variance (ANOVA) with repeated measures was performed by GraphPad Prism Version 5 program package (by considering time as a repeated factor and stress as an independent factor). Moreover, one-way and two-way ANOVA were performed and followed by post hoc multiple comparison tests with considering stress, time and/or glucose and insulin concentrations as a factor(s). A P value below 0.05 was considered to be statistically significant.
Results
The effect of interaction between early postnatal and young adulthood psychological stress on plasma corticosterone concentration
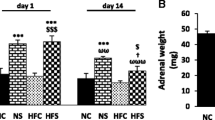
The two-way repeated-measures ANOVA analysis showed that adult psychological stress alone did not produce considerable changes in plasma corticosterone on day 1 or 5 of stress, while in combination with early life footshock exposure non-significantly increased the level of corticosterone on days 1 and 5 (Fig. 2). However, a downward trend in corticosterone levels was observed on days 1 and 5 that indicate an adaptation response to repeated stress during 5 consecutive days (Fig. 2).
The effect of early life stress and/or young adulthood chronic psychological stress on plasma corticosterone. Each column represents the mean ± SEM of 6 young adult male rats. Day 1, the first day of exposure to psychological stress. Day 5, the last day of exposure to psychological stress. Day 6, one day after stress duration. N STR none stress, E STR early life footshock stress, Psy STR young adulthood psychological stress, E + Psy STR early life + young adulthood psychological stress
The effect of interaction between early postnatal and young adulthood psychological stress on basal plasma glucose, insulin and TNF-alpha concentrations
The two-way repeated-measures ANOVA indicated that the day before the beginning of the stress procedure, basal plasma glucose levels did not show any significant difference between the present groups. While, after placing the animals of the Psy STR group in the communication box after exposure to psychological stress, basal plasma concentrations of glucose enhanced significantly in comparison with before stress (P = 0.001) at the same group; while, this impact was not observed in the E + Psy group (Fig. 3a).
The effect of early life stress and/or young adulthood chronic psychological stress on basal plasma glucose (a) and insulin (b) before and after exposure to psychological stress and on TNF-alpha (c). Each column represents the mean ± SEM of 8 young adult male rats. N STR none stress, E STR early life footshock stress, Psy STR young adulthood psychological stress, E + Psy STR early life + young adulthood psychological stress. *P = 0.03 versus respective basal before (a), *P = 0.001 versus respective basal before (b). *P = 0.03, **P = 0.009 versus N STR group (c)
Performing the two-way repeated-measures ANOVA the day before and after placing the young adult rats of all groups in the communication box with or without exposure to stress, showed no significant difference in basal insulin concentrations of plasma among study groups. On the other hand, after exposure to psychological stress in young adult rats which received stress in early life (the E + Psy STR group), basal plasma insulin levels enhanced significantly as compared with before exposing to stress at the same group (P = 0.03) but not with the other groups (Fig. 3b).
Performing the one-way ANOVA has demonstrated that exposure to early stress alone significantly increased TNF-alpha in plasma compared to N STR (P = 0.009); while, psychological stress in adult life alone did not show any difference. But psychological stress in young adult rats exposed to early stress could increase TNF-alpha in plasma in comparison with N STR (P = 0.02) (Fig. 3c).
The effect of interaction between early postnatal and young adulthood psychological stress on HOMA-IR and HOMA-B index
The two-way repeated-measures ANOVA indicated that after exposure to psychological stress in the Psy STR and the E + Psy STR groups, there was an increase in the level of HOMA-IR index in comparison with the same group before stress exposure (P = 0.05), (Table 1). But there was no difference between groups before or after stress exposure.
Also, after exposure to psychological stress in the E + Psy STR group, the HOMA-B index was increased just compared to the same group before stress (P = 0.02). However, there were no significant differences between groups before or after exposure to stress (Table 1).
The effect of interaction between early postnatal and young adulthood psychological stress on IPGTT
The two-way repeated-measures ANOVA showed that the levels of plasma glucose in the E STR group peaked at about 203.75 mg/dl at 15 min and 60 min were significantly higher than the N STR group (P = 0.0002). We expected that amount of glucose at 90 min to approach to the baseline level, while that was significantly greater than the N STR group (P = 0.009). Plasma glucose levels in the E + Psy STR group peaked at approximately 228.75 mg/dl at 15 min and remained slightly higher than the baseline level, but there was a significant difference between this group and the E STR group at 10 min (P = 0.01) (Fig. 4a).
The effect of early life stress and/or young adulthood chronic psychological stress on plasma glucose (a linear curve) and insulin (b linear curve) levels during IPGTT performance. Each point represents the mean ± SEM of 8 young adult male rats. N STR none stress, E STR early life footshock stress, Psy STR young adulthood psychological stress, E + Psy STR early life + young adulthood psychological stress. ***P = 0.0002, **P = 0.009 versus N STR group, $P = 0.01 versus E STR group
The two-way repeated-measures ANOVA was conducted to determine insulin changes during glucose tolerance. The peak of plasma insulin levels in the E + Psy STR group was approximately 3.93 µg/l at 15 min and remained slightly higher than the baseline level. The plasma insulin levels in this group were significantly higher than those of the N STR group at 15 min (P = 0.01) and E STR group at 60 min (P = 0.04) (Fig. 4b).
The effect of interaction between early postnatal and young adulthood psychological stress on insulin secretion from pancreatic isolated islets and pancreatic GLUT-2 levels
The two way ANOVA analysis revealed that insulin secreted from isolated islets of all groups in the presence of 5.6 mM glucose concentration was markedly lower than 16.7-mM glucose (P < 0.0001) (was not demonstrated in Fig. 5a). The insulin secretion from isolated islets showed a significant increase in response to 5.6-mM glucose concentration in the E + Psy STR group as compared to the E STR group (P = 0.02) (Fig. 5a). However, in response to 16.7-mM glucose concentration, the islets’ insulin output significantly enhanced in the E-STR group compared to the N STR group (P = 0.005) (Fig. 5a). Moreover, the combined early life stress and psychological young adulthood stress in the E + Psy STR group significantly increased the insulin release at the presence of 16.7-mM glucose as compared to the N STR group (P = 0.04) (Fig. 5a). However, insulin secretion in the response to 16.7-mM glucose level did not differ significantly in the Psy STR group in comparison with the N STR group (Fig. 5a).
The effect of early life stress and/or young adulthood chronic psychological stress on the isolated islets’ insulin secretion in response to basal (5.6-mM) and high (16.7-mM) glucose concentrations (a) and pancreatic Glut2 protein levels following western blot of pancreas tissue (b). Each column represents the mean ± SEM of 8 young adult male rats. N STR none stress, E STR early life footshock stress, Psy STR young adulthood psychological stress, E + Psy STR early life + young adulthood psychological stress. *P = 0.04, **P = 0.005 versus N STR group, $P = 0.01 versus E STR group (a). **P = 0.002, ***P = 0.0001 versus N STR group, $P = 0.01 versus E STR group, фффP = 0.0001 versus Psy STR group (b)
One-way ANOVA showed that the GLUT-2 protein level was decreased in the E STR group significantly compared to the N STR group (P < 0.0001) (Fig. 5b). Whereas, exposure to psychological stress in the Psy STR group significantly increased the level of the GLUT-2 protein in the pancreas as compared to the N STR group (P = 0.002) (Fig. 5b). Interestingly, early life stress in combination with young adulthood psychological stress in the E + Psy STR group, considerably decreased the amount of GLUT-2 as compared to the Psy STR (P < 0.0001) and N STR groups (P = 0.005), whereas significantly increased GLUT-2 levels in comparison with the E STR group (P < 0.01) (Fig. 5b).
Discussion
The present study results indicated that early stress in rats that exposed to psychological stress during young adulthood did not affect plasma corticosteron but it could increase plasma insulin and HOMA-IR, HOMA-B, TNF-alpha, impair the glucose tolerance, increase the in vivo glucose-stimulated insulin secretion and also could increase the in vitro insulin secretion from pancreatic isolated islets in response to 16.7-mM glucose but decrease pancreatic GLUT-2 levels. On the other hand, early stress alone increased the plasma TNF-alpha and the in vitro insulin secretion but decreased the GLUT-2 levels. Psychological stress during young adulthood alone increased the plasma glucose, HOMA-IR and GLUT-2 levels.
In this study, plasma corticosterone levels among groups showed no considerable changes immediately after second exposure to stress on days 1 and 5, although a downward trend was shown during the protocol of stress. In opposition to our study, maternal separation during 2 weeks of postnatal days (3 h/day) increased the corticosterone levels in exposure to acute psychological stress in adult animals [25, 26], while decreased it in response to chronic psychological stress [27]. In agreement with our results, Eiland et al. [28] and Fascolo et al. [29] found that postnatal stress did not change the serum corticosterone after induction of chronic psychological stress (restricted movement) in adult rats. It seems that mild or short-time early postnatal stress produces a persistent reduction in the HPA axis activity in response to stress later in life, while intense or long-term early postnatal stress persistently increases the HPA axis activity in a negative feedback circuit [25].
Perhaps, the first reason for unchanged corticosterone between the groups on the first day of stress (acute stress response) is due to the effect of injection of anesthetic compound on response to stress. The extra stress is produced by injection itself and this ceiling effect might be the reason why we did not see differences between the groups in plasma corticosterone levels [31]. The second possible reason for unchanged corticosterone on the first day of stress is an earlier adaptation of neuronal pathways is related to repeated psychological stress because we measured the level of corticosterone after the second exposure to psychological stress on the first day (stress was induced 2 times/day). De Boer et al. showed that the HPA axis response to second and third exposures to psychological stress was lower than the first exposure [32]. The development of a visible downward trend (on days 1 and 5) of corticosterone during days of stress indicates a habituation response to repeated psychological stress in adult rats that were exposed to early stress.
In this study, psychological stress alone enhanced basal plasma glucose but not insulin. On the other hand, early stress alone produced an increase in basal plasma glucose but not insulin. While the combination of these two stresses in the E + Psy STR group showed that basal plasma level of insulin was increased but the basal level of glucose remained at a normal level. In the previous studies, the interaction between early postnatal and adulthood stresses produced different changes in plasma concentrations of glucose and insulin [29, 32]. It seems that early postnatal stress in response to young adulthood psychological stress-induced insulin resistance; so that a more insulin release maintained the plasma glucose within the normal range. An increase in HOMA-IR as indicator of insulin resistance [21] and impaired glucose tolerance in the E + Psy STR group confirmed inducing insulin resistance. This impairment is probably in association with the defect of the glucose-stimulated insulin secretion. Our findings in this group revealed a significant increase in plasma glucose at 10 min after the glucose load. Also in this group, insulin secretion at 15 and 60 min after glucose load was higher than the other groups. It has been shown that stress may develop glucose intolerance [33, 34], which is associated with the HPA axis and/or a sympathoadrenal system activity [34]. It may be discussed that in the presence of both early life stress and young adulthood psychological stress, maintenance of plasma glucose within a normal range is due to increased insulin secretion in response to insulin resistance. There are several mechanisms involved in the development of insulin resistance that can be affected by stress [35] and impair insulin secretion in response to glucose [36]. Our results indicated that TNF-alpha enhanced following both early stress alone and also in combination with young adulthood psychological stress. It is well known that chronic stress exerts pro-inflammatory and/or anti-inflammatory effects, depending on the type or duration of stress [37, 38]. Inflammatory response of peripheral tissues to stress increases TNF-alpha as a pro-inflammatory cytokine and then it can impair insulin signaling and in turn, induce insulin resistance [39]. Impaired insulin signaling by TNF-alpha in muscles and adipose tissues is because of the impact of TNF-alpha on adipose tissue with the release of free fatty acids into the blood [40]. On the other hand, negative experiences in early life have reprogramming influences [41, 42] and epigenetic alterations on the function of immune systems and inflammation profiles [9, 43]. According to our results, Veenama et al. demonstrated that maternal isolation at 2 weeks after birth in exposure to psychological stress (subordinate colony housing) increased the plasma level of TNF-alpha [44]. In contrast to our findings, restriction of nesting material, as a kind of early life stress in the interaction of painless sound stress in adult Sprague–Dawley rats, showed no changes in TNF-alpha of plasma [45]. These variations in the results of various studies may be due to different designs of studies. Therefore, early life stress both alone and in combination with psychological stress in adult rats by increasing TNF-alpha could develop insulin resistance that in turn was resulted in a more glucose-stimulated insulin release in both in vivo (following IPGTT) and in vitro (insulin output from isolated islets).
In the present study, insulin output from isolated islets of endocrine pancreas following combination of early postnatal stress with psychological stress at adulthood in presence of both basal (5.6 mM) and high glucose (16.7 mM) concentrations was significantly high. Furthermore, HOMA-B as an index of β-call function, in the E + Psy STR group, was greater. Researchers of this study suggest that the reason for this increase in insulin secretion (secretory capacity) and β-call function may be a compensatory mechanism to overcome insulin resistance [6, 46] as was observed by increased HOMA-IR, glucose intolerance and high levels of basal plasma insulin.
The results of GLUT-2 measurement following western blotting exhibited that the mentioned transporter was increased by psychological stress alone; it was decreased by early life stress alone and finally ameliorated by the interaction of these two levels of stress. This effect partly corresponds to the profile of insulin secretion from Langerhans islets in response to the concentration of 5.6-mM glucose. Also, it appears that the increase in insulin output from the islets in response to 16.7-mM glucose in the E + Psy STR group is in the opposite direction of GLUT-2 levels changes. Therefore, the results of the present study and few several reports have confirmed that the levels of GLUT-2 protein and insulin secretion from islets of the pancreas may do not always change in the same direction [47,48,49]. Considering the available evidence at this research, it can be suggested that the increment of insulin secretion may be independent of GLUT-2 protein levels. It is possible that other mechanisms are involved in the biosynthesis of insulin content and insulin secretion inducing by early postnatal stress in the endocrine function of the pancreas [50, 51].
In summary, our results suggest that the combination of early footshock exposure and psychological stress during adulthood did not affect plasma corticosterone, but increased plasma insulin, HOMA-IR, HOMA-B and TNF-alpha levels. Plasma TNF-alpha was not only increased by the combination of both stressors, but also after only E STR exposure. HOMA-IR was increased in both Psy STR and E + Psy-STR groups. The combination of these two life stressors further increased the in vitro insulin secretion from isolated islets in response to 16.7-mM glucose. The level of Glut2 was increased in Psy STR and decreased in both E STR and E + Psy STR groups. Finally, glucose tolerance was impaired and glucose-stimulated insulin secretion was increased in E + Psy STR group.
It can be concluded that stress in early life can make the organism more susceptible to stressors to develop metabolic defects such as dysregulation in glucose homeostasis later in life. Further understanding the mechanisms of these changes needs more study in the future.
References
Friedman EM, Karlamangla AS, Gruenewald T, Koretz B, Seeman TE (2015) Early life adversity and adult biological risk profiles. Psychosom Med 77(2):176
Ilchmann-Diounou H, Olier M, Lencina C, Riba A, Barretto S, Nankap M, Sommer C, Guillou H, Ellero-Simatos S, Guzylack-Piriou L (2019) Early life stress induces type 2 diabetes-like features in ageing mice. Brain Behav Immun 80:452–463
Ruiz R, Roque A, Pineda E, Licona-Limón P, Valdéz-Alarcón JJ, Lajud N (2018) Early life stress accelerates age-induced effects on neurogenesis, depression, and metabolic risk. Psychoneuroendocrinology 96:203–211
Vargas J, Junco M, Gomez C, Lajud N (2016) Early life stress increases metabolic risk, HPA axis reactivity, and depressive-like behavior when combined with postweaning social isolation in rats. PLoS ONE 11(9):e0162665
Santarelli S, Zimmermann C, Kalideris G, Lesuis SL, Arloth J, Uribe A, Dournes C, Balsevich G, Hartmann J, Masana M (2017) An adverse early life environment can enhance stress resilience in adulthood. Psychoneuroendocrinology 78:213–221
Petersen MC, Shulman GI (2018) Mechanisms of insulin action and insulin resistance. Physiol Rev 98(4):2133–2223
Nolan CJ, Prentki M (2019) Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: tTime for a conceptual framework shift. Diabetes Vasc Dis Res 16(2):118–127
Whirledge S, DeFranco DB (2017) Glucocorticoid signaling in health and disease: insights from tissue-specific GR knockout mice. Endocrinology 159(1):46–64
Pedersen JM, Mortensen EL, Christensen DS, Rozing M, Brunsgaard H, Meincke RH, Petersen GL, Lund R (2018) Prenatal and early postnatal stress and later life inflammation. Psychoneuroendocrinology 88:158–166
Pedersen JM, Lund R, Andersen I, Clark AJ, Prescott E, Rod NH (2016) Psychosocial risk factors for the metabolic syndrome: a prospective cohort study. Int J Cardiol 215:41–46
Levine M, Cole S, Weir D, Crimmins E (2015) Childhood and later life stressors and increased inflammatory gene expression at older ages. Soc Sci Med 130:16–22
Kim W-H, Lee JW, Suh YH, Hong SH, Choi JS, Lim JH, Song JH, Gao B, Jung MH (2005) Exposure to chronic high glucose induces β-cell apoptosis through decreased interaction of glucokinase with mitochondria: downregulation of glucokinase in pancreatic β-cells. Diabetes 54(9):2602–2611
Navarro-Tableros V, Fiordelisio T, Hernández-Cruz A, Hiriart M (2007) Physiological development of insulin secretion, calcium channels, and GLUT2 expression of pancreatic rat β-cells. Am J Physiol-Endocrinol Metab 292(4):E1018–E1029
Farr OM, Ko B-J, Joung KE, Zaichenko L, Usher N, Tsoukas M, Thakkar B, Davis CR, Crowell JA, Mantzoros CS (2015) Posttraumatic stress disorder, alone or additively with early life adversity, is associated with obesity and cardiometabolic risk. Nutr Metab Cardiovas Dis 25(5):479–488
Nasca C, Watson-Lin K, Bigio B, Robakis TK, Myoraku A, Wroolie TE, McEwen BS, Rasgon N (2019) Childhood trauma and insulin resistance in patients suffering from depressive disorders. Exp Neurol 315:15–20
Zardooz H, Zahediasl S, Rostamkhani F, Farrokhi B, Nasiraei S, Kazeminezhad B, Gholampour R (2012) Effects of acute and chronic psychological stress on isolated islets’ insulin release. EXCLI J 11:163
Matsumoto M, Higuchi K, Togashi H, Koseki H, Yamaguchi T, Kanno M, Yoshioka M (2005) Early postnatal stress alters the 5-HTergic modulation to emotional stress at postadolescent periods of rats. Hippocampus 15(6):775–781
Andersen ML, Bignotto M, Machado RB, Tufik S (2004) Different stress modalities result in distinct steroid hormone responses by male rats. Braz J Med Biol Res 37(6):791–797
Zardooz H, Asl SZ, Naseri MG (2006) Effect of chronic psychological stress on insulin release from rat isolated pancreatic islets. Life Sci 79(1):57–62
Oosterlinck W, Vanderper A, Flameng W, Herijgers P (2011) Glucose tolerance and left ventricular pressure-volume relationships in frequently used mouse strains. BioMed Res Int 2011:1–7
Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R (1985) Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419
Krzyzanowska K, Zemany L, Krugluger W, Schernthaner G, Mittermayer F, Schnack C, Rahman R, Brix J, Kahn B, Schernthaner G (2008) Serum concentrations of retinol-binding protein 4 in women with and without gestational diabetes. Diabetologia 51(7):1115–1122
Lacy PE, Kostianovsky M (1967) Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes 16(1):35–39
Vélez-Granell CS, Arias AE, Torres-Ruíz JA, Bendayan M (1994) Molecular chaperones in pancreatic tissue: the presence of cpn10, cpn60 and hsp70 in distinct compartments along the secretory pathway of the acinar cells. J Cell Sci 107(3):539–549
Ladd CO, Huot RL, Thrivikraman K, Nemeroff CB, Plotsky PM (2004) Long-term adaptations in glucocorticoid receptor and mineralocorticoid receptor mRNA and negative feedback on the hypothalamo-pituitary-adrenal axis following neonatal maternal separation. Biol Psychiat 55(4):367–375
Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM (2007) Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci 25(10):3091–3098
Ladd CO, Thrivikraman K, Huot RL, Plotsky PM (2005) Differential neuroendocrine responses to chronic variable stress in adult Long Evans rats exposed to handling-maternal separation as neonates. Psychoneuroendocrinology 30(6):520–533
Eiland L, McEwen BS (2012) Early life stress followed by subsequent adult chronic stress potentiates anxiety and blunts hippocampal structural remodeling. Hippocampus 22(1):82–91
Fóscolo DRC, Fóscolo RB, Marubayashi U, Reis AM, Coimbra CC (2008) Neonatal maternal separation affects endocrine and metabolic stress responses to ether exposure but not to restraint exposure in adult rats. Metab Brain Dis 23(4):375
Wu XY, Hu YT, Guo L, Lu J, Zhu QB, Yu E, Wu JL, Shi LG, Huang ML, Bao AM (2015) Effect of pentobarbital and isoflurane on acute stress response in rat. Physiol Behav 145(1):118–121
De Boer S, Slangen J, Van der Gugten J (1988) Adaptation of plasma catecholamine and corticosterone responses to short-term repeated noise stress in rats. Physiol Behav 44(2):273–280
McPherson RJ, Mascher-Denen M, Juul SE (2009) Postnatal stress produces hyperglycemia in adult rats exposed to hypoxia-ischemia. Pediatr Res 66(3):278
Teague CR, Dhabhar FS, Barton RH, Beckwith-Hall B, Powell J, Cobain M, Singer B, McEwen BS, Lindon JC, Nicholson JK (2007) Metabonomic studies on the physiological effects of acute and chronic psychological stress in sprague− dawley rats. J Proteome Res 6(6):2080–2093
Eguchi R, Scarmagnani FR, Cunha CA, Souza GI, Pisani LP, Ribeiro EB, do Nascimento CMO, Spadari-Bratfisch RC, Oyama LM (2011) Fish oil consumption prevents glucose intolerance and hypercorticosteronemy in footshock-stressed rats. Lipids Health Dis 10(1):7
Wilcox G (2005) Insulin and insulin resistance. Clin Biochemist Rev 26(2):19
Wang Y, Nishi M, Doi A, Shono T, Furukawa Y, Shimada T, Furuta H, Sasaki H, Nanjo K (2010) Ghrelin inhibits insulin secretion through the AMPK–UCP2 pathway in β cells. FEBS Lett 584(8):1503–1508
Liu Y-Z, Wang Y-X, Jiang C-L (2017) Inflammation: the common pathway of stress-related diseases. Front Hum Neurosci 11:316
Pruett SB (2003) Stress and the immune system. Pathophysiology 9(3):133–153
Li J, Bai L, Wei F, Zhao J, Wang D, Xiao Y, Yan W, Wei J (2019) Therapeutic mechanisms of herbal medicines against insulin resistance: a review. Front pharmacol 10:661
Pittas AG, Joseph NA, Greenberg AS (2004) Adipocytokines and insulin resistance. J Clin Endocrinol Metab 89(2):447–452
Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB (2012) Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci 109(16):5995–5999
Hohmann CF, Odebode G, Naidu L, Koban M (2017) Early life stress alters adult inflammatory responses in a mouse model for depression. Ann Psychiatry Mental Health 5(2):1–12
Fagundes CP, Way B (2014) Early-life stress and adult inflammation. Curr Dir Psychol Sci 23(4):277–283
Veenema AH, Reber SO, Selch S, Obermeier F, Neumann ID (2008) Early life stress enhances the vulnerability to chronic psychosocial stress and experimental colitis in adult mice. Endocrinology 149(6):2727–2736
Alvarez P, Green PG, Levine JD (2013) Stress in the adult rat exacerbates muscle pain induced by early-life stress. Biol Psychiat 74(9):688–695
Courty E, Besseiche A, Do TTH, Liboz A, Aguid FM, Quilichini E, Buscato M, Gourdy P, Gautier J-F, Riveline J-P (2019) Adaptive β-cell neogenesis in the adult mouse in response to glucocorticoid-induced insulin resistance. Diabetes 68(1):95–108
Guillam M-T, Hümmler E, Schaerer E, Wu J-Y, Birnbaum MJ, Beermann F, Schmidt A, Dériaz N, Thorens B (1997) Early diabetes and abnormal postnatal pancreatic islet development in mice lacking Glut-2. Nat Genet 17(3):327
Tal M, Wu YJ, Leiser M, Surana M, Lodish H, Fleischer N, Weir G, Efrat S (1992) [Val12] HRAS downregulates GLUT2 in beta cells of transgenic mice without affecting glucose homeostasis. Proc Natl Acad Sci 89(13):5744–5748
De Vos A, Heimberg H, Quartier E, Huypens P, Bouwens L, Pipeleers D, Schuit F (1995) Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. J Clin Invest 96(5):2489–2495
Gluckman PD, Hanson MA, Cooper C, Thornburg KL (2008) Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359(1):61–73
Dhar A, Dhar I, Jiang B, Desai KM, Wu L (2011) Chronic methylglyoxal infusion by minipump causes pancreatic β-cell dysfunction and induces type 2 diabetes in Sprague-Dawley rats. Diabetes 60(3):899–908
Acknowledgements
We thank Dr. Hossein Khaleghzadeh-Ahangar, the Assistant Professor at Babol University of Medical Sciences for his aid in English editing. This work was supported by Diabetes Research Center (Grant 3069).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical standards
All procedures were approved by the Animal Care and Use Committee of the Diabetes Research Center of Research Deputy of Mazandaran University of Medical Sciences, Sari, Iran.
Informed consent
For this type of study formal consent in not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zardooz, H., Sadeghimahalli, F. & Khodagholi, F. Early postnatal stress impairs insulin secretion in response to psychological stress in adult rats. J Endocrinol Invest 44, 277–286 (2021). https://doi.org/10.1007/s40618-020-01291-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-020-01291-9