Abstract
Purpose
To explore the effects and mechanisms of anti-tumor necrosis factor-α (TNF-α) antibody on insulin resistance (IR) in rats with sepsis-induced stress hyperglycemia.
Methods
The sepsis-induced stress hyperglycemic rat model was constructed by cecal ligation and puncture combined with the intraperitoneal injection of lipopolysaccharide. The rats were randomly divided into six groups: normal control (NC) group, surgical rats (Cntl) group, high-dose anti-TNF-α antibody therapy (TNF, 6 mg/kg) group, low-dose anti-TNF-α antibody therapy (Tnf, 3 mg/kg) group, insulin therapy (INS) group, and INS + Tnf group. The blood glucose and serum insulin concentrations were detected, followed by analysis of intraperitoneal glucose tolerance test (IPGTT) and hyperinsulinemic–euglycemic clamp. Finally, the expression levels of phospho-Akt (p-Akt), Akt, p-mTOR, mTOR, nuclear factor-κB (NFκB), I kappa beta kinase (IKKβ), and suppressor of cytokine signaling (SOCS-3) were detected by western blotting.
Results
There was no significant difference in blood glucose concentrations among these groups, while the serum insulin concentration in TNF and Tnf groups was lower than that in the Cntl group at postoperative 6 h (P < 0.05). IPGTT analysis revealed that blood glucose level was lower in the TNF group than that in the Cntl group (P < 0.05). The glucose infusion rate in the Cntl group was lower than that in the Tnf and TNF groups (P < 0.05). The p-Akt/Akt, p-mTOR/mTOR ratio, and expression levels of NFκB, IKKβ and SOCS-3 were lower in the drug intervention than that in the Cntl group (P < 0.05).
Conclusions
Anti-TNF-α antibody could reduce IR by inhibiting AKt/mTOR signaling pathway and the expression levels of NFκB, IKKβ, and SOCS-3 in rats with sepsis-induced stress hyperglycemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stress hyperglycemia is associated with the elevation of blood glucose levels but not preexisting diabetes, and arises from various stresses such as trauma, surgery, burn injury, and sepsis. It is very common in patients with severe sepsis [1] and has deleterious effects on these patients [2, 3]. Acute insulin resistance (IR) is considered as one of the main causes of hyperglycemia [4]. A recent study has reported that intensive insulin therapy at normalization of blood glucose (4.4–6.1 mmol/L) is effective in reducing morbidity and mortality in critically ill patients [5]. However, other study has been reported that the use of intensive insulin therapy in patients with sepsis causes adverse events associated with hypoglycemia and its toxicity increases when the doses accumulated [6]. Therefore, an understanding of the molecular mechanisms of hyperglycemia and acute IR may provide new therapeutic approaches in treating the critically ill following injury.
Hyperglycemia is known to be implicated in many inflammatory pathways, such as nuclear factor-κB (NF-κB), Akt, mammalian target of rapamycin (mTOR), and I kappa beta kinase (IKKβ). Glucose intake can result in an increase of NFκB and a decrease in inhibiting IKKβ expression [7]. Besides, high glucose concentration may modulate insulin signaling by reducing Akt activity [8]. The mTOR pathway plays an important role in diabetic kidney disease [9]. IKKβ has been found as a target for insulin sensitization in type 2 diabetes mellitus [10]. Growing evidences also suggest that transcriptional factors regulating the expression of inflammatory cytokines such as c-Jun N-terminal kinase (JNK) and IKKβ could cause IR by activating tumor necrosis factor-α (TNF-α) [11, 12]. In cultured cells, TNF-α can induce IR by increasing serine phosphorylation of insulin receptor substrate-1 (IRS-1) and convert it to inhibit the activity of insulin receptor tyrosine kinase [13]. Under chronic hyperglycemia, TNF-α production is increased and its harmful effects on insulin sensitivity are found [14]. In addition, anti-inflammatory medications may have the opposite effect [10, 15], suggesting that inflammation cytokines may be involved in the development of IR.
TNF-α is produced by a variety of inflammatory cells and plays important roles in tumor development [16], inflammation [17], ischemia reperfusion [18], and critical illness [19]. The previous study has demonstrated that IR due to chronic inflammatory factors secreted by fat cells can be improved by anti-TNF-α antibodies [20]. Recent clinical trial has also revealed that anti-TNF-α antibodies can improve IR of patients with rheumatoid arthritis [21]. However, the specific underlying roles of anti-TNF-α antibody on IR and blood glucose in rats with sepsis-induced stress hyperglycemia are still poorly understood.
In this study, we established the models of sepsis-induced stress hyperglycemia in rats, and then, the model rats were treated with anti-TNF-α antibody or insulin. The blood glucose levels, serum insulin levels, serum lipopolysaccharide (LPS), C-reactive protein (CRP), inflammatory factors, and reactive oxygen species (ROS) production in liver tissue were detected, as well as IKKβ/NF-kB inflammatory pathway and Akt/mTOR insulin signaling pathway were evaluated, aiming to explore the mechanism of acute IR caused by the trauma and infection.
Materials and methods
Animal models
The healthy male Wister rats weighing 200–250 g were purchased from the Experimental Animal Center of Shandong University (Shandong, China). All the surgical procedures for animal use were approved by the University of Shandong Institutional Animal Care and Use Committee. The rats were maintained in a temperature- and light-controlled environment with free access to food and water. After 1 week of acclimatization, the sepsis-induced stress hyperglycemic rats model was constructed by cecal ligation and puncture (CLP) combined with the intraperitoneal injection of LPS. In brief, rats were fasted for 12 h prior to surgery. After ketamine anesthesia at room temperature, the abdominal cavity of the rats was opened. The terminal cecum was ligated using surgery line, and then, the intestinal wall was pierced by surgical needle. Subsequently, a little stool was extruded, and then, cecum was put into the abdominal cavity, followed by the suture of the abdominal wall. After the CLP surgery, the rats received the intraperitoneal injection of LPS (3 mg/kg) diluted with normal saline.
Study design
A total of 350 rats were randomly divided into six groups: normal control (NC) group (n = 54), surgical rats without antibody or insulin therapy (Cntl) group (n = 61), high-dose anti-TNF-α antibody therapy (TNF) group (n = 53), low-dose anti-TNF-α antibody therapy (Tnf) group (n = 59), insulin therapy (INS) group (n = 66), and insulin combined with low-dose anti-TNF-α antibody therapy (INS + Tnf) group (n = 57). After suturing the abdominal cavity, four drug treatment groups were treated as Table 1. Anti-TNF-α monoclonal antibody was purchased from infliximab (product name: Remicade, Janssen company, Xi’an, China).
Measurements of blood glucose concentration
Blood samples were obtained from the peripheral capillaries of tails at preoperative fasting, postoperative 1 h, every 2 h within 12 h, and 3–4 times daily until 7 days. Then, the blood glucose concentrations were measured by a blood glucose monitoring system of OneTouch® UltraVue™ (Johnson and Johnson, New Brunswick, NJ, USA). Hyperglycemia and hypoglycemia were defined as blood glucose >11.1 mmol/L and blood glucose <2.8 mmol/L, respectively.
Intraperitoneal glucose tolerance test (IPGTT)
The IPGTT was performed 24 h after the intraperitoneal injection of glucose (10 ml/kg, 10% w/v) in five rats of the NC, Cntl, TNF, and Tnf groups. The peripheral glucose levels from the peripheral capillaries of tails were recorded by the blood glucose monitoring system of OneTouch® UltraVue™ before intraperitoneal injection and 0.5, 1, 2, 3 h after injection. Each time point was measured two times in a row and taken the mean to reduce the error.
Hyperinsulinemic–euglycemic clamp analysis
Five rats in the NC, Cntl, TNF, and Tnf groups were anesthetized with ketamine (40 mg/kg i.p.). The analysis of euglycemic clamp was performed under non-stressful conditions with conscious rats after IPGTT. After the injection of 20% glucose, a hyperinsulinemic–euglycemic clamp was used for 50 min; meanwhile, insulin was infused at a rate of 20 mU/kg min. Plasma glucose concentration was maintained at basal concentrations by the infusion of 20% glucose at a variable rate. The glucose infusion rates (GIR) were recorded.
Enzyme-linked immune-sorbent assay (ELISA)
Serum insulin, TNF-α, IL-6, CRP, and LPS levels were measured by ELISA. At postoperative 0, 2, 6, 24, 72 h or 7 days, the abdominal cavity was opened, and then, the portal vein was exposed. Arterial blood from abdominal aortic was collected and then centrifuged at 2500 rpm for 10 min. Serum was removed and stored at −20 °C. Serum insulin, TNF-α, IL-6, CRP, and LPS concentrations were measured using the corresponding commercial kits (R&D Systems, Minneapolis, MN, USA; Luminex Corp., Austin, TX, USA; R&D Systems; Life Diagnostics, Inc., Westchester, PA, USA; R&D Systems) according to the manufacturer’s instructions.
ROS detection
At postoperative 0, 2, 6 h, 1, 3, or 7 days, the abdominal cavity was opened, and then, the livers were removed and frozen in liquid nitrogen. ROS content was detected using Reactive Oxygen Species Assay Kit (KeyGen BioTECH, Nanjing, China). Briefly, 1 g liver samples were collected and washed with cleaning liquid. After centrifuged at 300g for 5 min, ROS content was detected using fluorescence spectrophotometer according to the manufacturer’s instructions.
Western blotting
Liver tissues were homogenized at 4 °C in lysis buffer. Totally, 30 μg proteins per lane was separated by 7.5% SDS-PAGE and transferred to polyvinylidene fluoride membranes. Then, the membranes were immunoblotted with primary antibodies: anti-phospho (p)-mTOR (Invitrogen, Carlsbad, CA, USA), anti-mTOR (Invitrogen), and anti-p-S473-Akt (Cell Signaling Technology, Danvers, MA, USA), anti-Akt (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-NF-κB-P65 (Invitrogen), and anti-SOCS-3 (Cell Signaling Technology), and anti-IKKß (Santa Cruz Biotechnology) at 4 °C overnight, followed by the incubation of Horseradish peroxidase-conjugated secondary antibody (Pierce Chemical Co., Rockford, IL, USA). Related expression was quantified using LabWorks 4.5 software (BioImaging Systems, Upland, CA, USA) and calculated according to the β-actin reference bands.
Statistical analysis
Data were presented as mean ± standard deviation (SD) and analyzed by one-way ANOVA followed by Tukey–Kramer Multiple Comparisons Test or Fisher exact test. Kaplan–Meier curve of rats was analyzed and multivariate cox regression analysis was used to estimate the prognostic factors of survival. All tests were two-tailed and computed using SPSS v13.0 (SPSS Inc., Chicago, IL). P value <0.05 was considered statistically significant.
Results
Effects of anti-TNF-α antibody or insulin therapy on blood glucose level and survival in rats with sepsis-induced stress hyperglycemia
As shown in Fig. 1a, blood glucose levels raised in rats at 1 h after surgery, reached the peak at 2–4 h, and gradually reduced from 6 h. Glucose levels of rats were always higher than pre-operation. There was no significant difference of blood glucose concentrations at the same time among non-insulin-treated groups (Cntl, TNF, tnf) or insulin-treated groups (INS, INS + Tnf). The results showed that the incidences of hyperglycemia in the insulin-treated groups were significantly lower than other groups (P < 0.05, Table 2). The incidences of hypoglycemia and 7-day mortality in each group were also not significantly different (Table 2). In spite of no statistically significant, 7-day mortality in the TNF-α group was slightly lower than other groups (Table 2). Further Kaplan–Meier analysis revealed the highest cumulative survival probability rate in the TNF group and the lowest cumulative survival probability in the INS group, while no difference was found among these groups (P = 0.142, Fig. 1b). Multivariate cox regression analysis showed that both insulin-treatment and anti-TNF-α antibody treatment were not related to survival, while both hyperglycemia and hypoglycemia accidents were the prognostic factors of survival [hazard ratio (HR): 2.306, 95% confidence interval (CI): 5.085–19.796, P < 0.001, and HR: 2.594, 95% CI 7.116–25.161, P < 0.001, Table 3].
Anti-TNF-α antibody or insulin therapy had no influence on blood glucose level and survival in rats with sepsis-induced stress hyperglycemia. a Blood glucose levels in the Cntl, Tnf, TNF, INS, and Tnf + INS groups at postoperative 0, 2, 6, 8, 12 h, 1, 2, 3, 4, 5, 6, and 7 days; b cumulative survival probability in the Cntl, Tnf, TNF, INS, and Tnf + INS groups by Kaplan–Meier analysis. TNF-α Tumor necrosis factor-α, Cntl group surgical rats without antibody or insulin therapy group, TNF group high-dose anti-TNF-α antibody therapy group, Tnf group low-dose anti-TNF-α antibody therapy group, INS group insulin therapy group, INS + Tnf group insulin combined with low-dose anti-TNF-α antibody therapy group
Effect of anti-TNF-α antibody on glucose tolerance and IR in rats with sepsis-induced stress hyperglycemia
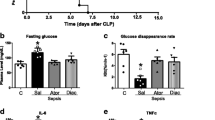
IPGTT analysis was performed to examine the effect of anti-TNF-α antibody on glucose control. As shown in Fig. 2a, there was a rise in blood glucose level, with the peak occurring at 60 min after glucose injection in each group. Compared with the NC group, blood glucose levels in rats of each surgery group were significantly increased (P < 0.01). In addition, compared with the Cntl group, blood glucose levels were obviously decreased in the TNF group at 60, 120, and 180 min after surgery (P < 0.05), while only slight reduction of blood glucose levels was found without statistical difference in the Tnf group (Fig. 2a). Moreover, ELISA results revealed that the serum insulin level showed a peak at postoperative 6 h in all these three groups (Fig. 2b). The serum insulin concentration in the TNF (3.02 ± 0.48 ng/mL) and Tnf groups (3.36 ± 0.64 ng/mL) was significantly lower than that in the Cntl group (4.53 ± 1.02 ng/mL, P < 0.05, Fig. 2b) at postoperative 6 h. The hyperinsulinemic–euglycemic clamp analysis revealed that the GIR level was higher in the NC group (26.80 ± 2.54 mg/kg min) than that in the Cntl (11.96 ± 2.85 mg/kg min), Tnf (15.94 ± 3.17 mg/kg min), and TNF (19.76 ± 3.48 mg/kg/min, P < 0.01, Fig. 2c) groups. Besides, the GIR level was significantly elevated in the Tnf and TNF groups compared with the Cntl group (P < 0.05, Fig. 2c).
Anti-TNF-α antibody could inhibit glucose tolerance and improve insulin resistance in rats with sepsis-induced stress hyperglycemia. a Blood glucose levels in the NC, Cntl, TNF, and Tnf groups using intraperitoneal glucose tolerance test; b serum insulin concentrations in the Cntl, TNF, and Tnf groups at postoperative 0, 2, 6 h, 1, 3, and 7 days; c glucose infusion rate (GIR) in the NC, Cntl, TNF, and Tnf groups by hyperinsulinemic–euglycemic clamp. *P < 0.05, **P < 0.01 vs. the Cntl group, △ P < 0.01 vs. the NC group. TNF-α Tumor necrosis factor-α, NC group normal control group, Cntl group surgical rats without antibody or insulin therapy group, TNF group high-dose anti-TNF-α antibody therapy group, Tnf group low-dose anti-TNF-α antibody therapy group
Effect of anti-TNF-α antibody or insulin therapy on the levels of LPS, TNF-α, IL-6, CRP, and ROS in rats with sepsis-induced stress hyperglycemia
The levels of serum LPS, TNF-α, and CRP elevated at postoperative 2 h and reached to a peak at postoperative 1 day, and then slightly decreased in the following time (Fig. 3a–c). Compared with the Cntl group, serum LPS level was significantly decreased in four drug treatment groups at postoperative 1 day (P < 0.05), in the TNF, INS, and Tnf + INS groups at postoperative 3 days (P < 0.05), and in the TNF and Tnf + INS groups at postoperative 7 days (P < 0.05), respectively (Fig. 3a). Serum TNF-α levels in the TNF and Tnf + INS groups at postoperative 2 h, in the TNF group at postoperative 6 h, and in four drug treatment groups at postoperative 1, 3, and 7 days, respectively, were significantly lower than those in the Cntl group (P < 0.05, Fig. 3b). Serum CRP levels in the TNF group at postoperative 6 h, in the TNF and Tnf + INS groups at postoperative 1 day, in four drug treatment groups at postoperative 3 days, and in the INS, TNF, and Tnf + INS groups at postoperative 7 days, respectively, were significantly lower than those in the Cntl group (P < 0.05, Fig. 3c). The levels of serum IL-6 elevated at postoperative 2 h and approved to a peak at postoperative 3 days, and then slightly decreased in the following time. Serum IL-6 levels in four drug treatment groups at postoperative 6 h, 3, and 7 days, in the TNF and Tnf + INS groups at postoperative 1 day, respectively, were significantly lower than those in the Cntl group (P < 0.05, Fig. 3d). ROS content in liver tissues elevated at postoperative 2 h and approved to a peak at postoperative 6 h, and then slightly decreased in the following time. Compared with the Cntl group, ROS content was reduced in the Tnf, TNF, and Tnf + INS groups at postoperative 6 h (P < 0.05, Fig. 3e).
Anti-TNF-α antibody or insulin therapy could inhibit the levels of LPS, TNF-α, CRP, IL-6, and ROS in rats with sepsis-induced stress hyperglycemia. The levels of LPS, TNF-α, CRP, IL-6, and ROS GIR in the Cntl, TNF, Tnf, INS, and Tnf + INS groups at postoperative 0, 2, 6 h, 1, 3, and 7 days using enzyme-linked immune-sorbent assay. *P < 0.05 vs. the Cntl group. LPS Lipopolysaccharide, TNF-α tumor necrosis factor-α, CRP C-reactive protein, IL-6 interleukin-6, ROS reactive oxygen species, Cntl group surgical rats without antibody or insulin therapy group, TNF group high-dose anti-TNF-α antibody therapy group, Tnf group low-dose anti-TNF-α antibody therapy group, INS group insulin therapy group, INS + Tnf group insulin combined with low-dose anti-TNF-α antibody therapy group
Effect of anti-TNF-α antibody or insulin therapy on AKt/mTOR and IKKβ/NF-κB signaling pathway in rats with sepsis-induced stress hyperglycemia
Western blotting analysis revealed that p-Akt/Akt ratio was significantly increased in the Cntl group compared to the NC group (P < 0.001), while obviously reduced in Tnf (P < 0.05), TNF (P < 0.01) and Tnf + INS (P < 0.05) groups compared with the Cntl group (Fig. 4a). Likewise, p-mTOR/mTOR ratio was remarkably higher in the Ctnl group than that in the NC group, while lower in the TNF (P < 0.05), Tnf + INS (P < 0.01), and INS (P < 0.05) groups than that in the Ctnl group (Fig. 4b). In addition, the expression levels of IKKβ, NF-κB, and SOCS-3 were obviously increased in the Cntl group compared to the NC group (P < 0.05). The expression levels of IKKβ and SOCS-3 were significantly reduced in the TNF group compared with the Cntl group, and NF-κB expression was remarkably lower in four drug treatment groups than that in the Cntl group (P < 0.05, Fig. 4c).
Anti-TNF-α antibody or insulin therapy could inhibit AKt/mTOR and IKKβ/NF-κB signaling pathways in rats with sepsis-induced stress hyperglycemia. a Expression of Akt, p-Akt, and analysis of p-Akt/Akt ratio in the NC, Cntl, TNF, Tnf, INS, and Tnf + INS groups using western blotting; b expression of mTOR, p-mTOR, and analysis of p-mTOR/mTOR ratio in the NC, Cntl, TNF, Tnf, INS, and Tnf + INS groups using western blotting; c expression of NF-κB, IKKβ, and SOCS-3 in the NC, Cntl, TNF, Tnf, INS, and Tnf + INS groups using western blotting; *P < 0.05, **P < 0.01, ***P < 0.001 vs. the Cntl group. TNF-α Tumor necrosis factor-α, p-Akt phospho-Akt, Akt serine/threonine kinase, mTOR mammalian target of rapamycin, NF-κB nuclear factor-κB, IKKβ I kappa beta kinase, SOCS-3 suppressor of cytokine signaling, NC group normal control group, Cntl group surgical rats without antibody or insulin therapy group, TNF group high-dose anti-TNF-α antibody therapy group, Tnf group low-dose anti-TNF-α antibody therapy group, INS group insulin therapy group, INS + Tnf group insulin combined with low-dose anti-TNF-α antibody therapy group
Discussion
As a systemic inflammatory responding to severe infections, sepsis could cause high morbidity and mortality rates [22]. The present study revealed that anti-TNF-α antibody or insulin therapy had no influence on blood glucose level and survival in rats with sepsis-induced stress hyperglycemia, while hypoglycemia or hyperglycemia accident was the prognostic factors of survival. Anti-TNF-α antibody could inhibit glucose tolerance and improve IR. In addition, anti-TNF-α antibody or insulin therapy inhibited the levels of LPS, TNF-α, CRP, IL-6, and ROS; meanwhile, AKt/mTOR and IKKβ/NF-κB signaling pathways were inhibited by anti-TNF-α antibody or insulin therapy.
Stress hyperglycemia had been known to be involved in the molecular mechanism of stress hormones, and pro-inflammatory cytokines could cause hyperglycemia through various kinds of mechanisms [2, 23]. For example, TNF-α had been found to induce IR by increasing the secretion of stress hormones [24]. Study had shown that the elevated blood TNF-α concentration could reduce glucose uptake in skeletal muscle in a rat model of zymosan-induced inflammatory response [25]. In this study, we found that after treatment with intraperitoneal injection anti-TNF-α antibody of either 6 or 3 mg/kg, the level of serum insulin was significantly lower than that in the Cntl group at postoperative 6 h. A previous study had reported that insulin sensitivity had not changed when given an infusion of 5 mg/kg body weight of recombinant-engineered human TNF-α neutralizing antibody [26]. Nevertheless, other report identified that when anti-TNF-α antibodies were infused into patients with rheumatoid arthritis, a secondary benefit of enhanced insulin sensitivity was observed [27]. Similarly, our study showed an improved IR after the treatment of anti-TNF-α antibody. These findings suggested the inhibiting effect of anti-TNF-α antibody on IR in rats with sepsis-induced stress hyperglycemia.
The previous study had suggested that IR could be induced by mTOR pathway [28]. Akt, as the upstream factor of mTOR signaling pathways, was proved to be associated with insulin signaling [8]. This study revealed that both anti-TNF-α antibody and insulin significantly downregulated p-Akt and p-mTOR, indicating that the inhibiting effect of anti-TNF-α antibody on IR was associated with AKt/mTOR signaling pathway. Inflammation was thought to be an important driver of obesity-induced IR [29]. It had been reported that TNF-α, IL-6, CRP, LPS, and ROS were involved in the pro-inflammatory response and oxidative stress [30]. Our study found that anti-TNF-α antibody inhibited the levels of TNF-α, IL-6, CRP, LPS, and ROS, suggesting the association of the inhibition of inflammation and improved IR. Another finding of the present study was that anti-TNF-α antibody reduced the expression of NF-κB, IKKβ, and SOCS-3 in rats with sepsis-induced stress hyperglycemia. Consistently, inhibiting IKKβ/NF-κB pathway could improve IR [31]. Hyperglycemia-induced oxidative stress has the ability to activate stress-sensitive signaling pathways including NF-κB [32]. Studies have found that TNF-α could activate the IKKβ pathways [10]. Conversely, NF-κB regulated TNF-α by activating IKKβ, which indicated that TNF-α was associated with IKKβ to induce NF-κB activation in type 2 diabetes [33]. SOCS-3 belonged to insulin signaling system and functions as an insulin-induced negative regulator [34]. In the liver, TNF-α might induce the expression of SOCS-3 [35]. TNF-α-induced SOCS-3 was upregulated in the adipose tissue of obese mice and could inhibit insulin signaling [36]. These results indicated that anti-TNF-α antibody could reduce IR by inhibiting the activation of IKK-β, NF-κB, and SOCS-3 in sepsis-induced stress hyperglycemia.
However, there are still many limitations in the current study. First, although we found the relationship between anti-TNF-α antibody and sepsis-induced stress hyperglycemia, it is difficult to elucidate the interaction between them and more studies are still needed to verify these mechanisms. Second, the activation assays of NF-κB, IKKβ, and SOCS3 were not performed. Third, the hyperinsulinemic–euglycemic clamp is commonly used to distinguish between hepatic and peripheral IR. However, in this study, we only analyzed the peripheral IR using the hyperinsulinemic–euglycemic clamp. Thus, to compare the results between hepatic and peripheral IR, more investigations are required. Last but not the least, the observed difference of anti-TNF-α antibody dose in analysis of IPGTT and hyperinsulinemic–euglycemic clamp does not reveal the specific effectiveness of dose in IR. Additional studies based on different dose of anti-TNF-α antibody may be needed to further elucidate the relationship between anti-TNF-α antibody and IR in sepsis-induced stress hyperglycemia.
Conclusion
This study indicated that anti-TNF-α antibody and insulin could reduce IR by inhibiting AKt/mTOR signaling pathway as well as the expression levels of NF-κB, IKKβ, and SOCS-3 in rats with sepsis-induced stress hyperglycemia.
References
Leonidou L, Michalaki M, Leonardou A, Polyzogopoulou E, Fouka K, Gerolymos M, Leonardos P, Psirogiannis A, Kyriazopoulou V, Gogos CA (2008) Stress-induced hyperglycemia in patients with severe sepsis: a compromising factor for survival. Am J Med Sci 336(6):467–471
Marik PE, Raghavan M (2004) Stress-hyperglycemia, insulin and immunomodulation in sepsis. Intensive Care Med 30(5):748–756
Van Cromphaut SJ, Vanhorebeek I, Van den Berghe G (2008) Glucose metabolism and insulin resistance in sepsis. Curr Pharm Des 14(19):1887–1899
Li L, Messina JL (2009) Acute insulin resistance following injury. Trends Endocrinol Metab Tem 20(9):429–435
Van den Berghe G, Wouters PJ, Bouillon R, Weekers F, Verwaest C, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P (2003) Outcome benefit of intensive insulin therapy in the critically ill: insulin dose versus glycemic control. Crit Care Med 31(2):359–366
Lambert K, Ward J (2009) The use of thalidomide in the management of bleeding from a gastric cancer. Palliat Med 23(5):473–475
Dhindsa S, Tripathy D, Mohanty P, Ghanim H, Syed T, Aljada A, Dandona P (2004) Differential effects of glucose and alcohol on reactive oxygen species generation and intranuclear nuclear factor-κB in mononuclear cells. Metabolism 53(3):330–334
Hernandez R, Teruel T, Lorenzo M (2001) Akt mediates insulin induction of glucose uptake and up-regulation of GLUT4 gene expression in brown adipocytes. FEBS Lett 494(3):225–231
Lloberas N, Cruzado JM, Franquesa M, Herrero-Fresneda I, Torras J, Alperovich G, Rama I, Vidal A, Grinyó JM (2006) Mammalian target of rapamycin pathway blockade slows progression of diabetic kidney disease in rats. J Am Soc Nephrol 17(5):1395–1404
Yuan M, Konstantopoulos N, Lee J, Hansen L, Li Z-W, Karin M, Shoelson SE (2001) Reversal of obesity-and diet-induced insulin resistance with salicylates or targeted disruption of Ikkβ. Science 293(5535):1673–1677
Hotamisligil GS (2003) Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord 27(3):S53–S55
Moller DE (2000) Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab 11(6):212–217
Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM (1996) IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 271(5249):665–668
Fukuzawa M, Satoh J, Qiang X, Miyaguchi S, Sakata Y, Nakazawa T, Ikehata F, Ohta S, Toyota T (1999) Inhibition of tumor necrosis factor-α with anti-diabetic agents. Diabetes Res Clin Pract 43(3):147–154
Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, Shulman GI (2002) Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest 109(10):1321–1326
Waters JP, Pober JS, Bradley JR (2013) Tumour necrosis factor and cancer. J Pathol 230(3):241–248
Coskun M, Nielsen OH (2013) Tumor necrosis factor inhibitors for inflammatory bowel disease. N Engl J Med 369(8):754–762
Zhang Y, Liu R (2014) The effect of valsartan on tumor necrosis factor-alpha (TNF-alpha) in rat hearts during myocardial ischemia reperfusion. Cardiology 129:50
Chu W-M (2013) Tumor necrosis factor. Cancer Lett 328(2):222–225
Liang H, Yin B, Zhang H, Zhang S, Zeng Q, Wang J, Jiang X, Yuan L, Wang CY, Li Z (2008) Blockade of tumor necrosis factor (TNF) receptor type 1-mediated TNF-alpha signaling protected Wistar rats from diet-induced obesity and insulin resistance. Endocrinology 149(6):2943
Burska AN, Sakthiswary R, Sattar N (2015) Effects of tumour necrosis factor antagonists on insulin sensitivity/resistance in rheumatoid arthritis: a systematic review and meta-analysis. PLoS One 10(6):e0128889
Hotchkiss RS, Karl IE (2003) The pathophysiology and treatment of sepsis. N Engl J Med 348(2):138–150
Losser MR, Damoisel C, Payen D (2010) Bench-to-bedside review: glucose and stress conditions in the intensive care unit. Crit Care 14(4):231
Grimble RF (2002) Inflammatory status and insulin resistance. Curr Opin Clin Nutr Metab Care 5(5):551–559
Petit F, Bagby GJ, Lang CH (1995) Tumor necrosis factor mediates zymosan-induced increase in glucose flux and insulin resistance. Am J Physiol 268(2 Pt 1):E219–E228
Ofei F, Hurel S, Newkirk J, Sopwith M, Taylor R (1996) Effects of an engineered human anti-TNF-alpha antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM. Diabetes 45(7):881–885
Gonzalez-Gay MA, De Matias JM, Gonzalez-Juanatey C, Garcia-Porrua C, Sanchez-Andrade A, Martin J, Llorca J (2006) Anti-tumor necrosis factor-alpha blockade improves insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol 24(1):83–86
Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS (2012) Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 335(6076):1638–1643
Odegaard JI, Chawla A (2013) Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science 339(6116):172–177
Fischer R, Maier O (2015) Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF. Oxid Med Cell Longev 2015:610813
De LC, Olefsky JM (2006) Inflammation and insulin resistance. J Clin Investig 116(7):1793–1801
Evans JL, Goldfine ID, Maddux BA, Grodsky GM (2002) Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 23(5):599–622
Yang J, Park Y, Zhang H, Xu X, Laine GA, Dellsperger KC, Zhang C (2009) Feed-forward signaling of TNF-alpha and NF-kappaB via IKK-beta pathway contributes to insulin resistance and coronary arteriolar dysfunction in type 2 diabetic mice. Am J Physiol Heart Circ Physiol 296(6):10
Emanuelli B, Peraldi P, Filloux C, Sawka-Verhelle D, Hilton D, Van Obberghen E (2000) SOCS-3 is an insulin-induced negative regulator of insulin signaling. J Biol Chem 275(21):15985–15991
Hong FNV, Gao B (2001) Tumor necrosis factor alpha attenuates interferon alpha signaling in the liver: involvement of SOCS3 and SHP2 and implication in resistance to interferon therapy. FASEB J 15(9):1595–1597
Emanuelli B, Peraldi P, Filloux C, Chavey C, Freidinger K, Hilton DJ, Hotamisligil GS, Van Obberghen E (2001) SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-α in the adipose tissue of obese mice. J Biol Chem 276(51):47944–47949
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study was supported by China Postdoctoral Science Foundation (No. 20080441294).
Conflict of interest
The author declares that they have no conflict of interest.
Ethical approval
All the surgical procedures for animal use were approved by the University of Shandong Institutional Animal Care and Use Committee.
Informed consent
No informed consent needed.
Additional information
W. Qu and C. Han are regarded as co-first authors.
Rights and permissions
About this article
Cite this article
Qu, W., Han, C., Li, M. et al. Anti-TNF-α antibody alleviates insulin resistance in rats with sepsis-induced stress hyperglycemia. J Endocrinol Invest 41, 455–463 (2018). https://doi.org/10.1007/s40618-017-0742-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-017-0742-7