Abstract
Background
Sepsis is a systemic inflammatory response usually correlated with multi-organ failure. Myocardial dysfunction is one of the adverse outcomes in septic patients and results in high mortality rates. The aim of this study was to investigate the impact of irbesartan in attenuation of cardiac depression during polymicrobial sepsis via decreased activation of the phospho-p38MAPK/nuclear factor (NF)-κB signaling pathway.
Materials and methods
A model of polymicrobial sepsis induced via cecal ligation and puncture (CLP) with 8- to 12-week-old albino mice was used. Mice were treated with i.p. irbesartan (3 mg/kg) 1 h before CLP. Using a micro-tipped transducer catheter, the following hemodynamic parameters were evaluated after CLP: heart rate, ejection fraction, left ventricular (LV) end-diastolic pressure, LV systolic pressure, and cardiac output. Plasma levels of proinflammatory cytokines, including tumor necrosis factor (TNF)-alpha, interleukin (IL)-1 beta, IL-6, monocyte chemoattractant protein-1 (MCP-1), and cardiac troponin I (cTn-I), were measured via ELISA analysis. The degree of p38MAPK and NF-κB phosphorylation was assessed via Western blotting.
Results
Mice treated with irbesartan displayed improvement in LV function (ejection fraction: 42.4 ± 1.1% vs. 27.8 ± 3% in CLP mice). The attenuation of cardiac depression in irbesartan-treated mice was associated with lower levels of MCP-1 in plasma and a reduction in the levels of TNF-alpha, IL-1beta, and IL-6. Furthermore, irbesartan-treated mice displayed lower expression levels of p38-MAPK and NF-κB phosphorylation.
Conclusion
Irbesartan can attenuate cardiac dysfunction during polymicrobial sepsis possibly via a reduction of proinflammatory cytokines through decreased activation of the p38MAPK/NF-κB pathways.
Zusammenfassung
Hintergrund
Die Sepsis stellt eine systemische entzündliche Reaktion dar, die gewöhnlich mit Multiorganversagen einhergeht. Myokardfunktionsstörungen sind eine der ungünstigen Folgen bei septischen Patienten und führen zu einer hohen Mortalitätsrate. Ziel der vorliegenden Studie war es, den Einfluss von Irbesartan auf die Abschwächung der Kreislaufdepression bei polymikrobieller Sepsis über eine verminderte Aktivierung des Signalwegs von Phospho-p38MAPK/Nuclear-Factor(NF)-κB zu untersuchen.
Material und Methoden
Es wurde ein Modell der polymikrobiellen Sepsis verwendet, bei dem die Sepsis über eine Ligatur und Punktur des Zoekums („cecal ligation and puncture“, CLP) bei 8–12 Wochen alten Albinomäusen induziert wurde. Die Mäuse wurden i.p. mit Irbesartan (3 mg/kg) 1 h vor CLP behandelt. Unter Einsatz eines Mikrotip-Transducer-Katheters wurden die folgenden hämodynamischen Parameter nach CLP gemessen: Herzfrequenz, Ejektionsfraktion, linksventrikulärer (LV) enddiastolischer Druck, LV systolischer Druck und Herzzeitvolumen. Mittels ELISA-Test wurden die Plasmaspiegel proinflammatorischer Zytokine einschließlich Tumornekrosefaktor-alpha (TNF-α), Interleukin-1beta (IL-1β), IL-6, Monozyten-chemoattraktives Protein-1 (MCP-1) und kardiales Troponin I (cTn-I) bestimmt. Der Grad der Phosphorylierung von p38MAPK und NF-κB wurde per Western-Blot-Test ermittelt.
Ergebnisse
Die mit Irbesartan behandelten Mäuse wiesen eine Verbesserung der LV-Funktion auf (Ejektionsfraktion: 42,4 ± 1,1% vs. 27,8 ± 3% bei CLP-Mäusen). Die Abschwächung der Kreislaufdepression bei den mit Irbesartan behandelten Mäusen ging mit niedrigeren Plasmaspiegeln von MCP-1 und einer Senkung der Werte für TNF-α, IL-1β und IL-6 einher. Darüber hinaus zeigten die mit Irbesartan behandelten Mäuse eine geringere Expression der Phosphorylierung von p38-MAPK und NF-κB.
Schlussfolgerung
Irbesartan kann möglicherweise über eine Verminderung proinflammatorischer Zytokine durch geringere Aktivierung des p38MAPK/NF-κB-Signalwegs zu einer Abschwächung der kardialen Funktionsstörung bei polymikrobieller Sepsis führen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Sepsis is a systemic inflammatory reaction results from bacterial infection and is considered a main cause of death in critically ill patients [1, 2]. Myocardial dysfunction is one of the major signs of adverse outcomes in septic patients. It is usually correlated with decreased cardiac contractility, diastolic impairment, and cardiac injury, leading to a hypotensive condition. Approximately one fourth of patients with sepsis have cardiovascular complications, associated with elevated mortality rates of up to 70% [2–4]. Endotoxemia may decrease cardiac work via an increased expression level of proinflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6. These cytokines act as cardiodepressant proinflammatory mediators [5], resulting in cardiac contractile dysfunction [6], cardiac hypertrophy, and heart failure [7, 8]. Furthermore, an increased cardiac troponin I (cTn-I) level during endotoxemia will decrease myofilament calcium responsiveness to a large extent and subsequently impairment of cardiac contractile function will occur [9–11]. We proposed that irbesartan plays a role in attenuation of cardiac depression during polymicrobial sepsis via decreased expression of the phospho-p38MAPK/ nuclear factor (NF)-κB signaling pathway.
Materials and methods
Experimental animals
A total of 32 adult male Swiss albino mice aged 8–12 weeks, weighing 20–30 g, were obtained from the College of Science, Babylon University and housed in the animal house of the College of Science of Kufa University. They were kept in cages under a 12 h light: 12 h dark cycle, with room temperature kept at 25 °C and humidity at 60–65%, with free access to food and water.
Study design
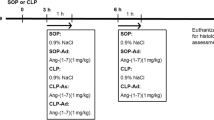
Mice were assigned randomly to one of the following groups (n = 8 in each group): control group (cecal ligation and puncture [CLP]), sham group (negative control), irbesartan-pretreated group (3 mg/kg of irbesartan 1 h prior to CLP), and vehicle (PBS)-pretreated group.
CLP procedure
In the present study, mice were selected to induce polymicrobial sepsis in a model based on previous studies [12–14]. In brief, polymicrobial sepsis was induced by a double-puncture technique using 20-guage needles. Mice were anesthetized using ketamine/xylazine solution [15]. Laparotomy was performed via a 1.5-cm midline incision and the cecum was exposed. The cecum was ligated just below the ileocecal valve and punctured; it was then placed back in its anatomical position. The abdomen was sutured, using a 5.0 surgical suture (Ethicon, Norderstedt, Germany), and 1 ml of Ringer’s solution was given s.c. for resuscitation. Mice were monitored for signs of disease every 4 h for 24 h. Sham-operated mice (anesthesia and laparotomy) served as the surgical control group.
Hemodynamic measurements
We assessed cardiac functions as described previously [16–18]. Briefly, mice were anesthetized intraperitoneally with ketamine at a dose of 50 mg/kg after CLP. Animals were placed supine on a heating blanket and body temperature was maintained at 37 °C ± 0.5 °C. The external right carotid artery was exposed, and a micro-tipped transducer catheter (1.4 F; Millar Instrument Inc., Houston, TX) was placed into the artery and then advanced into the left ventricle (LV). The other end of the catheter was connected to an electrostatic chart recorder (model ES 2000, Gould, Cleveland, Ohio) and pressure-volume loops were recorded to measure the maximum rate of change in ventricular pressure and ejection fraction by using an MPVS-400 system with the aid of PVAN software (Conductance Technologies, San Antonio, Tex., and Millar, Houston, Tex., respectively). Heart rates, LV end-diastolic pressure (LVEDP), and LV systolic pressure (LVSP) measurements were recorded.
ELISA analysis
The blood samples of mice were centrifuged (10,000 rpm for 10 min) and myocardial tissue was homogenized and treated in PBS containing 0.5% Triton X100 with a protease inhibitor cocktail. Commercial ELISA kits (Bosterbio Corp., Pleasanton, CA) were utilized to quantify monocyte chemoattractant protein (MCP)-1, TNF-α, IL-1β, and IL-6 as well as plasma cTn-I levels. Samples and standards were prepared according to the manufacturer’s instructions. Absorbance values of standards and samples were determined spectrophotometrically at 450 nm, using a microplate reader (Bio-Rad Laboratories, Hercules, California, United States). The data obtained were plotted against the linear portion of a standard curve [19].
Western blot
Myocytes of cardiac tissue were harvested with ice-cold PBS and centrifuged at 13,000 g for 3 min at 4 °C. Nuclear and cytosolic extracts were prepared using a Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime Institute of Biotechnology, Jiangsu, China) according to the manufacturer’s instructions. Protein concentrations were measured using a bicinchoninic acid protein assay kit (Beyotime). Equal amounts of lysates (50 μg) were separated on 10% SDS-PAGE. Proteins were transferred onto immunoblot polyvinylidene difluoride membranes (Chemicon International, Millipore, Billerica, Mass), and the membranes were blocked with 5% BSA in Tris-buffered saline with 0.1% Tween (TBS-T) for 2 h and incubated overnight at 4 °C with the following primary antibodies; MAPK (1:1000), phospho-p38 (1:1000), p38 (1:1000), rabbit anti-mouse NF-κB (1:1000; Santa Cruz Biotechnology, Delaware Ave, Santa Cruz, CA), β‑actin (1:2000; Santa Cruz Biotechnology). Blots were washed four times for 15 min each in TBS-T and incubated with horseradish peroxidase-labeled secondary goat anti-rabbit (1:2000; Santa Cruz Biotechnology) or rabbit anti-goat (1:2000; Santa Cruz Biotechnology) for 1 h. Blots were again washed four times for 15 min each in TBS-T. Finally, blots were developed using the enhanced chemiluminescence (Pplygen Co., PPLYGEN, Beijing, China) method.
Statistical analysis
Statistical analysis of the data was performed using StatView software (Abacus Concepts, USA). Analysis of variance (ANOVA) with Fisher’s post-hoc test was used to investigate differences between the mice, and differences were confirmed using the Mann–Whitney U test. Statistical significance was set at p ≤ 0.05.
Results
Effect of irbesartan pretreatment on LV function after CLP
LV function was assessed 24 h after CLP to investigate the effect of treatment with irbesartan on sepsis-induced myocardial dysfunction. The results in Table 1 show that both the CLP and vehicle groups had significantly (p < 0.05) attenuated LV function with decreased ejection fraction, cardiac output, and LVSP and an increase in heart rate and LVEDP as compared with the sham group. Further, the irbesartan-treated group had improved LV function through increased ejection fraction, cardiac output, and LVSP as well as reduced heart rate and LVEDP.
Effect of irbesartan pretreatment on plasma level of proinflammatory cytokines after CLP
At the end of the experiment (24 h after CLP), the levels of plasma proinflammatory cytokines (TNF-α, IL-1β, and IL-6) were increased in the CLP and vehicle groups compared with the irbesartan treatment group.
The changes in plasma proinflammatory cytokines (TNF-α, IL-1β, IL-6) levels are shown in Fig. 1.
Effect of irbesartan pretreatment on the plasma level of MCP-1 after CLP
At the end of the experiment (24 h after CLP), the plasma MCP-1 level was increased in the CLP and vehicle groups compared with the irbesartan treatment group. The changes in the plasma MCP-1 level are shown in Fig. 2.
Effect of irbesartan pretreatment on myocardial injury after CLP
The plasma level of cTn-I was significantly (p < 0.05) increased in the CLP and vehicle groups as compared with the sham group. The cTn-I level of the irbesartan-treated group was significantly (p < 0.05) lower than that of the CLP group. The changes in cTn-I level are presented in Fig. 3.
Irbesartan pretreatment attenuates phosphorylation of p38MAPK/NF-κB in cardiomyocytes after CLP
Myocardial tissue homogenates were analyzed using the Western blot technique. The p38MAPK/NF-κB phosphorylation in myocardial cells was significantly (p < 0.05) increased in the CLP and vehicle groups as compared with the sham group. The NF-κB phosphorylation in the irbesartan-treated group was significantly (p < 0.05) lower than that of the CLP group: This indicates the involvement of NF-κB in the mechanistic action of irbesartan. The phosphorylated p38MAPK level of the irbesartan-treated group was significantly (p < 0.05) lower than that of the CLP group: This indicates the involvement of p38MAPK in the mechanistic action of irbesartan. The changes in p38MAPK and NF-κB level are presented in Fig. 4.
Discussion
During sepsis, the inflammatory responses mediate myocardial injury, including LV dysfunction and cardiac pathophysiological changes [20–22]. Previous studies reported that plasma levels of inflammatory mediators (IL-1β, TNF-α, and IL-6) were higher following myocardial injury and sepsis [23–25]. Furthermore, another study confirmed in vivo increased MCP-1 levels in both plasma and myocardial tissue in a mouse model after sepsis [26]. To understand the pathway of sepsis in the context of endotoxemic cardiac depression, the present study investigated the role of irbesartan pretreatment in improving cardiac function following sepsis. According to our knowledge, there are no published data discussing the relationship between the p38MAPK/NF-κB pathway and the effective role of irbesartan on improved cardiac function following sepsis in a CLP mouse model.
Sepsis attenuated myocardial function via elevation of inflammatory mediators
A number of published studies have confirmed that myocardial dysfunction during sepsis is related to the expression of inflammatory mediators, including IL-6, TNF-α, and IL-1β [26, 27]. Furthermore, inflammatory cytokines have been up-regulated in myocardial dysfunction after acute injuries caused by sepsis, myocardial ischemia, and reperfusion [26, 28, 29]. Additionally, intravenous administration of either TNF-α or IL-1β in animal experiments can evoke a similar process to that caused by sepsis leading to cardiac comorbidity and mortality [26]. The aforementioned adverse effects of proinflammatory cytokines can be ameliorated by antibodies that antagonize the effects of these molecules [29–31]. Other studies have demonstrated that TNF-α also plays an important role in septic myocardial dysfunction and that TNF-α is linked to the TLR4 activation pathway [30, 32]. In the present study, we demonstrated that sepsis increases the levels of inflammatory mediators (IL-1β, TNF-α, and IL-6) in both plasma and cardiac tissue of mice, and that it is associated with worse LV function performance as reflected in the hemodynamic measurements (heart rate, ejection fraction). Furthermore, these results are associated with increased levels of circulating cTn-I in mice exposed to CLP. Our data suggest that significantly higher levels of myocardial depressant proinflammatory cytokines in the heart directly attenuated cardiac contractility and induced myocardial injury. Taken together, these results contribute partly to the mechanism of exaggerated cardiac depression in an experimental sepsis mouse model. Interestingly, we observed that pre-treatment with irbesartan resulted in a greater reduction in cytokines with an improvement in LV function: Ejection fraction was improved (40.4 ± 1.1) in irbesartan-treated mice. Additionally, pretreatment with irbesartan improved other LV function parameters, such as LVESP (82.5 ± 3.2) and cardiac output (3.4 ± 1.9).
Sepsis up-regulated myocardial MCP-1 expression level
Many studies have demonstrated that MCP-1 decreased the recruitment of neutrophils and reduced tissue injury in many animal models of sepsis-induced organ injury [33–35]. In the present study, we found that MCP-1 levels in plasma and cardiac tissue were significantly higher in the CLP group than in the sham group.
Down-regulation of p38MAPK/NF-κB improved LV function
The intracellular downstream signaling pathway of TLR4 includes phosphorylation of p38MAPK and activation of NF-κB [10, 11, 36, 37]. In the present work, we investigated the mechanisms of action of irbesartan. Our findings suggest that irbesartan decreased cardiac injury in the irbesartan-pretreated group through its ability to decrease both the degree of p38MAPK phosphorylation and NF-κB activation as compared with the CLP group, which showed an elevated level of p38MAPK phosphorylation and NF-κB activation.
Conclusion
This study found that both p38MAPK phosphorylation and NF-κB activation are increased during sepsis and lead to attenuation of LV function. Additionally, it was found that both p38MAPK phosphorylation and NF-κB activation are closely related to increased plasma and tissue levels of proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6, which leads to decreased LV function. This finding suggests that both p38MAPK phosphorylation and NF-κB activation could be biomarkers and novel targets for therapy in patients with cardiac complications during sepsis via improvement of LV function. These experimental results indicate that endogenous p38MAPK phosphorylation and NF-κB activation mediate the expression of MCP-1, leading to increased levels of cTn-I and subsequently myocardial cell injury. Western blot analysis proved the low levels of both p38MAPK and NF-κB in irbesartan-pretreated mice compared with the sham or vehicle-treated groups. The effects of p38 MAPK or NF-κB inhibitors during sepsis remain to be further studied and tested.
References
Drosatos K et al (2015) Pathophysiology of sepsis-related cardiac dysfunction: driven by inflammation, energy mismanagement, or both? Curr Heart Fail Rep 12(2):130–140
Wang J et al (2016) Clinical significance of plasma levels of brain natriuretic peptide and cardiac troponin T in patients with sepsis. Exp Ther Med 11(1):154–156. doi:10.3892/etm.2015.2863
Drosatos K et al (2013) PPARγ activation prevents sepsis-related cardiac dysfunction and mortality in mice: drosatos et al: PPARγ treats septic cardiac dysfunction. Circ Heart Fail 6(3):550–562
Romero-Bermejo FJ et al (2011) Sepsis-induced cardiomyopathy. Curr Cardiol Rev 7(3):163–183
Kumar A et al (1996) Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med 183(3):949–958
Prabhu SD (2004) Cytokine-induced modulation of cardiac function. Circ Res 95(12):1140–1153
Higashikuni Y et al (2013) Toll-like receptor-2 mediates adaptive cardiac hypertrophy in response to pressure overload through Interleukin-1β upregulation via nuclear factor κB activation. J Am Heart Assoc 2(6):e000267. doi:10.1161/jaha.113.000267
Layland J et al (2005) Protection against endotoxemia-induced contractile dysfunction in mice with cardiac-specific expression of slow skeletal troponin I. FASEB J 19(9):1137–1139
Zhang N et al (2015) Pharmacological TLR4 inhibition protects against acute and chronic fat-induced insulin resistance in rats. PLOS ONE 10(7):e0132575
Zbinden-Foncea H et al (2012) TLR2 and TLR4 activate p38 MAPK and JNK during endurance exercise in skeletal muscle. Med Sci Sports Exerc 44(8):1463–1472
Kim HM et al (2007) Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell 130(5):906–917
Dejager L et al (2011) Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol 19(4):198–208
Chang CL et al (2012) Impact of apoptotic adipose-derived mesenchymal stem cells on attenuating organ damage and reducing mortality in Rat sepsis syndrome induced by cecal puncture and ligation. J Transl Med 10:244
Coletta C et al (2014) Endothelial dysfunction is a potential contributor to multiple organ failure and mortality in aged mice subjected to septic shock: preclinical studies in a murine model of cecal ligation and puncture. Crit Care. doi:10.1186/s13054-014-0511-3
Khan AI et al (2013) Erythropoietin attenuates cardiac dysfunction in experimental sepsis in mice via activation of the β‑common receptor. Dis Model Mech 6(4):1021–1030
Williams DL et al (1999) Early activation of hepatic NFkappaB and NF-IL6 in polymicrobial sepsis correlates with bacteremia, cytokine expression, and mortality. Ann Surg 230(1):95–104
Yousif NG, Al-Amran FG (2011) Novel toll-like receptor-4 deficiency attenuates trastuzumab (Herceptin) induced cardiac injury in mice. BMC Cardiovasc Disord 11:62
Slimani H et al (2014) Enhanced monocyte chemoattractant protein-1 production in aging mice exaggerates cardiac depression during endotoxemia. Crit Care 18(5):527
Turler M, Thoma S, Graves M, Lyman SM, Kasper JH (2015) Dopamine signaling attenuated myocardial injury during endotoxemia. Am J Biomed 3(7):381–391
Slimani H, Zhai Y, Yousif NG, Ao L, Zeng Q (2014) Enhanced monocyte chemoattractant protein-1 production in aging mice exaggerates cardiac depression during endotoxemia. Crit Care Med 18(5):527
Hullmann G et al (2015) Role of IL-1B in TLR4-mediated MCP-1expression: renal sepsis. Am J Biomed 3(1):22–31
Tracey SG et al (2014) Critical role of macrophage migration inhibitory factor in sepsis via interfere with MCP-1/CCL2 signaling pathway. Am J Biomed 2(6):688–701
Deshmane SL, Kremlev S, Amini S, Sawaya BE (2009) Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 29(6):313–26; doi:10.1089/jir.2008.0027
Turler M, Thoma S, Graves M, Lyman SM, Kasper JH (2015) Dopamine signaling attenuated myocardial injury during endotoxemia. Am J Biomed 3(7):381391
Fukumoto J et al (2015) Sepsis and signal transduction pathway: cross-talk TLR4/ MyD88/TRIF. Am J Biomed 3(4):150–163
Colorado University, Everett A, Yousif N, Ao L, Cleveland J, Fullerton D, Meng X (2013) Ghrelin reduces myocardial injury following global ischemia and reperfusion via suppression of myocardial inflammatory response. Am J Biomed 1(2):38–48
Al-amran FG, Yousif NG, Meng XM (2011) A TLR4-MCP-1-macrophage IL18 cascade plays a major role in myocardial injury and cardiac dysfunction after permanent Ischemia. J Surg Res 165(2):265–266
Nasser YG, Cleveland JC Jr, Fullerton DA Jr, Meng X Jr (2012) Aging augments myocardial inflammatory response to ischemia and reperfusion: an obligatory role of TLR4. Shock 37:31–119
Yousif NG et al (2011) Expression of human interleukine-37 protects mouse heart against Ischemic injury through suppression of monocyte chemoattractant protein-1-mediated mononuclear cell accumulation. Circulation 124(Suppl 21):A8603
Yousif NG (2014) Novel therapeutic role of siglec-E in down-regulation TLR4-mediated inflammatory response after global myocardial ischemia and reperfusion. Cardiovasc Res 103:s90
Stamm C et al (2001) Inhibition of tumor necrosis factor-alpha improves postischemic recovery of hypertrophied hearts. Circulation 104(12 Suppl 1):I-350–I-355
Slimani HS et al (2014) Critical role IL-37 to ameliorate endotoxemic cardiac depression in aging mice: a critical role of suppression cardiodepressant cytokines. Cardiovasc Res 103:S92–S92
Woo J‑I et al (2010) Spiral ligament fibrocyte-derived MCP-1/CCL2 contributes to inner ear inflammation secondary to nontypeable H. influenzae-induced otitis media. BMC Infect Dis 10:314–314
Grupper R et al (2015) Critical role of microRNAs after global myocardial ischemia and reperfusuion. Am J Biomed 3(7):451–467
Gao Y‑J et al (2009) JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. Neuroscience 29(13):4096–4108
Chang JH, Park JY, Kim SK (2006) Dependence on p38 MAPK signalling in the up-regulation of TLR2, TLR4 and TLR9 gene expression in Trichomonas vaginalis-treated HeLa cells. Immunology 118(2):164–170
O’Neill LA, Bowie AG (2007) The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol 7(5):353–364
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
N.G. Yousif, N.R. Hadi, F. Al-Amran, and Q.A. Zigam declare that they have no competing interests.
All national guidelines on the care and use of laboratory animals have been followed and that the necessary approval was obtained from the relevant authorities.
Rights and permissions
About this article
Cite this article
Yousif, N.G., Hadi, N.R., Al-Amran, F. et al. Cardioprotective effects of irbesartan in polymicrobial sepsis. Herz 43, 140–145 (2018). https://doi.org/10.1007/s00059-017-4537-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-017-4537-6