Abstract
Purpose of Review
Copper, zinc, iron, and manganese are essential micronutrients for all living organisms. Microbial pathogens must acquire these elements from their host. Through a process termed nutritional immunity, animal hosts seek to withhold these vital nutrients from the microbe and the competition for metals can influence survival outcomes during infection. Much is known about the battle for iron, copper, and zinc during fungal infections, but a picture is just now beginning to emerge for manganese.
Recent Findings
Pathogenic fungi utilize manganese for antioxidant defense, cell wall construction, morphogenesis, and survival in animal and plant hosts. The animal host can limit manganese availability for invading fungi at the macrophage, neutrophil, and whole tissue levels.
Summary
Here, we review the role of manganese as an essential nutrient for pathogenic fungi and the ways an animal host can withhold this vital metal from infectious fungi of clinical and agricultural importance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metals such as Fe, Cu, Zn, and Mn play important roles in biology and serve as co-factors for nearly half of all enzymes [1]. Both participants in infection, the host and the pathogen, must acquire these micronutrients to perform necessary cellular functions. While animal hosts obtain these nutrients through diet, pathogens must scavenge trace metal nutrients from their host. The mammalian host exploits this nutritional dependence of the microbial pathogen and limits metal availability at sites of infection to effectively starve the invaders and impede their growth. Such regulation of metal availability by the host is known as nutritional immunity [2•]. Successful pathogens have evolved with specialized mechanisms to tolerate changes in metal availability. In the case of fungal pathogens, there have been many reports of the battle for micronutrients Fe, Zn, and Cu at the host–pathogen interface, and several excellent reviews have been written on the topic [3,4,5]. Comparatively, little, however, is known about Mn, and until very recently, an essential role for Mn in fungal pathogenesis had not been characterized. Here, we provide an overview of Mn as an essential micronutrient for fungal growth and fitness in the mammalian host and describe ways the host itself can manipulate this metal at the fungal infection battleground.
Manganese as a Micronutrient for Fungi and the Hosts They Infect
Manganese is the 12th most abundant element in the earth’s crust and accumulates in biological systems as divalent and trivalent Mn+2 and Mn+3. Mn binds selectively to oxygen and imidazolate nitrogen ligands and therefore coordinates well in enzyme active sites harboring aspartates, glutamates, and histidines. Because of its participation in redox chemistry, Mn serves as an excellent co-factor for enzymes involved in oxygen chemistry. Mn can also serve in non-redox catalytic roles for a variety of enzymes involved in metabolism and signaling. In fungi and animals, the best-characterized roles of Mn as a micronutrient involve the protein families of superoxide dismutases and protein glycosyltransferases.
Superoxide dismutases (SODs) are antioxidant metalloenzymes that disproportionate superoxide, a potentially toxic-free radical and reactive oxygen species (ROS) generated by metabolism. Fungi and animals typically use the Mn- and Cu-containing enzymes. Of these, a Mn-SOD generally resides in the mitochondrial matrix (so-called SOD2) to remove electron transport chain–generated superoxide, while the cytosol contains a Cu- and Zn-containing SOD1 [6]. Certain pathogenic fungi such as the opportunistic fungal pathogen Candida albicans uniquely express a second Mn requiring SOD3 in the cytosol that represents a clever adaptation to nutritional immunity for Cu. When C. albicans is limited for Cu as occurs in the kidney during infection, the fungus substitutes its Cu/Zn SOD1 with Mn-SOD3 to maintain antioxidant protection [7, 8].
Protein glycosyltransferases that reside in the Golgi decorate proteins with chains of glucose, galactose, or mannose, and this broad class of enzymes is Mn-dependent [9]. Mannosylation is prevalent among fungi, and mannosylated proteins at the cell surface create a thick mannose layer that represents the outer coat of the fungal cell wall. The Mn-dependent mannosyltransferases (MNTs) that create these elaborate chains of mannose are essential for the virulence of fungal pathogens [10, 11]. In addition to the primary roles of Mn in fungal protein mannosylation and antioxidant function, a number of kinases and phosphatases use Mn (or Mg) as co-factors [9, 12], and Mn is also believed to be the physiological substrate of the TOR kinase used in nutrient signaling [13••].
Mn Uptake and Homeostasis: Lessons Learned from Bakers’ Yeast
By far, the vast majority of what is known about Mn in fungi stems from older studies in bakers’ yeast Saccharomyces cerevisiae. Mn uptake in S. cerevisiae is largely accomplished by NRAMP (natural resistance-associated macrophage protein, see ahead) divalent metal transporters. Across eukaryotes and bacteria, NRAMP transporters co-transport divalent metals such as Co2+, Fe2+, Cd2+, Mn2+, and Zn2+ together with protons [14•]. In S. cerevisiae, the SMF1 and SMF2 NRAMP transporters specifically function in Mn uptake and utilization. (Note: The name SMF derives from its original isolation as a suppressor of mif1 mutant affecting mitochondrial protein processing [15].) S. cerevisiae SMF1 and SMF2 have been localized to the cell surface and endosomes where they function in high affinity cell surface uptake of Mn that is then delivered to Mn-SOD2 in the mitochondria and Mn-MNTs in the Golgi (Fig. 1) [16,17,18,19]. The only other source of Mn for S. cerevisiae is the Mn-phosphate transporter PHO84 that operates under low-affinity (high Mn) conditions [20, 21].
Mn transport and trafficking in a fungal cell: Mn ion uptake is mediated by NRAMP Mn transporters that based on studies in S. cerevisiae, localize to the cell surface and endosomes. The Mn is taken up by the Golgi through Mn and Ca transporters PMR1 and GDT1 that activate Mn requiring mannosyl transferases (MNT) in the secretory pathway. Mn is also delivered to the mitochondria to activate Mn-containing SOD2. The mechanism may involve an interaction with NRAMP-containing endosomes (see main text). In certain fungi (e.g., C. albicans), Mn is delivered to the cytosol to activate a cytosolic Mn-SOD3
S. cerevisiae SMF1 and SMF2 are negatively regulated by Mn ions in order to prevent Mn hyperaccumulation when the metal is abundant and to maximize Mn uptake when the metal is low. Such negative feedback control of metal transport is not unique to Mn and the SMF transporters. Fungal transporters for Zn, Cu, and Fe are all regulated by their cognate metal substrate at the transcriptional level using Zn, Cu, or Fe sensing transcription factors [3,4,5]. However, there is no known trans-regulator for Mn in yeast species, and SMF1 and SMF2 transporters are not regulated at the mRNA level. Instead, Mn regulates SMF1 and SMF2 at the post-translational level through changes in SMF protein localization and protein turnover. Under Mn-replete conditions, the transporters are targeted directly to the vacuole for degradation. Under Mn starvation conditions, the two proteins are diverted from the vacuole and accumulate at the cell surface and endosomes to facilitate Mn uptake and utilization. The sensing of Mn and control of SMF1 and SMF2 localization are accomplished by BSD2, an adaptor for the RSP5 ubiquitin ligase. BSD2 in the endoplasmic reticulum detects conformations in SMF1 that alter with Mn, and through ubiquitination of the metal transporter, SMF1 is directed to the vacuole for degradation [16, 17, 19, 22,23,24,25,26].
In addition to Mn uptake at the cell surface, Mn needs to be transported into the Golgi for activation of MNTs, and this is accomplished in part by a Mn2+ and Ca2+ transporting ATPase first identified in S. cerevisiae as PMR1 (for plasma membrane–related ATPase) [27, 28]. Loss of PMR1 results in defective protein mannosylation without impaired mitochondrial Mn SOD2, in addition to hyperaccumulation of Mn in the cytosol. These phenotypes are consistent with a role for PMR1 in delivering Mn specifically from the cytosol to the Golgi (Fig. 1) [18, 27,28,29]. More recently, a second Golgi transporter has been identified that functions in Mn delivery to the Golgi for protein mannosylation, namely yeast GDT1, a proton and Ca/Mn exchanger [30, 31•]. Yeast GDT1 appears to serve as a backup to PMR1 for supplying the secretory pathway with Mn, as well as Ca (Fig. 1).
Regarding mitochondrial uptake of Mn, we previously identified mitochondrial solute transporter MTM1 as necessary for Mn incorporation into SOD2 [32]. However, MTM1 was subsequently found to function in mitochondrial homeostasis of Fe, not Mn, and yeast mutants for mtm1 mis-incorporate Fe into the active site of SOD2 rather than Mn [33, 34]. How then does Mn reach the mitochondria for activation of Mn SOD2? Interestingly, studies in mammalian cells have implicated a NRAMP transporter in the process, namely DMT1, that like S. cerevisiae SMF1 and SMF2 localizes to the cell surface and endosomes. DMT1-containing endosomes can physically interact with mitochondria and deliver Mn [35, 36, 37•]. A similar scenario may occur in fungi with SMF1- or SMF2-containing endosomes (depicted in Fig. 1).
Conserved Mn Trafficking Pathways Across the Fungal Kingdom
Many of the same aforementioned Mn transport and trafficking pathways first discovered in S. cerevisiae have recently been identified in diverse fungal species, from environmental non-pathogenic species to pathogens that infect plants and animals. Below, we highlight commonalities identified in NRAMP transporters for Mn and in the Golgi Mn transport systems involving PMR1 and GDT1.
By genome inspections, the vast majority of fungi examined have one to five NRAMP candidates, exceptions include plant pathogens Magnaporthe grisea and Alternaria brassicicola [38]. The human pulmonary pathogen Cryptococcus neoformans contains a single NRAMP, denoted Cramp, that when expressed in oocytes, exhibits Mn uptake activity and is therefore likely to be a Mn transporter for the fungus. However, Cramp also has the capacity to transport Fe, Co, and Ni in this oocyte system [39], and the precise metal substrate in C. neoformans cells warrants investigation. Indeed, NRAMP transporters from bacteria to humans can display a great deal of promiscuity in their metal ion substrate, and it is often difficult to predict the physiological metal for transport [37•]. Perhaps, the best evidence for Mn selectivity in fungal NRAMPs has emerged through studies of the single NRAMP in filamentous Aspergillus sp and a pair of NRAMPs in C. albicans. In pathogenic Aspergillus niger, loss of the single NRAMP DmtA drastically reduced Mn uptake and resulted in growth defects that were rescued by supplements of Mn salts [40]. Deletion of the analogous AoNramp1 in the non-pathogenic Aspergillus oryzae also resulted in growth deficiencies correctable by Mn or Zn supplements [41]. C. albicans contains four NRAMPs: SMF3 for vacuolar Fe transport [42], SMF11 of unknown function, and SMF12 and SMF13 that operate in Mn transport [43••, 44•]. Loss of either SMF12 or SMF13 effects a drastic reduction in total cellular Mn accumulation [43••, 44•]. The activity of both the mitochondrial MnSOD2 and the cytosolic MnSOD3 are inhibited, as are MNTs in the Golgi [43••]. Total cell surface phosphomannans are decreased, and protein mannosylation is severely impaired, including that of a vacuolar acid phosphatase as well as a cell wall Cu-containing superoxide dismutase (SOD5) needed for antioxidant defense [43••]. Interestingly, mannosylation is not totally eliminated, but the size of the mannose chains on cell wall proteins such as SOD5 is greatly reduced. C. albicans SMF12 and SMF13 appear to be specific for Mn, as all the defects associated with loss of these transporters were rescuable by supplements of Mn, but not other metals [43••]. Furthermore, mutations in SMF12 and SMF13 resulted in no loss of Fe, Zn, or Cu accumulation in C. albicans, only Mn was impacted [43••].
As mentioned above, the Mn NRAMP transporters in S. cerevisiae are negatively regulated by Mn through degradation in the vacuole involving BSD2 and ubiquitination [16, 17, 19, 22,23,24,25,26]. It is not currently clear whether NRAMPs in other fungal species are similarly regulated at the post-translational level. In Aspergillus nidulans, the BsdA homologue has been shown to target misfolded proteins to the vacuole for degradation, and the metal-bound forms of NRAMP transporters may be one such cargo [45].
Are the NRAMPs the only source of fungal Mn? With both C. albicans and A. niger, the defects associated with loss of the Mn transporting NRAMPs can be rescued by supplementation with high levels of Mn [40, 43••], implying a second Mn uptake system. It was proposed that the A. niger homologue to S. cerevisiae PHO84 (metal-phosphate transporter) accounts for this low-affinity Mn uptake, although this has yet to be verified [40]. In C. albicans, mutants of pho84 showed no deficits in Mn uptake in strains that express SMF12 and SMF13 [46], yet it remains possible that PHO84 becomes operative for Mn uptake when SMF12 and SMF13 are defective.
The PMR1 (Mn and Ca P-type ATPase) and GDT1 (Mn and Ca/proton exchanger) transport systems for delivering Mn to the Golgi for MNTs are also conserved across fungi. In C. albicans, loss of PMR1 causes decreases in cell surface phosphomannans and in mannosylation of acid phosphatase [47], similar to the Mn deficiency phenotypes of C. albicans smf12 and smf13 mutants [43••]. In the plant pathogen Aspergillus flavus, mutants of PMR1 and GDT1 exhibit defects in protein mannosylation [48••], and an analogous role for PMR1 and GDT1 in Golgi Mn uptake was described for the plant pathogen Fusarium graminearum [49•]. While PMR1 and GDT1 are predicted to transport Ca as well as Mn into the Golgi, the protein mannosylation defects associated with loss of these transporters were rescued by Mn supplements in A. flavus [48••], strongly pointing to a role for Mn transport in protein mannosylation.
The Role of Fungal Mn in Cell Wall Integrity, Morphogenesis, and Pathogenesis
The fungal cell wall maintains cell shape and integrity and helps accommodate morphological changes that occur during fungal differentiation and filamentation. With pathogenic fungi, the cell wall layers are important for immune recognition [50••, 51, 52]. The wall is typically composed of an inner layer of beta-glucan and chitin covered by an outer mannose layer derived from mannosylated cell wall proteins (depicted in Fig. 2). With the aforementioned role of Mn in protein mannosylation, any disruptions in Mn homeostasis could potentially impact cell wall integrity, morphogenesis, and pathogenesis. Below, we provide examples to support this role for Mn in fungi.
The effects of fungal Mn deficiency on the cell wall. Shown are the layers of the fungal cell wall atop the plasma membrane lipid bilayer. The fungal cell wall can be comprised of inner beta-glucan (green balls) and chitin (brown horizontal lines) layers and an outer mannose layer (blue) derived from heavily mannosylated cell wall proteins (CWP) such as Cu-only superoxide dismutases (Cu-SOD). Left—under Mn-replete conditions the fungal cell wall is intact. Right—under Mn starvation conditions, the loss in activity of mannosyl transferases results in very short mannose chains on cell wall proteins and an attenuated mannose layer. The potential implications of such Mn deficiency are listed
Several studies have linked Mn to fungal cell wall maintenance. During Mn deficiency, the outer mannose layer can become thin as indicated by the dramatic loss in cell surface phosphomannans and short-chain mannosylation of cell wall proteins seen with C. albicans smf12 and smf13 mutants (Fig. 2) [43••]. Additionally, pmr1 mutants of C. albicans, Aspergillus fumigatus, and A. flavus show changes in cell wall thickness, an increased hypersensitivity to cell wall perturbing agents, and elevated beta-glucan and chitin content, perhaps to compensate for losses in the mannose layer [47, 48••, 53].
Regarding the role of Mn in differentiation and morphogenesis, mutants of Mn NRAMP transporters in A. niger and A. oryzae show defects in germination and hyphal morphology [40, 41], and C. albicans smf12 and smf13 mutants form abnormal hyphae, a defect that is corrected by Mn supplementation to the growth media [43••]. PMR1 of the plant pathogen Magnaporthe oryzae is essential for morphogenesis [54], and mutants of pmr1 and gdt1 in F. graminearum and A. flavus likewise show defects in morphology that are rescued by high Mn supplementation [48••, 49•]. Even Mn in the growth media can impact the morphology of wild-type fungi, as has been reported with filamentation of Rhizophagus irregularis [55] and biofilm formation in Candida parapsilosis [56].
With several pathogenic species, morphogenesis and differentiation are keys for fungal invasion of cells and tissues. Accordingly, mutants of Mn homeostasis have displayed losses in virulence with both plant and animal pathogens. C. albicans mutants of smf12, smf13, and pmr1 show decreased virulence both in a mouse model for disseminated candidiasis [43••, 47] and in an insect model for fungal infection [44•]. In addition, pmr1 mutants in the plant pathogens Fusarium graminearum and A. flavus have exhibited decreased virulence in plant infection models, and with A. flavus, this virulence defect was rescued by high Mn [48••, 49•]. Altogether these studies underscore the notion that proper Mn homeostasis is essential for fungal survival during infection.
Manganese on the Host Side of the Infection Battleground—Nutritional Immunity for Manganese
With the importance of Mn in fungal growth and pathogenesis, host manipulation of this metal may greatly influence outcomes in fungal infections. Here, we review what is currently known about host nutritional immunity for Mn in animals as applies to pathogenic fungi.
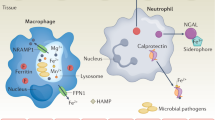
Macrophages can withhold Mn from invading microbes once they are engulfed into the phagolysosome. Pathogens housed in phagolysosomes are then attacked by a myriad of harsh conditions including hydrolytic enzymes, oxidative stress conditions, and acidic pH. Pathogens face Mn and Fe starvation in this compartment through the action of NRAMP1 [57]. NRAMP1 in fact is the prototype of the NRAMP family of divalent metal transporters named for “natural resistance-associated macrophage protein” or for its ability to enhance mouse resistance to microbial infection [57]. NRAMP1 is effective in promoting resistance to a wide array of bacterial and parasitic pathogens [14•, 57, 58], but limited information is available on fungi. During macrophage invasion by C. neoformans, NRAMP1 improved macrophage anti-cryptococcal activity at early, but not late stages of fungal invasion [59].
Neutrophils can also restrict Mn from pathogens using calprotectin (Cp). Calprotectin is highly expressed in neutrophils, making up about 40% of the total cytoplasmic protein content in these immune cells [60]. The metal binding properties of Cp are well defined, and this protein is widely recognized for its ability to restrict microbial access to Mn, Zn, Cu, and Fe [61,62,63,64]. Cp can withhold multiple metals from fungi in culture, including Mn, Zn, and Cu, and in animals, Cp is released in abundance at tissue sites of infection with C. albicans and A. fumigatus [64,65,66]. Cp-deficient mice display an increased fungal burden during A. fumigatus corneal infection [66] and also show an impaired ability to starve C. albicans for Zn during disseminated candidiasis [64]. When C. albicans invades the kidney, fungal mRNA markers for Zn starvation are strongly upregulated in WT mice, but not in Cp-deficient mice [64]. A similar effect of Cp on fungal Mn in vivo could not be examined due to the lack of any known fungal mRNA markers for Mn deficiency. Based on the strong effects of Cp on fungal Mn in vitro [64,65,66], this host protein is likely to restrict Mn from the pathogen in vivo as well.
In addition to these macrophage and neutrophil effects on Mn, recent studies have demonstrated whole tissue restriction of Mn during fungal invasion. Specifically, in a mouse model for disseminated candidiasis where the kidney is the major target tissue, whole kidney Mn levels decline during C. albicans invasion of the tissue [43••]. The liver is a secondary site of C. albicans infection, and like the kidney, liver Mn declines during fungal invasion [67••]. The mechanism for the loss in tissue Mn during fungal infection is not known. In the kidney, Mn levels are controlled by several Zn/Mn transporters including ZIP8. Interestingly, mutations in ZIP8 have been associated with higher susceptibility to inflammatory bowel disease and to infection by Streptococcus pneumoniae [68•, 69]. Future studies are warranted to address the possible role of ZIP8 or other host metal transporters in Mn limitation in the kidney during fungal invasion.
Conclusion
Like other eukaryotes, fungi rely on Mn to activate numerous enzymes including superoxide dismutases and glycosyltransferases. What sets fungi apart is their dependence on this metal for the formation of the fungal cell wall, particularly the outer mannose-containing layer that contributes to cell shape and integrity and modulates fungal recognition by the host immune system. With the importance of the cell wall in fungal growth and survival, it is not surprising that Mn deficiencies have been associated with defects in fungal morphogenesis and pathogenesis. In addition to cell wall effects, Mn has a key role as an antioxidant, which can be of particular importance to pathogenic fungi attacked by ROS from host immune cells. Mn is a co-factor for SOD enzymes, and in C. albicans, Mn drives maturation (through mannosylation) of extracellular Cu-SODs that detoxify host ROS [43••, 70]. Mn is also necessary for TOR nutrient signaling [13••], a process important for fungal morphogenesis [71]. Because fungi rely on Mn for such diverse processes, host withholding of this metal may be an effective antifungal tactic. Several immune responses are consistent with nutritional immunity for Mn. Macrophages can withhold Mn (and Fe) from fungi in the phagolysosome [59], and neutrophils release abundant metal-binding calprotectin at sites of infection [64,65,66]. Moreover, total kidney and liver Mn drop in response to tissue invasion by C. albicans [43••, 67••].
While a role for Mn in fungal pathogenesis is now established, there are still many unknowns. For example, how do pathogenic fungi control Mn homeostasis? Transporters for Zn, Fe, and Cu in pathogenic fungi are regulated at the mRNA level by transcription factors that sense these metals [3,4,5], but there is no known fungal transcription factor that senses and responds to Mn. Based on the Irving-Williams series [72], Mn is predicted to have a relatively low affinity for biological ligands compared to other metals, and a Mn-specific trans-regulator may not be thermodynamically feasible. Rather than transcription, Mn transport in pathogenic fungi may be regulated in the same fashion as described for S. cerevisiae, namely by post-translational control of NRAMP transporter protein localization and turnover [16, 17, 19, 22,23,24,25,26]. Future studies are needed to understand how pathogenic fungi adapt to changes in environmental Mn, whether this occurs by regulating Mn transport and trafficking or by modulating the usage of Mn as enzymatic co-factor. There is also still much to be learned on how the host controls Mn availability to the fungus during infection. What is the mechanism underlying the total drop in kidney and liver Mn when C. albicans invades these tissues? With the availability of mouse mutants for Mn transport, an understanding may be imminent.
Lastly, the role of Mn in fungal infections is expected to have important implications regarding the increasing rise in fungal infections. Recent estimations show human fungal infections now affecting ~ 300 million people annually, with over 1 million deaths worldwide [73]. Fungal infections can be challenging to diagnose, and with a rising incidence of drug resistance, many fungal infections are difficult to treat [74]. Of particular concern is the recent emergence of new fungal pathogens, such as Cryptococcus gattii and the multidrug-resistant Candida auris [75,76,77]. Since fungi are also pathogens for non-human animals and plants, fungal infections are greatly impactful in agriculture and the ecosystem [78, 79]. There is clearly an urgent need for an improved understanding of fungal disease and for the development of novel therapeutic avenues. Could the fungal reliance on Mn represent an Achilles’ heel worth exploring? Future studies should shed light on ways to exploit fungal dependence on Mn and other metals in tackling infectious fungi.
Data Availability
No datasets were generated or analyzed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Waldron KJ, Rutherford JC, Ford D, Robinson NJ. Metalloproteins and metal sensing. Nature. 2009;460(7257):823–30. https://doi.org/10.1038/nature08300nature08300.
• Murdoch CC, Skaar EP. Nutritional immunity: the battle for nutrient metals at the host-pathogen interface. Nat Rev Microbiol. 2022;20(11):657–70. https://doi.org/10.1038/s41579-022-00745-6. Comprehensive review on nutritional immunity involving metals including manganese.
Wilson D. Chapter two - the role of zinc in the pathogenicity of human fungal pathogens. In: Gadd GM, Sariaslani S, editors. Advances in applied microbiology. Academic Press; 2021. p. 35–61.
Gupta M, Outten CE. Iron-sulfur cluster signaling: the common thread in fungal iron regulation. Curr Opin Chem Biol. 2020;55:189–201. https://doi.org/10.1016/j.cbpa.2020.02.008.
Smith AD, Logeman BL, Thiele DJ. Copper acquisition and utilization in fungi. Annu Rev Microbiol. 2017;71:597–623. https://doi.org/10.1146/annurev-micro-030117-020444.
Sheng Y, Abreu IA, Cabelli DE, Maroney MJ, Miller AF, Teixeira M, et al. Superoxide dismutases and superoxide reductases. Chem Rev. 2014;114(7):3854–918. https://doi.org/10.1021/cr4005296.
Culbertson EM, Bruno VM, Cormack BP, Culotta VC. Expanded role of the Cu-sensing transcription factor Mac1p in Candida albicans. Mol Microbiol. 2020;114(6):1006–18. https://doi.org/10.1111/mmi.14591.
Li CX, Gleason JE, Zhang SX, Bruno VM, Cormack BP, Culotta VC. Candida albicans adapts to host copper during infection by swapping metal cofactors for superoxide dismutase. Proc Natl Acad Sci U S A. 2015;112(38):E5336–42. https://doi.org/10.1073/pnas.1513447112.
Avila DS, Puntel RL, Aschner M. Manganese in health and disease. Met Ions Life Sci. 2013;13:199–227. https://doi.org/10.1007/978-94-007-7500-8_7.
Bates S, Hughes HB, Munro CA, Thomas WP, MacCallum DM, Bertram G, et al. Outer chain N-glycans are required for cell wall integrity and virulence of Candida albicans. J Biol Chem. 2006;281(1):90–8. https://doi.org/10.1074/jbc.M510360200.
Bai C, Xu XL, Chan FY, Lee RT, Wang Y. MNN5 encodes an iron-regulated alpha-1,2-mannosyltransferase important for protein glycosylation, cell wall integrity, morphogenesis, and virulence in Candida albicans. Eukaryot Cell. 2006;5(2):238–47. https://doi.org/10.1128/ec.5.2.238-247.2006.
Jomova K, Makova M, Alomar SY, Alwasel SH, Nepovimova E, Kuca K, et al. Essential metals in health and disease. Chem Biol Interact. 2022;367: 110173. https://doi.org/10.1016/j.cbi.2022.110173.
•• Nicastro R, Gaillard H, Zarzuela L, Péli-Gulli MP, Fernández-García E, Tomé M, et al. Manganese is a physiologically relevant TORC1 activator in yeast and mammals. Elife. 2022;11. https://doi.org/10.7554/eLife.80497. First to show a role for Mn in fungal TOR signaling.
• Cellier MFM. Nramp: Deprive and conquer? Front Cell Dev Biol. 2022;10:988866. https://doi.org/10.3389/fcell.2022.988866. Role for NRAMP transporters in nutritional immunity including manganese.
West AH, Clark DJ, Martin J, Neupert W, Hart FU, Horwich AL. Two related genes encoding extremely hydrophobic proteins suppress a lethal mutation in the yeast mitochondrial processing enhancing protein. J Biol Chem. 1992;267:24625–33.
Liu XF, Culotta VC. Post-translational control of Nramp metal transport in yeast: role of metal ions and the BSD2 gene. J Biol Chem. 1999;274:4863–8.
Liu XF, Supek F, Nelson N, Culotta VC. Negative control of heavy metal uptake by the Saccharomyces cerevisiae BSD2 gene. J Biol Chem. 1997;272:11763–9.
Luk E, Culotta VC. Manganese superoxide dismutase in S. cerevisiae acquires its metal co-factor through a pathway involving the Nramp metal transproter, Smf2p. J Biol Chem. 2001;276:47556–62.
Liu XF, Culotta VC. Mutational analysis of Saccharomyces cerevisiae Smf1p, a member of the Nramp family of metal transporters. J Mol Biol. 1999;289(4):885–91. https://doi.org/10.1006/jmbi.1999.2815.
Jensen LT, Ajua-Alemanji M, Culotta VC. The Saccharomyces cerevisiae high affinity phosphate transporter encoded by PHO84 also functions in manganese homeostasis. J Biol Chem. 2003;278:42036–40.
Trilisenko L, Zvonarev A, Valiakhmetov A, Penin AA, Eliseeva IA, Ostroumov V, et al. The reduced level of inorganic polyphosphate mobilizes antioxidant and manganese-resistance systems in Saccharomyces cerevisiae. Cells. 2019;8(5). https://doi.org/10.3390/cells8050461.
Stimpson HE, Lewis MJ, Pelham HR. Transferrin receptor-like proteins control the degradation of a yeast metal transporter. Embo j. 2006;25(4):662–72. https://doi.org/10.1038/sj.emboj.7600984.
Sullivan JA, Lewis MJ, Nikko E, Pelham HR. Multiple interactions drive adaptor-mediated recruitment of the ubiquitin ligase rsp5 to membrane proteins in vivo and in vitro. Mol Biol Cell. 2007;18(7):2429–40. https://doi.org/10.1091/mbc.e07-01-0011.
Nikko E, Sullivan JA, Pelham HR. Arrestin-like proteins mediate ubiquitination and endocytosis of the yeast metal transporter Smf1. EMBO Rep. 2008;9(12):1216–21. https://doi.org/10.1038/embor.2008.199.
Nikko E, Pelham HR. Arrestin-mediated endocytosis of yeast plasma membrane transporters. Traffic. 2009;10(12):1856–67. https://doi.org/10.1111/j.1600-0854.2009.00990.x.
Jensen LT, Carroll MC, Hall MD, Harvey CJ, Beese SE, Culotta VC. Down-regulation of a manganese transporter in the face of metal toxicity. Mol Biol Cell. 2009;20(12):2810–9. https://doi.org/10.1091/mbc.E08-10-1084.
Durr G, Strayle J, Plemper R, Elbs S, Klee SK, Catty P, et al. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Molec Biol Cell. 1998;9:1149–62.
Rudolph HK, Antebi A, Fink GR, Buckley CM, Dorman TE, LeVitre J, et al. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca+2-ATPase family. Cell. 1989;58:133–45.
Lapinskas PJ, Cunningham KW, Liu XF, Fink GR, Culotta VC. Mutations in PMR1 suppress oxidative damage in yeast cells lacking superoxide dismutase. Mol Cell Biol. 1995;15:1382–8.
Thines L, Deschamps A, Sengottaiyan P, Savel O, Stribny J, Morsomme P. The yeast protein Gdt1p transports Mn(2+) ions and thereby regulates manganese homeostasis in the Golgi. J Biol Chem. 2018;293(21):8048–55. https://doi.org/10.1074/jbc.RA118.002324.
• Deschamps A, Thines L, Colinet AS, Stribny J, Morsomme P. The yeast Gdt1 protein mediates the exchange of H(+) for Ca(2+) and Mn(2+) influencing the Golgi pH. J Biol Chem. 2023;299(5):104628. https://doi.org/10.1016/j.jbc.2023.104628. Description of the mechanism of action of the Golgi Mn and Ca transporter GDT1.
Luk E, Carroll M, Baker M, Culotta VC. Manganese activation of superoxide dismutase 2 in Saccharomyces cerevisiae requires MTM1, a member of the mitochondrial carrier family. Proc Natl Acad Sci USA. 2003;100:10353–7.
Yang M, Cobine PA, Molik S, Naranuntarat A, Lill R, Winge DR, et al. The effects of mitochondrial iron homeostasis on cofactor specificity of superoxide dismutase 2. EMBO J. 2006;25:1775–83.
Naranuntarat A, Jensen LT, Pazicni S, Penner-Hahn JE, Culotta VC. The interaction of mitochondrial iron with manganese superoxide dismutase. J Biol Chem. 2009;284(34):22633–40. https://doi.org/10.1074/jbc.M109.026773.
Wolff NA, Garrick MD, Zhao L, Garrick LM, Ghio AJ, Thévenod F. A role for divalent metal transporter (DMT1) in mitochondrial uptake of iron and manganese. Sci Rep. 2018;8(1):211. https://doi.org/10.1038/s41598-017-18584-4.
Wolff NA, Garrick LM, Zhao L, Garrick MD, Thévenod F. Mitochondria represent another locale for the divalent metal transporter 1 (DMT1). Channels (Austin). 2014;8(5):458–66. https://doi.org/10.4161/19336950.2014.956564.
• Bozzi AT, Gaudet R. Molecular mechanism of Nramp-family transition metal transport. J Mol Biol. 2021;433(16):166991. https://doi.org/10.1016/j.jmb.2021.166991. Review showing functions of NRAMP transporters including interactions with the mitochondria and delivery of metals to mitochondria including Mn and Fe.
Diss L, Blaudez D, Gelhaye E, Chalot M. Genome-wide analysis of fungal manganese transporters, with an emphasis on Phanerochaete chrysosporium. Environ Microbiol Rep. 2011;3(3):367–82. https://doi.org/10.1111/j.1758-2229.2010.00235.x.
Agranoff D, Collins L, Kehres D, Harrison T, Maguire M, Krishna S. The Nramp orthologue of Cryptococcus neoformans is a pH-dependent transporter of manganese, iron, cobalt and nickel. Biochem J. 2005;385(Pt 1):225–32. https://doi.org/10.1042/bj20040836.
Fejes B, Ouedraogo JP, Fekete E, Sándor E, Flipphi M, Soós Á, et al. The effects of external Mn(2+) concentration on hyphal morphology and citric acid production are mediated primarily by the NRAMP-family transporter DmtA in Aspergillus niger. Microb Cell Fact. 2020;19(1):17. https://doi.org/10.1186/s12934-020-1286-7.
Fan J, Zhang H, Li Y, Chen Z, Chen T, Zeng B, et al. Identification and characterization of Nramp transporter AoNramp1 in Aspergillus oryzae. 3 Biotech. 2021;11(10):452. https://doi.org/10.1007/s13205-021-02998-z.
Xu N, Dong Y, Cheng X, Yu Q, Qian K, Mao J, et al. Cellular iron homeostasis mediated by the Mrs4-Ccc1-Smf3 pathway is essential for mitochondrial function, morphogenesis and virulence in Candida albicans. Biochim Biophys Acta. 2014;1843(3):629–39. https://doi.org/10.1016/j.bbamcr.2013.12.009.
•• Wildeman AS, Patel NK, Cormack BP, Culotta VC. The role of manganese in morphogenesis and pathogenesis of the opportunistic fungal pathogen Candida albicans. PLoS Pathog. 2023;19(6):e1011478. https://doi.org/10.1371/journal.ppat.1011478. First evidence showing a role for a Mn-specific fungal NRAMP in fungal pathogenesis of a mammalian host and a host response involving lowering of kidney Mn.
• Henry M, Khemiri I, Tebbji F, Abu-Helu R, Vincent AT, Sellam A. Manganese homeostasis modulates fungal virulence and stress tolerance in Candida albicans. bioRxiv. 2023:2023.10.12.562042. https://doi.org/10.1101/2023.10.12.562042. Fungal Mn NRAMP transporter important for pathogenesis in an insect model.
Evangelinos M, Martzoukou O, Chorozian K, Amillis S, Diallinas G. BsdA(Bsd2) -dependent vacuolar turnover of a misfolded version of the UapA transporter along the secretory pathway: prominent role of selective autophagy. Mol Microbiol. 2016;100(5):893–911. https://doi.org/10.1111/mmi.13358.
Liu NN, Uppuluri P, Broggi A, Besold A, Ryman K, Kambara H, et al. Intersection of phosphate transport, oxidative stress and TOR signalling in Candida albicans virulence. PLoS Pathog. 2018;14(7): e1007076. https://doi.org/10.1371/journal.ppat.1007076.
Bates S, MacCallum DM, Bertram G, Munro CA, Hughes HB, Buurman ET, et al. Candida albicans Pmr1p, a secretory pathway P-type Ca2+/Mn2+-ATPase, is required for glycosylation and virulence. J Biol Chem. 2005;280(24):23408–15.
•• Qu S, Chi SD, He ZM. The development of Aspergillus flavus and biosynthesis of aflatoxin B1 are regulated by the Golgi-localized Mn(2+) transporter Pmr1. J Agric Food Chem. 2024;72(2):1276–91. https://doi.org/10.1021/acs.jafc.3c06964. Evidence supporting a role for Golgi Mn in morphogenesis and pathogenesis in a filamentous fungus.
• Wu C, Guo Z, Zhang M, Chen H, Peng M, Abubakar YS, et al. Golgi-localized calcium/manganese transporters FgGdt1 and FgPmr1 regulate fungal development and virulence by maintaining Ca2+ and Mn2+ homeostasis in Fusarium graminearum. Environ Microbiol. 2022;24(10):4623–40. https://doi.org/10.1111/1462-2920.16128. Golgi Mn and Ca transporter are important for fungal morphogenesis and pathogenesis.
Briard B, Fontaine T, Kanneganti TD, Gow NAR, Papon N. Fungal cell wall components modulate our immune system. Cell Surf. 2021;7: 100067. https://doi.org/10.1016/j.tcsw.2021.100067.Thefungalcellwallplaysaroleinimmunerecognition.
Yadav B, Mora-Montes HM, Wagener J, Cunningham I, West L, Haynes K, et al. Differences in fungal immune recognition by monocytes and macrophages: N-mannan can be a shield or activator of immune recognition. Cell Surf. 2020;6: 100042. https://doi.org/10.1016/j.tcsw.2020.100042.
Hopke A, Brown AJP, Hall RA, Wheeler RT. Dynamic fungal cell wall architecture in stress adaptation and immune evasion. Trends Microbiol. 2018;26(4):284–95. https://doi.org/10.1016/j.tim.2018.01.007.
Pinchai N, Juvvadi PR, Fortwendel JR, Perfect BZ, Rogg LE, Asfaw YG, et al. The Aspergillus fumigatus P-type Golgi apparatus Ca2+/Mn2+ ATPase PmrA is involved in cation homeostasis and cell wall integrity but is not essential for pathogenesis. Eukaryot Cell. 2010;9(3):472–6. https://doi.org/10.1128/ec.00378-09.
Nguyen QB, Kadotani N, Kasahara S, Tosa Y, Mayama S, Nakayashiki H. Systematic functional analysis of calcium-signalling proteins in the genome of the rice-blast fungus, Magnaporthe oryzae, using a high-throughput RNA-silencing system. Mol Microbiol. 2008;68(6):1348–65. https://doi.org/10.1111/j.1365-2958.2008.06242.x.
López-Lorca VM, Molina-Luzón MJ, Ferrol N. Characterization of the NRAMP gene family in the arbuscular mycorrhizal fungus Rhizophagus irregularis. J Fungi (Basel). 2022;8(6). https://doi.org/10.3390/jof8060592.
Shafeeq S, Pannanusorn S, Elsharabasy Y, Ramírez-Zavala B, Morschhäuser J, Römling U. Impact of manganese on biofilm formation and cell morphology of Candida parapsilosis clinical isolates with different biofilm forming abilities. FEMS Yeast Res. 2019;19(6). https://doi.org/10.1093/femsyr/foz057.
Jabado N, Jankowski A, Dougaparsad S, Picard V, Grinstein S, Gros P. Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J Exp Med. 2000;192(9):1237–48. https://doi.org/10.1084/jem.192.9.1237.
Vidal S, Tremblay ML, Govoni G, Gauthier S, Sebastiani G, Malo D, et al. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J Exp Med. 1995;182(3):655–66. https://doi.org/10.1084/jem.182.3.655.
Blasi E, Colombari B, Mucci A, Cossarizza A, Radzioch D, Boelaert JR, et al. Nramp1 gene affects selective early steps in macrophage-mediated anti-cryptococcal defense. Med Microbiol Immunol. 2001;189(4):209–16. https://doi.org/10.1007/s004300100066.
Hood MI, Skaar EP. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol. 2012;10(8):525–37. https://doi.org/10.1038/nrmicro2836.
Nakashige TG, Zhang B, Krebs C, Nolan EM. Human calprotectin is an iron-sequestering host-defense protein. Nat Chem Biol. 2015;11(10):765–71. https://doi.org/10.1038/nchembio.1891.
Brophy MB, Nakashige TG, Gaillard A, Nolan EM. Contributions of the S100A9 C-terminal tail to high-affinity Mn(II) chelation by the host-defense protein human calprotectin. J Am Chem Soc. 2013;135(47):17804–17. https://doi.org/10.1021/ja407147d.
Damo SM, Kehl-Fie TE, Sugitani N, Holt ME, Rathi S, Murphy WJ, et al. Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proc Natl Acad Sci U S A. 2013;110(10):3841–6. https://doi.org/10.1073/pnas.1220341110.
Besold AN, Gilston BA, Radin JN, Ramsoomair C, Culbertson EM, Li CX, et al. Role of calprotectin in withholding zinc and copper from Candida albicans. Infect Immun. 2018;86(2). https://doi.org/10.1128/iai.00779-17.
Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5(10): e1000639. https://doi.org/10.1371/journal.ppat.1000639.
Clark HL, Jhingran A, Sun Y, Vareechon C, de Jesus CS, Skaar EP, et al. Zinc and manganese chelation by neutrophil S100A8/A9 (calprotectin) limits extracellular Aspergillus fumigatus hyphal growth and corneal infection. J Immunol. 2016;196(1):336–44. https://doi.org/10.4049/jimmunol.1502037.
•• Sunuwar L, Tomar V, Wildeman A, Culotta V, Melia J. Hepatobiliary manganese homeostasis is dynamic in the setting of inflammation or infection in mice. Faseb j. 2023;37(9):e23123. https://doi.org/10.1096/fj.202300539R. First to show a host response involving loss of liver Mn during fungal infection.
• Hall SC, Smith DR, Dyavar SR, Wyatt TA, Samuelson DR, Bailey KL, et al. Critical role of zinc transporter (ZIP8) in myeloid innate immune cell function and the host response against bacterial pneumonia. J Immunol. 2021;207(5):1357–70. https://doi.org/10.4049/jimmunol.2001395. A mammalian Mn (and Zn) transporter that responds to infection and inflammation.
Sunuwar L, Frkatović A, Sharapov S, Wang Q, Neu HM, Wu X, et al. Pleiotropic ZIP8 A391T implicates abnormal manganese homeostasis in complex human disease. JCI Insight. 2020;5(20). https://doi.org/10.1172/jci.insight.140978.
Frohner IE, Bourgeois C, Yatsyk K, Majer O, Kuchler K. Candida albicans cell surface superoxide dismutases degrade host-derived reactive oxygen species to escape innate immune surveillance. Mol Microbiol. 2009;71(1):240–52.
Zacchi LF, Gomez-Raja J, Davis DA. Mds3 regulates morphogenesis in Candida albicans through the TOR pathway. Mol Cell Biol. 2010;30(14):3695–710. https://doi.org/10.1128/mcb.01540-09.
Irving H, Williams RJP. 637. The stability of transition-metal complexes. J Chem Soc. 1953;3192–210. https://doi.org/10.1039/JR9530003192.
Almeida F, Rodrigues ML, Coelho C. The still underestimated problem of fungal diseases worldwide. Front Microbiol. 2019;10:214. https://doi.org/10.3389/fmicb.2019.00214.
Denning DW, Bromley MJ. Infectious disease. How to bolster the antifungal pipeline. Science. 2015;347(6229):1414–6. https://doi.org/10.1126/science.aaa6097.
Casadevall A, Kontoyiannis DP, Robert V. On the emergence of Candida auris: climate change, azoles, swamps, and birds. mBio. 2019;10(4). https://doi.org/10.1128/mBio.01397-19.
Du H, Bing J, Hu T, Ennis CL, Nobile CJ, Huang G. Candida auris: epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020;16(10): e1008921. https://doi.org/10.1371/journal.ppat.1008921.
Dixit A, Carroll SF, Qureshi ST. Cryptococcus gattii: an emerging cause of fungal disease in North America. Interdiscip Perspect Infect Dis. 2009;2009: 840452. https://doi.org/10.1155/2009/840452.
Savary S, Ficke A, Aubertot J-N, Hollier C. Crop losses due to diseases and their implications for global food production losses and food security. Food Security. 2012;4(4):519–37. https://doi.org/10.1007/s12571-012-0200-5.
Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, et al. Emerging fungal threats to animal, plant and ecosystem health. Nature. 2012;484(7393):186–94. https://doi.org/10.1038/nature10947.
Funding
ASW and VCC are supported by the National Institutes of Health Grants R21AI154726 and R35GM136644.
Author information
Authors and Affiliations
Contributions
AW and VC wrote the main text and VC prepared figures. Both authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
All studies performed by the authors that were reported to involve animals have been published and were compliant with Johns Hopkins Institutional Animal Care and Use Committee and with the guidelines of the Animal Welfare Act and Public Health Service Policy.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Fungal Pathogenesis
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wildeman, A.S., Culotta, V.C. Nutritional Immunity and Fungal Pathogens: A New Role for Manganese. Curr Clin Micro Rpt 11, 70–78 (2024). https://doi.org/10.1007/s40588-024-00222-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40588-024-00222-z