Abstract
Long-term glucocorticoid (GC) therapy is frequently indicated to treat autoimmune and chronic inflammatory diseases in daily clinical practice. Two of the most devastating untoward effects are bone loss and fractures. Doses as low as 2.5 mg of prednisone for more than 3 months can impair bone integrity. Population at risk is defined based on the dose and duration of GC therapy and should be stratified according to FRAX (Fracture Risk Assessment Tool), major osteoporotic fracture, prior fractures, and bone mineral density values (BMD). General measures include to prescribe the lowest dose of GC to control the underlying disease for the shortest possible time, maintain adequate vitamin D levels and calcium intake, maintain mobility, and prescribe a bone acting agent in patients at high risk of fracture. These agents include oral and intravenous bisphosphonates, denosumab, and teriparatide.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucocorticoid (GC) therapy is widely used in daily clinical practice to treat several diseases, such as chronic arthritis, systemic lupus, polymyalgia rheumatica, allergies, chronic pulmonary entities (asthma, emphysema), and inflammatory bowel diseases, among others. Most frequent prescribers are internal medicine specialists, rheumatologists, general/family practitioners, gastroenterologists, pulmonologists, and dermatologists.
GC is associated with several untoward effects, such as fluid retention, body fat redistribution, hyperglycemia, dyslipidemia, cataracts, and bone loss with an increased risk of fractures that has been reported to occur with doses as low as 2.5–7.5 mg of prednisone or equivalent daily for more than 3 months. This increased risk of fractures occurs at higher bone mineral density (BMD) values than occur in postmenopausal osteoporosis.
High risks of fractures are related to older age and prior fragility fractures. General measures include to assure an adequate calcium intake (1000–1200 mg/day), levels of vitamin D > 30 ng/ml, maintain mobility, prescribe the lowest GC dose for the shortest time to control the underlying disease and prevent falls. Risk assessment based on the clinical evaluation, bone mineral density (BMD), fracture risk factors, and FRAX will define the need of pharmacological interventions. In this review, we will discuss all these concepts.
Epidemiology
GCs are frequently used for the treatment of a variety of inflammatory and autoimmune diseases. A meta-analysis showed that 3% of the population aged 50 years or more have ever been treated with GCs, and this percentage increases to 5.2% among those who are 80 years old and older [1]. In the Global Longitudinal Study of Osteoporosis in Women, a multinational population-based observational study of 60,393 postmenopausal women who had visited their primary care practice within the preceding 2 years, up to 3.1% were currently treated with oral GCs [2]. GC therapy induces bone loss, and is associated with an increased risk for vertebral and nonvertebral fractures [3].
Bone loss starts within months after initiating GC treatment, and is more pronounced in trabecular bone, which is predominantly present in spine and ribs [3, 4]. Earlier studies have demonstrated that GC-induced bone loss is biphasic, with a rapid initial phase of 3–5% in the first year of GC use, followed by a slower phase of 0.5–1.0% bone loss per year during continued GC therapy [5,6,7]. Besides the adverse effects of GCs on bone mass, the underlying disease for which GCs were prescribed may also contribute to bone loss.
In a meta-analysis of data from 42,500 men and women, previous or current GC use was associated with an increased risk of fractures at all ages, from 50 years old upwards, and was similar between men and women [1]. The increased fracture risk during GC therapy was demonstrated to be dose dependent, and is increased even with low doses of prednisolone (2.5–7.5 mg daily) [3, 8, 9]. Van Staa et al. demonstrated that hip fracture risk is 1.77 [95% confidence interval (CI) 1.55–2.02] times increased in patients taking a standardized daily dose of 2.5–7.5 mg prednisolone, rising to 2.27 (1.94–2.66) times increased at doses of 7.5 mg daily or greater [8]. The relative risk of vertebral fractures is particularly increased during GC therapy, ranging from 1.55 (1.20–2.01) in patients using less than 2.5 mg prednisolone daily rising to 5.18 (4.25–6.31) in those using 7.5 mg daily or greater [8]. After initiation of GC therapy, a rapid onset of the increased risk for fractures has been reported. A large, population-based cohort study in adults aged < 64 years from the United States demonstrated an increase in fracture incidence [incidence rate ratio 1.87 (1.69–2.07)] within 30 days of initiation of GC therapy [10], which underlines the importance of initiating anti-osteoporotic therapy when prescribing GCs.
Fracture risk in GIOP also depends on cumulative GC dose; a recent study from Denmark demonstrated that a cumulative GC dosage ≥ 1000 mg is more strongly associated with fractures compared with a smaller cumulative dose (< 1000 mg) [11]. For hip fractures a 60% increased risk was observed for cumulative GC dosage ≥ 1000 mg (adj. OR 1.64 [95% CI 1.54–1.74]) while a cumulative dosage < 1000 mg was associated with a 30% increased risk (adj.OR 1.28 [95% CI 1.14–1.44]). The risk for symptomatic vertebral fractures was 2.5-fold increased for cumulative GC dosage ≥ 1000 mg (adj.OR 2.57 [95% CI 1.49–2.17]) while a 1.8-fold increased risk for cumulative GC dosage < 1000 mg (adj.OR 1.80 [95% CI 2.30–2.87]) was demonstrated [11].
Most studies demonstrating an association between GC use and fracture risk were performed in subjects receiving chronic daily oral GC therapy, but an increased fracture risk has also been reported from patients on intermittent high-dose oral GC treatment [12].
After discontinuation of GC therapy, fracture risk decreases gradually towards baseline, and it is; therefore, supposed to be partially reversible [12]. However, a residual increased risk remains, which might be related to the underlying disease for which GC therapy was initiated, which is most frequently a chronic systemic inflammatory disorder, and might result from previous GC therapy. Previous studies have demonstrated that even slightly increased levels of systemic inflammation are associated with increased bone loss and may in turn increase fracture risk [13].
GIOP and the associated increased occurrence of fractures are a major public health problem, causing functional impairment, mortality, and significant costs. A recent study on costs of adverse events related to GC use in patients with rheumatoid arthritis established an incremental cost of $3,201 per fracture at cumulative doses greater than 1800 mg [14].
Pathogenesis of GIOP: novel insights
Direct effects of glucocorticoids on bone
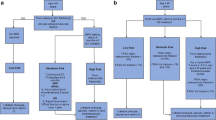
In recent years, several studies have provided more insight into the mechanisms involved in the development of GIOP, including increased apoptosis of mature osteoblasts and osteocytes, impaired differentiation of osteoblasts, and an increased lifespan of osteoclasts [15] (see Fig. 1). The increased apoptosis of osteoblasts leads to reduced bone formation and an impaired response to bone damage, which may lead to reduced bone strength [16]. GCs suppress the Wnt signaling pathway, which plays an important role in bone metabolism and especially in osteoblastogenesis. Recent animal in vivo and in vitro studies demonstrated a central role of the Wnt pathway in the pathogenesis of GIOP. Wnt16 expression is suppressed by GCs in a time-dependent and dose-dependent manner [17], while overexpression of Wnt16 partly protects against GC-induced bone loss [18].
GCs suppress the Wnt signaling pathway by increasing the production of Wnt pathway inhibitors, such as dickkopf-1 (Dkk-1) and sclerostin [19, 20], leading to decreased osteogenesis and an impaired capacity to bone regeneration [21]. In addition, inhibition of DKK-1 reduces the GC-induced suppression of osteoblast differentiation [22]. Furthermore, a high dose of GCs was demonstrated to induce a shift from mesenchymal stem cells, the precursor cells of osteoblasts, to differentiate towards adipocytes instead of osteoblasts [23]. In contrast to the increased apoptosis of osteoblasts and osteocytes, the lifespan of osteoclasts is extended during GC therapy due to an upregulation of receptor activator for nuclear factor-κB ligand (RANKL) and suppression of osteoprotegerin (OPG) [24], which may be associated with impaired osteoclast function.
In the last few years, more insight has been gained in the role of the Notch signaling pathway in the pathogenesis of GIOP. The Notch pathway consists of four transmembrane receptors that control the differentiation of osteoblasts and other cell lines. Recent studies demonstrated that GCs suppress the expression of Notch target genes in osteoblasts, which leads to reduced bone formation [25, 26]. Interestingly, the effect of GCs on the Notch pathway was demonstrated to be dose dependent in experimental studies. Osteoblast cultures exposed to physiological GC doses showed increased osteoblast viability, whereas high dose GCs induced osteoblast apoptosis [27]. Moreover, another animal study demonstrated GCs specifically induce bone loss at the femoral head and not at the distal femur, this finding was suggested as an explanation for the development of avascular necrosis of the femoral head in association with GC therapy [28].
Indirect effects of GCs on bone
Apart from direct effects on osteoblasts, osteocytes, and osteoclasts, GCs have indirect effects on muscles, calcium metabolism, and bone mass. An adverse effect of GC therapy on muscle mass and muscle strength has been demonstrated in an early study [4]. GC-induced muscle atrophy is attributed to both increased muscle protein breakdown and decreased muscle protein synthesis, which is supposed to result from inhibition of insulin-like growth factor I, a muscle anabolic growth factor, and by stimulating muscles to produce the muscle catabolic growth factor myostatin [29]. The reduced muscle mass and muscle strength associated with GC use has been related with poor structural bone parameters, decreased bending strength, and impaired balance, which in turn indirectly increase the risk for fractures by enhancing fall risk [30].
In addition, GCs impair bone metabolism by inhibition of the intestinal calcium absorption and by inhibiting the renal tubular calcium reabsorption, which may lead to hypocalcemia and subsequently hyperparathyroidism [31]. Furthermore, GCs were demonstrated to impair bone mineralization by transrepression of osteocalcin and collagen, two important bone matrix proteins [32].
Risk assessment and candidates for pharmacologic therapy
GC therapy is associated with multiple risk factors for osteoporosis and fractures, including substantial effects on BMD, alterations in bone quality that are not detected by DXA scan and increased falls risk. The fracture risk depends on the GC dosage, the duration of therapy and cumulative dosage [33,34,35,36].
An initial baseline evaluation of the fracture risk is recommended when oral glucocorticoid therapy was considered for 3 months or longer. In most guidelines, the daily glucocorticoid dose for intervention threshold is generally 7.5 mg daily of prednisolone or equivalent. In the absence of a baseline evaluation, the fracture risk assessment should be performed as soon as possible, preferably within the first 6 months of starting the glucocorticoid treatment, and the risk must be reassessed periodically [35, 37, 38].
The initial fracture risk assessment should include a full clinical history including the dose and duration of glucocorticoid therapy, evaluation of previous low-energy fractures, evaluation of falls, alcohol intake, smoking, height loss, secondary causes of bone loss, family history of osteoporosis, and hip fracture. Other risk factors and comorbidities should be assessed and ensure adequate calcium intake and correct vitamin D deficiency [37, 38].
Vertebral fracture assessment (VFA) or lateral spinal X-ray is recommended to assess vertebral deformities by the International Society for Clinical Densitometry when glucocorticoid therapy equivalent to ≥ 5 mg of prednisone or equivalent per day for ≥ 3 months is indicated [39]. However, we must consider that at any time in patients with GIOP who start anti-osteoporotic treatment, the radiological study of the spine (antero-posterior and lateral) would help to identify pre-existing or incident vertebral fractures and it would also allow to identify additional pathology (e.g., osteoarthritis).
It is important to consider that fractures occur with a higher-than-expected BMD in glucocorticoid treated patients and bone loss does not fully explain the high fracture risk observed in glucocorticoid treated patients. BMD has certain limitations as a risk fracture assessment tool due to bone microarchitectural impairment rather than a reduced bone density in glucocorticoid users [33,34,35,36,37,38,39,40].
Trabecular bone score (TBS) is a gray-level texture measurement that can be applied to lumbar spine DXA images and provide an indirect quantified information of trabecular bone microarchitecture and has predictive value for fracture independent of BMD [41]. Several cross-sectional and retrospective studies showed an association between TBS and daily glucocorticoid dose and TBS have a higher diagnostic accuracy than BMD for identifying glucocorticoid-related fractures [42,43,44]. In addition, TBS also appears to discriminate the treatment effect of an anabolic versus an anti-resorptive agent in patients receiving glucocorticoids [45]. TBS in combination with BMD as a complementary measure, may be a useful tool to assess bone status in GIOP; however, TBS has no role in the identification of patients who should be treated, and further studies are needed to confirm the role of TBS in the treatment and monitoring of GIOP.
FRAX is a computer-based algorithm that calculates the risk of suffering fractures in the next 10 years (10-year probability) of a major osteoporotic fracture (hip, clinical spine, humerus, or wrist fracture) and hip fracture, based on the individual analysis of clinical risk factors with and without femoral neck BMD for each patient, among subjects who are at least 40 years of age. [46, 47]. The FRAX algorithm includes only a dichotomous variable for oral glucocorticoids (answer as yes or no) if patient is currently exposed to oral glucocorticoids or has been exposed for more than 3 months at a dose of prednisolone of 5 mg daily or more (or equivalent doses of other glucocorticoids). However, FRAX does not consider treatment duration, dosage concurrently or other risk factors for fractures in glucocorticoids users such as the severity of the underlying inflammatory disease, vertebral deformities, or fall risk [34, 46, 48]. Another limitation of the FRAX is the use of BMD at the femoral neck instead of at the lumbar spine, since GC have a more negative effect on trabecular bone in the spine [49].
FRAX may underestimate fracture risk in patients receiving higher doses and overestimate risk in those receiving lower doses of GC [50]. A modification of the FRAX algorithm has been developed to assesses the possible impact of different doses of glucocorticoids. Patients receiving low dose prednisolone (< 2.5 mg daily or equivalent), the probability of major fracture is decreased by 20%, for medium doses (2.5–7.5 mg of prednisolone daily) no modification is necessary and unadjusted FRAX can be used. For high doses (> 7.5 mg of prednisolone daily), the fracture risk should be increased by about 15% [48].
Several national and professional societies guidelines for the prevention and management of GIOP are available, most of which are primarily aimed for postmenopausal women and men ≥ 50 years. Most guidelines address users of long-term oral glucocorticoids (≥ 3 months), and although the daily threshold dose varies among guidelines, it has consistently proposed a dose threshold between 5 and 7.5 mg daily of prednisolone or equivalent [37, 38, 50, 51].
BMD measurement is recommended in all patients starting oral glucocorticoid therapy, but at least within 6 months after the initiation of therapy [49]. The BMD threshold for pharmacological intervention is generally a T score between − 1.0 and − 1.5. FRAX assessment has already been included in most guidelines at different steps for risk stratification and treatment decision. A common recommendation in all guidelines in that bone-protective therapies should be started at the same time as GC therapy is initiated [36, 38, 51]
Most guidelines concern GIOP management in postmenopausal women and in men ≥ 50 years old, but there are no tools available for the prediction of absolute fracture risk in patient younger than 40 years old and in children [38, 52].
The 2017 American College of Rheumatology (ACR) guidelines provides recommendations in accordance with a subject’s individual profile and categorizes patients into two groups by age (adults < 40 years and > 40 years of age), glucocorticoids status (initiating or continuing) and fracture risk. For patient´s over 40 years, the risk is categorized as low, moderate, or high, by history of prior osteoporotic fracture (s), BMD, and 10-year fracture risk calculated by adjusted FRAX® [38] (see Table 1).
In adults of 40 years of age ACR guidelines consider that there is a high risk of fracture if they had a prior osteoporotic fracture. The risk is moderate if they were expected to continue glucocorticoid treatment at ≥ 7.5 mg/day for ≥ 6 months and had either: a hip or spine BMD Z score of < − 3 or a rapid decline in hip or spine BMD equivalent to ≥ 10% in one year. Low fracture risk is considered when none of the above-mentioned risk factors other than GC treatment are present [38].
The recommendations between the different guides for bone-protective therapy have similarities but also some differences. For postmenopausal women and men ≥ 50 years, the International Osteoporosis Foundation (IOF)-European Calcified Tissue Society (ECTS) recommends bone-protective therapy in patients aged ≥ 70 years, those with previous fragility fracture or incident fragility fracture during GC therapy, prednisolone dose > 7.5 mg/day, BMD T score ≤ − 1.5 or adjusted FRAX® fracture probability above the intervention threshold of the general population. In premenopausal women and men aged < 50 years bone-protective therapy is recommended when GC use ≥ 3 months plus fragility fracture [37].
Risk stratification of fractures (adults < 40 years)
High | Moderate | Low |
|---|---|---|
History of osteoporotic fracture | Hip or spine Z score < − 3 or Rapid bone loss of ≥ 10% (at the hip or spine) over 1 year and Continuing GC treatment of ≥ 7.5 mg/day for ≥ 6 months | No of the above risk factors |
The UK National Osteoporosis Guideline Group (NOGG) suggests bone-protective therapy for women and men aged ≥ 70 years old, with a previous fragility fracture, or those who are taking large doses of glucocorticoids (≥ 7.5 mg/day prednisolone). These guidelines also recommend using the adjusted FRAX for assessing the risk in all other individuals [50].
As we have reviewed, there are similarities between the different guidelines, particularly regarding the dose of corticosteroids and the duration of treatment to recommend interventions, but risk stratification is different in some of these guidelines, particularly in the new ACR guideline risk stratification which is complex and very difficult to simplify (Table 2).
Choices of pharmacological therapy in glucocorticoid-induced osteoporosis
Bisphosphonates in GIOP
As stated above, chronic prednisolone use increases fracture risk. Therefore, it is important to decrease the global burden of fracture risk in GC treated patients. Fracture risk management should involve both nonpharmacological measures as pharmacological therapy. In general, all guidelines regarding GIOP agree that in GC treated patients with moderate to high risk for fractures bisphosphonates (BP) are first-line anti-osteoporotic drugs in preventing new fractures [37, 38, 50]. Nowadays, oral BP are widely used in daily clinical practice and alendronate and risedronate are the most common used oral BP and have an anti-resorptive mode of action mainly by direct inhibiting effects on osteoclasts [53]. Some years ago, Allen et al. performed a Cochrane systematic review, including 23 randomized trials and more than 2000 GIOP patients, on the efficacy of BP in GIOP as compared to calcium and vitamin D supplementation alone [54]. This study yielded an improvement of 3.5% [95% confidence interval (CI) 2.9–4.1] in BMD at lumbar level in the patient group treated with BP for a year, while GIOP patients in the control groups had a deterioration of BMD of approximately − 3.2% (95% CI − 8.1 to 1.7%). The minority of the included studies had a longer follow-up than 12 months, as only nine studies looked for a follow up duration with a maximum of 2 years. Combining the results of these studies with a longer follow up, a sustained effect of BP on lumbar BMD level in GIOP patients with a calculated BMD increase of 5.5% (95% CI 3.5–7.5%) was observed.
The abovementioned studies were performed in placebo-controlled design or in a design with calcium and vitamin D treated GIOP patients as controls. Nowadays, it is not ethical to treat GIOP without BP; therefore, modern studies must be performed with anti-osteoporotic medication like BP as active comparators. One of the landmark studies in the modern era in GIOP treatment is the Horizon trial, a 1-year randomized, double-blind noninferiority trial comparing the effectiveness of 5 mg zoledronic acid intravenous infusion once yearly with oral risedronate with approximately 415 patients in both treatment arms [7]. It was found that both zoledronic acid and risedronate increased BMD levels at lumbar spine, but the rise in BMD at lumbar spine level was statistically significantly larger in the zoledronate group compared with the risedronate group. Hence, zoledronic acid is at least noninferior in preventing bone loss in GIOP patients. The beneficial effects observed on lumbar BMD were also found for hip BMD in the systematic review; 1-year BP use increased BMD at hip level for approximately 2.1% (95% CI 1.5–2.7) and was sustained after 1.5–2 years [54].
The goal of preserving and improving BMD in GIOP patients is to prevent incident fractures. This question was also covered in the systematic review by Allen and colleagues. In this analysis approximately 1300 GIOP patients were included to calculate the vertebral risk reduction of BP. It was found that the incidence rate was 44 per 1000 in the BP group compared to 77 per 1000 patients (RR 0.57 95% CI 0.35–0.91) in the control group [54]. This observation is confirmed in more recent observational studies in oral BP (i.e., alendronate, risedronate, and etidronate) treated GIOP patients, as it was observed that these oral BP were able to decrease the incidence rate of vertebral fractures with approximately 40% (HR 0.6 95% CI 0.5–0.7) [55].
Looking at the evidence of nonvertebral fracture risk reduction, the systematic review by Allen et al. observed no statistically significant beneficial effect of oral BP in fracture rate fall [54]. It is Important to mention that most studies included in this systematic review had a short follow-up duration and the included studies were not designed and powered to detect fracture rate reduction. Interestingly, some more recent observational cohort studies with longer follow-up showed a decline in fracture rate (including both vertebral and nonvertebral fractures) [56, 57]. All these studies strongly support the beneficial effects of BP on fracture risk reduction.
Denosumab in GIOP
Another option for GIOP patients with moderate to high fracture risk is denosumab (DMAB). DMAB acts like BP as an anti-resorptive drug, and is a fully human monoclonal antibody to an activator of osteoclastic differentiation and proliferation, soluble RANK-L. Some years ago, it was shown in a randomized controlled trial that DMAB subcutaneously was noninferior to risedronate in preserving BMD at lumbar spine in patients (n = ~ 800) suffering from GIOP [58, 59]. In these studies, two subgroups were compared; the first group (glucocorticoid initiating patients) included patients who were receiving glucocorticoid therapy (prednisone or its equivalent) at a dose of ≥ 7.5 mg for < 3 months, the other group patients (glucocorticoid continuing patients) were receiving ≥ 3 months (prednisone or its equivalent) at a dose of ≥ 7.5 mg. The glucocorticoid initiating patients included 290 patients and approximately 89% of the patients were postmenopausal; the glucocorticoid continuing group included 505 patients and approximately 85% of the patients were postmenopausal. In both groups GIOP patients were 1:1 randomized to either risedronate or denosumab. In this study it was clearly shown that after two years, the rise in BMD was larger in the DMAB group compared to the risedronate group in GIOP patients, who recently started GC, + 3.1% and + 0.0% at total hip level and + 6.2% and + 1.7% at lumbar spine level, respectively. The same improvements in BMD were seen in patients who were longer on GC treatment (at least 3 months); + 2.9% at total hip level and + 6.4% at lumbar spine level in DMAB treated group and + 0.5% at total hip level and 3.2% at lumbar spine level in the risedronate group. This trial was not powered to detect fracture risk reduction and only looked from a safety point of view to fracture rate. No differences in fracture occurrence were observed in GC patients treated with DMAB or risedronate as in both groups the fracture rate was reported to be approximately 9%. From these studies it can be concluded that in GIOP patients denosumab has larger effects on BMD at all sites compared to risedronate, although no fall in fracture rate could be demonstrated.
Teriparatide in GIOP
In contrast to BP and DMAB, which are anti-resorptive drugs, teriparatide is an osteoanabolic drug. The daily pulsatile subcutaneously injected synthetic form of parathormone (PTH), teriparatide, stimulates osteoblast activity, and induces increases in BMD. Since inhibition of bone formation is a hallmark in the pathogenesis of GIOP, anabolic therapy seems an attractive therapy for GIOP therapy in fracture risk management. In a randomized controlled trial in approximately 430 GIOP patients teriparatide was compared with alendronate with lumbar spine at BMD level as primary outcome. Approximately 80% of the patients were postmenopausal with a comparable mean age of 57.3 and 56.1 in the alendronate and teriparatide group, respectively.
In this study, it was observed that the teriparatide group had significantly larger improvements in lumbar spine BMD than the alendronate group, + 7.2% and + 3.4% respectively [60]. The same effect was seen on hip BMD levels; + 3.8% in the teriparatide group and + 2.4% in the alendronate group. Moreover, a significant reduction in vertebral fracture rate was observed in the teriparatide group as compared to the alendronate group: 0.6% and 6.1%, respectively. In the extension study with 3 years follow up, the same vertebral fracture protection was observed in favor of teriparatide, but no beneficial effect was seen on nonvertebral fractures compared to alendronate [61]. These observations are in line with observational studies in daily clinical practice that found a fall in vertebral fracture rate in teriparatide treated GIOP patients [62]. These studies clearly demonstrate that teriparatide treated subjects had significantly greater increases in spine and hip BMD compared with subjects receiving alendronate during 36 months of therapy. Therefore, beside the substantial differences in cost price, teriparatide is another good option for high fracture risk GIOP patients.
Modern treatment of GIOP, how to optimize prevention of fractures in daily practice
The first step to optimize prevention of bone loss in GC-treated patients is awareness and identification of patients at risk. Since GIOP is the most common form of secondary osteoporosis, it is a major health problem, and physicians should discuss the prevention of GC-induced osteoporotic fractures in all of their GC users, thus in many patients and particularly in the elderly. Unfortunately, undertreatment still occurs in many patients: in the GLOW study, a 5-year observational study of 60,393 postmenopausal women enrolled in 17 sites in ten countries in Europe, North America, and Australia, around 893 (2%) reported continuous use of glucocorticoids over the past 2 or more years. In this large study in a real-life setting, calcium, and/or vitamin D were only prescribed in around 67% of patients, while anti-osteoporotic drugs were prescribed in (only) 42% of patients [63]. In fact, this is remarkable, since a questionnaire by EULAR investigated which side effects of GC are the clinically most relevant: both for clinicians and for patients, osteoporotic fractures are among the top three of the most important reported side effects [64].
Adequate fracture risk estimation is after awareness and identification the second step. Fracture risk in GC-treated patients is dependent on the well-known classical risk factors for osteoporotic fractures, such as high advanced age, low BMI, familiar osteoporosis, which can be estimated with the FRAX score, but also on the severity of the underlying disease and the dosage of GC [65]. Thus, it is important to realize that preventive measures are particularly necessary in patients with a high FRAX score; thus, often in the elderly, but also in the early phase of therapy, when the activity of the underlying disease is high, and high dosages of GC are warranted, while usually later in the disease, the disease activity is under control and the dosages of GC lower.
Life style factors are an important cornerstone of the prevention of GC-induced fractures, and should start with lowering the dosage of GC as soon as possible to the lowest possible dosage, and by the co-prescription of anti-inflammatory drugs, such as azathioprine, methotrexate, and mycophenolate mofetil [66]. Calcium and vitamin D are crucial for building up strong bones in young individuals to build up their peak bone mass, but adequate calcium and vitamin D intake is also crucial in the elderly [67]. For GC-treated patients, calcium supplementation is even more necessary than in postmenopausal women, since GC lower the intestinal absorption of calcium and elevate the urinary excretion of calcium [66]. Thus, in GC-treated patients, the daily calcium intake should be above 1000–1200 mg per day [38], or even 1000–1500 mg per day [68]. Adequate vitamin D levels are also important in GC-treated patients, for several reasons: vitamin D increases the intestinal calcium absorption, but it counteracts also GC-induced myopathy, and it might have an immunosuppressive effect [69]. A 25(OH) vitamin D level above 30 ng/ml is advised throughout the whole year which is particularly critical in the elderly. Exercise therapy has a favorable effect on muscle strength and neuromuscular coordination, and as a consequence, lowers fall risk [70]. The use of nicotine and alcohol both have a negative effect on bone [71, 72], while alcohol might also increase fall risk.
In addition to life-style factors, anti-osteoporotic drug therapy is necessary in GC-treated patients: the oral bisphosphonates alendronate and risedronate are usually first choice, because of studies showing reductions in vertebral fractures in GC-treated patients, and the relative safety, low-cost price, and the long-term experience with these drugs over more than 15 years [37, 38, 50, 73]. These oral bisphosphonates should be preferably prescribed in patients who tolerate it adequately throughout the whole period of GC-treatment. After stopping the GCs, a risk calculation whether or not to continue anti-osteoporotic drugs should be made based on clinical risk factors without GC use, and a BMD measurement in combination with VFA. There is no consensus what to do in patients who are on long-term treatment (e.g., 8–10 years) with GCs and oral bisphosphonates, since the risk of atypical femoral fractures, which is generally very low, is increasing in long-term bisphosphonate users [74].
However, for three drugs superiority above oral bisphosphonates have been documented: both zoledronic acid [7] and denosumab [59] have shown to induce a larger increase in BMD of both lumbar spine and hips than risedronate. No differences in fracture rates were observed, probably related to the active drug comparator (versus placebo) and lower number of patients in comparison to phase III trials in postmenopausal women with these drugs. The third drug with proven superiority is teriparatide, which is theoretically a very attractive option, since it has an anabolic effect, while inhibition of bone formation plays a central role in the pathogenesis of GC-induced osteoporosis [61, 62]. Indeed, teriparatide has a favorable effect on BMD versus alendronate in a direct comparison, but also reduces vertebral fractures [61]. We expect that the role of osteoanabolic drugs in GC users will increase, due to the introduction of biosimilars for teriparatide, which have a much lower cost price, the availability of abaloparatide, a daily subcutaneous parathyroid hormone-related protein analog, in some countries (but not in Europe) and because of the introduction of romosozumab, a powerful monoclonal antibody against sclerostin [59]. All three drugs have an anabolic window, which is theoretically attractive in GC-treated patients with inhibited bone formation.
Fracture reduction has been demonstrated for romosozumab versus alendronate, an active comparator, but no data are available yet in GC-treated patients. Ronate, an active comparator, but no data is available yet in GC-treated patients. However, romosozumab is also associated with an increased risk of cardiovascular risk in the ARCH study, a study with romosozumab versus alendronate, but not in the FRAME study, romosozumab versus placebo [76, 77]. Thus, in GC users, the favorable effect on bone in patients at high risk for fractures, should be balanced against the cardiovascular risk, which is also elevated in GC users.
In men, there is much less data available in GC users than in females. Men are often undertreated, probably because physicians hesitate to treat osteoporosis in men, probably due to a lack of awareness and because men have a lower life-time fracture risk; however, the risk of a second fracture is the same in women and in men [75]. Increases in BMD have been described for alendronate and risedronate versus placebo in male GC users. For teriparatide, an increase in BMD was found in comparison with risedronate, but favorable effects were also shown in bone quality, measured by HRpQCT [78].
In GC-treated premenopausal women even less data is available; physicians try to avoid the prescription of drug treatment in (potentially) pregnant women. Because of the young age of premenopausal women, anti-osteoporotic drugs are often not necessary, but in some GC-treated premenopausal women, a cascade of (vertebral) fractures may occur, and anti-osteoporotic drugs should be prescribed. Because of the long term maternal skeletal retention, which may affect later the fetal skeleton, bisphosphonates have a relatively contraindication in premenopausal women [79].
The third subgroup is elderly women, aged e.g., 80 + and over. Sometimes anti-osteoporotic drugs are not prescribed to them, because of assumptions such as “low bone mass is physiological”, or because of using multiple other drugs or because of an estimated low life expectancy. However, on the other hand, fracture risk is extremely high in elderly women with GC use and an underlying inflammatory disease, and thus a relative risk reduction of 50–75% of vertebral fractures corresponds with a large reduction in absolute risk. In addition, in postmenopausal women a reduction in fractures was already demonstrated 6 months after initiating alendronate [80]. In other words, the elderly individuals treated with GCs y are the preferred target group for anti-osteoporotic drugs.
Although several schemes are available for GIOP patients around starting anti-osteoporotic drug treatment, the Working Group Osteoporosis of the Dutch Society of Rheumatology, propose a new scheme, more or less in line with earlier schemes, but incorporating VFA [81] (see Fig. 2). Other new points are the incorporation of a new recent sentinel fracture (< 2 years) and a rule for stopping. As in other schemes, anti-osteoporotic drugs should be started independent from DXA results in patients aged 40 years and over, treated with 7.5 mg prednisone per day or more for at least 3 months, and 1 or more clinically relevant risk factor: postmenopausal status, a vertebral sentinel fracture in the scheme, and a rule for stopping. As in other schemes, anti-osteoporotic drugs should be started independent from DXA results in patients aged 40 years and over, treated with 7.5 mg prednisone per day or more for at least 3 months, and 1 or more clinically relevant risk factor: postmenopausal status, a vertebral fracture (Genant grade 2 or more, > 25% height loss) or a recent (< 2 years) nonvertebral fracture. In patients above 40 years of age and treated with (only) 2.5 mg prednisone per day, the decision to start should be based on additional data: a low T score (< − 2.0), a prior vertebral fracture (Genant grade 2 or more, > 25% height loss) [82, 83] or a high FRAX score (10 years hip-fracture risk > 3% and MOF > 15%) [48]. We suppose that the strength of the scheme is to categorize the patients in high and low risk, starting directly in high-risk patients, and only when additional risk factors can be documented in the low-risk group: a simple scheme, and the simplicity will probably facilitate the implementation [84].

Adapted from reference [75]
Scheme of management of glucocorticoid-induced osteoporosis.
There is nowadays some discussion about the order of prescription of anti-osteoporotic drugs in GC-treated patients: in many guidelines the oral bisphosphonates alendronate and risedronate are first choice, but what is the position of denosumab, zoledronic acid and teriparatide? Usually, these drugs are regarded as second-line therapy in GC users. However, fracture risk might be very high in GC users, particularly in the elderly and in the early phase of GC treatment when disease activity is high. Thus, in our opinion, there are also arguments to prescribe these so-called second line drugs as initial therapy in high-risk patients, e.g., in the elderly, with a low BMI, a vertebral fracture and a relatively high dose GC [66]. Since there are no head-to-head studies, we do not have preferences between these three drugs.
Bone loss and fractures are among the most devastating side effects of long-term GC therapy. Despite available, effective preventive measures, many patients receiving GC therapy are not appropriately evaluated or treated, to preserve bone integrity and to prevent fractures. Patients with GIOP should be treated as early as possible based on the bone mineral density and risk factors. FRAX algorithm provides a 10-year probability of fractures that can be adjusted according to GC dose. Available anti-osteoporotic agents, such as denosumab, bisphosphonates, and teriparatide are effective in the management of GIOP. General measurements, such as physical activity, calcium intake, and adequate levels of Vitamin D should be assured. Medical education must be highlighting the need of detection, prevention, and treatment of patients receiving GC for more than 3 months and doses higher than 5 to 7.5 mg of prednisone or equivalent.
Change history
23 July 2021
A Correction to this paper has been published: https://doi.org/10.1007/s40520-021-01937-5
References
Kanis JA, Johansson H, Oden A et al (2004) A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res 19:893–899
Díez-Pérez A, Hooven FH, Adachi JD et al (2011) Regional differences in treatment for osteoporosis. The Global Longitudinal Study of Osteoporosis in Women (GLOW). Bone 49:493–498
Van Staa TP, Leufkens HG, Cooper C (2002) The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 13:777–787
Natsui K, Tanaka K, Suda M et al (2006) High-dose glucocorticoid treatment induces rapid loss of trabecular bone mineral density and lean body mass. Osteoporos Int 17:105–108
Adachi JD, Saag KG, Delmas PD et al (2001) Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum 44:202–211
Cohen S, Levy RM, Keller M et al (1999) Risedronate therapy prevents corticosteroid-induced bone loss: a twelve month, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 42:2309–2318
Reid DM, Devogelaer JP, Saag K et al (2009) Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 373:1253–1263
van Staa TP, Leufkens HG, Abenhaim L et al (2000) Use of oral corticosteroids and risk of fractures. J Bone Miner Res 15:993–1000
van Staa TP, Geusens P, Pols HA et al (2005) A simple score for estimating the long-term risk of fracture in patients using oral glucocorticoids. QJM 98:191–198
Waljee AK, Rogers MA, Lin P et al (2017) Short term use of oral corticosteroids and related harms among adults in the United States: population-based cohort study. BMJ 357:j1415
Amiche MA, Abtahi S, Driessen JHM et al (2018) Impact of cumulative exposure to high-dose oral glucocorticoids on fracture risk in Denmark: a population-based case-control study. Arch Osteoporos 13:30
De Vries F, Bracke M, Leufkens HG et al (2007) Fracture risk with intermittent high-dose oral glucocorticoid therapy. Arthritis Rheum 56:208–214
Schett G, Saag KG, Bijlsma JW (2010) From bone biology to clinical outcome: state of the art and future perspectives. Ann Rheum Dis 69:1415–1419
Best JH, Kong AM, Lenhart GM et al (2018) Association between glucocorticoid exposure and health care expenditures for potential glucocorticoid-related adverse events in patients with rheumatoid arthritis. J Rheumatol 45:320–328
Buttgereit F, Straub RH, Wehling M et al (2004) Glucocorticoids in the treatment of rheumatic diseases: an update on the mechanisms of action. Arthritis Rheum 50:3408–3417
O’Brien CA, Jia D, Plotkin LI et al (2004) Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology 145:835–841
Hildebrandt S, Baschant U, Thiele S et al (2018) Glucocorticoids suppress Wnt16 expression in osteoblasts in vitro and in vivo. Sci Rep 8:8711
Ohlsson C, Nilsson KH, Henning P et al (2018) WNT16 overexpression partly protects against glucocorticoid-induced bone loss. Am J Physiol Endocrinol Metab 314:E597–E604
Ohnaka K, Tanabe M, Kawate H et al (2005) Glucocorticoid suppresses the canonical Wnt signal in cultured human osteoblasts. Biochem Biophys Res Commun 329:177–181
Wang FS, Ko JY, Yeh DW et al (2008) Modulation of Dickkopf-1 attenuates glucocorticoid induction of osteoblast apoptosis, adipocytic differentiation, and bone mass loss. Endocrinology 149:1793–1801
Kato T, Khanh VC, Sato K et al (2018) Elevated expression of Dkk-1 by glucocorticoid treatment impairs bone regenerative capacity of adipose tissue-derived mesenchymal stem cells. Stem Cells Dev 27:85–99
Butler JS, Queally JM, Devitt BM et al (2010) Silencing Dkk1 expression rescues dexamethasone-induced suppression of primary human osteoblast differentiation. BMC Musculoskelet Disord 11:210
Cárcamo-Orive I, Gaztelumendi A, Delgado J et al (2010) Regulation of human bone marrow stromal cell proliferation and differentiation capacity by glucocorticoid receptor and AP-1 crosstalk. J Bone Miner Res 25:2115–2125
Hofbauer LC, Gon F, Riggs BL et al (1999) Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology 140:4382–4389
Pereira RMR, Delany AM, Durant D et al (2002) Cortisol regulates the expression of notch in osteoblasts. J Cell Biochem 85:252–258
Zanotti S, Yu J, Adhikari S et al (2018) Glucocorticoids inhibit notch target gene expression in osteoblasts. J Cell Biochem 119:6016–6023
Zhang S, Liu Y, Liang Q (2018) Low-dose dexamethasone affects osteoblast viability by inducing autophagy via intracellular ROS. Mol Med Rep 17:4307–4316
Weinstein RS, Hogan EA, Borelli MJ et al (2017) The pathophysiological sequence of glucocorticoid-induced osteonecrosis of the femoral head in male mice. Endocrinology 158:3817–3831
Schakman O, Kalista S, Barbe C et al (2013) Glucocorticoid-induced skeletal muscle atrophy. Int J Biochem Cell Biol 45:2163–2172
Sculz P, Beck TJ, Marchand F et al (2005) Low skeletal muscle mass is associated with poor structural parameters of bone and impaired balance in elderly men—the MINOS study. J Bone Miner Res 20:721–729
Weinstein RS (2011) Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med 365:62–70
Canalis E, Mazziotti G, Giustina A et al (2007) Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 18:1319–1328
Hu K, Adachi JD (2019) Glucocorticoid induced osteoporosis. Expert Rev Endocrinol Metabol 14:259–266
Lane NE (2019) Glucocorticoid-induced osteoporosis: new insights into the pathophysiology and treatments. Curr Osteoporos Rep 17:1–7
Compston J (2018) Glucocorticoid-induced osteoporosis: an update. Endocrine 61:7–16
Adami G, Saag KG (2019) Glucocorticoid-induced osteoporosis: 2019 concise clinical review. Osteoporos Int 30:1145–1156
Lekamwasam S, Adachi JD, Agnusdei D et al (2012) A framework for the development of guidelines for the management of glucocorticoid-induced osteoporosis. Osteoporos Int 23:2257–2276
Buckley L, Guyatt G, Fink HA et al (2017) 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol 69:1521–1537
Shuhart CR, Yeap SS, Anderson PA et al (2019) Executive summary of the 2019 ISCD position development conference on monitoring treatment, DXA cross-calibration and least significant change, spinal cord injury, peri-prosthetic and orthopedic bone health, transgender medicine, and pediatrics. J Clin Densitom 22:453–471
Iacopo C, Alberto F, Daniela M et al (2020) Updates in epidemiology, pathophysiology and management strategies of glucocorticoid-induced osteoporosis. Expert Rev Endocrinol Metabol 15:283–298
Silva BC, Leslie WD, Resch H et al (2014) Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res 29:518–530
Dubrovsky AM, Maricic M, Lane NE (2020) Glucocorticoid-induced osteoporosis. In: Leaer BZ, Wein MN (eds) Osteoporosis: pathophysiology and clinical management, 3rd edn. Humana, Cham, pp 407–418
Leib ES, Winzenrieth R (2016) Bone status in glucocorticoid-treated men and women. Osteoporos Int 27:39–48
Florez H, Hernández-Rodríguez J, Muxi A et al (2020) Trabecular bone score improves fracture risk assessment in glucocorticoid-induced osteoporosis. Rheumatology 59:1574–1580
Saag KG, Agnusdei D, Hans D et al (2016) Trabecular bone score in patients with chronic glucocorticoid therapy-induced osteoporosis treated with alendronate or teriparatide. Arthritis Rheumatol 68:2122–2128
Leib ES, Saag KG, Adachi JD et al (2011) Official positions for FRAX® clinical regarding glucocorticoids: the impact of the use of glucocorticoids on the estimate by FRAX® of the 10-year risk of fracture: from Joint Official Positions Development Conference of the International Society for Clinical Densitometry and International Osteoporosis Foundation on FRAX®. J Clin Densitom 14:212–219
Güler-Yüksel M, Hoes JN, Bultink IE et al (2018) Glucocorticoids, inflammation and bone. Calcif Tissue Int 102:592–606
Kanis JA, Johansson H, Oden A et al (2011) Guidance for the adjustment of FRAX according to the dose of glucocorticoids. Osteoporos Int 22:809–816
Buckley L, Humphrey MB (2018) Glucocorticoid-induced osteoporosis. New Engl J Med 379:2547–2556
Compston J, Cooper A, Cooper C et al (2017) UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos 12:43
Briot K, Cortet B, Roux C et al (2014) 2014 update of recommendations on the prevention and treatment of glucocorticoid-induced osteoporosis. J Bone Spine 81:493–501
Rizzoli R, Biver E (2015) Glucocorticoid-induced osteoporosis: who to treat with what agent? Nat Rev Rheumatol 11:98
Drake MT, Clarke BL, Khosla S (2008) Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc 83:1032–1045
Allen CS, Yeung JH, Vandermeer B et al (2016) Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev 10:CD001347
Amiche MA, Levesque LE, Gomes T et al (2018) Effectiveness of oral bisphosphonates in reducing fracture risk among oral glucocorticoid users: three matched cohort analyses. J Bone Miner Res 33:419–429
Axelsson KF, Nilsson AG, Wedel H et al (2017) Association between alendronate use and hip fracture risk in older patients using oral prednisolone. JAMA 318:146–155
Bergman J, Nordstrom A, Nordstrom P (2018) Alendronate use and the risk of nonvertebral fracture during glucocorticoid therapy: a retrospective cohort study. J Clin Endocrinol Metab 103:306–313
Saag KG, Wagman RB, Geusens P et al (2018) Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol 6:445–454
Saag KG, Pannacciulli N, Geusens P et al (2019) Denosumab vs risedronate in glucocorticoid-induced osteoporosis: final results of a 24-month randomized, double-blind, double-dummy trial. Arthritis Rheumatol 71:1174–1184
Saag KG, Shane E, Boonen S et al (2007) Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med 357:2028–2039
Saag KG, Zanchetta JR, Devogelaer JP et al (2009) Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: thirty-six-month results of a randomized, double-blind, controlled trial. Arthritis Rheum 60:3346–3355
Karras D, Stoykov I, Lems WF et al (2012) Effectiveness of teriparatide in postmenopausal women with osteoporosis and glucocorticoid use: 3-year results from the EFOS study. J Rheumatol 39:600–609
Silverman S, Curtis J, Saag K et al (2015) International management of bone health in glucocorticoid-exposed individuals in the observational GLOW study. Osteoporos Int 26:419–420
Van der Goes MC, Jacobs JWG, Boers M et al (2010) Patient and rheumatologist perspectives on glucocorticoids: EULAR recommendations on the management of glucocorticoid therapy in rheumatic diseases. Ann Rheum Dis 69:1015–1021
Briot K, Geusens P, Bultink IEM et al (2017) Inflammatory Diseases and bone fragility. Osteoporos Int 28:3301–3314
Raterman H, Bultink IEM, Lems WF (2019) Current treatments and new developments in the management of glucocorticoid-induced osteoporosis. Drugs 79:1065–1087
Weaver CM, Gordon CM, Janz KF et al (2016) The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int 27:1281–1386
Briot K, Roux C (2015) Glucocorticoid-induced osteoporosis. RMD Open 1:e000014
Lips P (2007) Relative value of 25(OH)D and 1,25(OH)2D measurements. J Bone Miner Res 22:1668–1671
Gillespie LD, Robertson MC, Gillespie WJ et al (2012) Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD007146.pub3
Kanis JA, Johnell O, Oden A et al (2005) Smoking and fracture risk: a meta-analysis. Osteoporos Int 16:155–162
Maurel DB, Boisseau N, Benhamou CL et al (2012) Alcohol and bone: review of dose effects and mechanisms. Osteoporos Int 23:1–16
Hoes JN, Jacobs JW, Boers M et al (2007) EULAR evidence-based recommendations on the management of systemic glucocorticoid therapy in rheumatic diseases. Ann Rheum Dis 66:1560–1567
Black DM, Abrahamsen B, Bouxsein ML et al (2018) Atypical femur fractures—review of epidemiology, relationship to bisphosphonates, prevention and clinical management. Endocr Rev 40:333–368
Raterman HG, de Nijs R, Lems WF (2020) Standpunt van de Nederlandse Vereniging voor Reumatologie inzake Glucocorticoïd-geïnduceerde Osteoporose. GIOP guideline Dutch Society Rheumatology. https://www.nvrnl/wp-content/uploads/2020/01/NVR-Concept-standpunt-GIOPpdf.2020
Saag KG, Petersen J, Brandi ML et al (2017) Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med 377:1417–1427
Cosman F, Crittenden DB, Adachi JD et al (2016) Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med 375:1532–1543
Bliuc D, Alarkawi D, Nguyen TV et al (2015) Risk of subsequent fractures and mortality in elderly women and men with fragility fractures with and without osteoporotic bone density: the Dubbo Osteoporosis Epidemiology Study. J Bone Miner Res 30:637–646
Gluer CC, Marin F, Ringe JD et al (2013) Comparative effects of teriparatide and risedronate in glucocorticoid-induced osteoporosis in men: 18-month results of the EuroGIOPs trial. J Bone Miner Res 28:1355–1368
Pepe J, Body JJ, Hadji P et al (2020) Osteoporosis in premenopausal women: a clinical narrative review by the ECTS and the IOF. J Clin Endocrinol Metab 105:2487–2506
Van de Glind EMM, Willems HC, Eslami S et al (2016) Estimating the time to benefit for preventive drugs with the statistical process control method: an example with alendronate. Drugs Aging 33:347–353
Lems WF, Dreinhofer KE, Bischoff-Ferrari H et al (2017) EULAR/EFORT recommendations for management of patients older than 50 years with a fragility fracture and prevention of subsequent fractures. Ann Rheum Dis 76:802–810
Genant HK, Wu CY, van Kuijk C et al (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148
Kanis JA, Hans D, Cooper C et al (2011) Interpretation and use of FRAX in clinical practice. Osteoporos Int 22:2395–2411
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
Authors declare no conflicts of interest.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Messina, O.D., Vidal, L.F., Wilman, M.V. et al. Management of glucocorticoid-induced osteoporosis. Aging Clin Exp Res 33, 793–804 (2021). https://doi.org/10.1007/s40520-021-01823-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-021-01823-0