Abstract
Glucocorticoids can be lifesaving for patients with chronic inflammatory diseases and allergic conditions yet have devastating effects on the skeleton. Chronic glucocorticoid therapy is a common cause of drug-induced osteoporosis. This is a review of the epidemiology and pathophysiology of glucocorticoid-induced osteoporosis, the risk of bone loss and fractures, and strategies to reduce fracture risk in patients receiving glucocorticoid therapy. Patients on chronic glucocorticoids tend to fracture at higher bone density levels than those with postmenopausal osteoporosis and may have comorbidities, including sarcopenia and the underlying disease being treated, that contribute to falls risk and skeletal fragility. Fractures are associated with significant morbidity and mortality. Despite the availability of evidence-based clinical practice guidelines providing physicians with recommendations for interventions proven to reduce fracture risk, many patients are currently not being treated. Patients receiving long-term glucocorticoid therapy should be evaluated for fracture risk and treated appropriately to reduce the risk of fractures.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Glucocorticoid excess, whether generated endogenously or administered exogenously, is known to have many adverse effects, including harm to the skeleton. In his seminal report published over 85 years ago, Harvey Cushing analyzed the clinical and pathological findings of 12 patients with pituitary adenomas [1]. In what came to be called “Cushing’s disease” (hypercortisolism caused by a pituitary tumor), he described “softening affecting the entire skeleton but more particularly the vertebrae, leading to multiple fractures.” Today, hypercortisolism of any cause (“Cushing’s syndrome”) is most often due to exogenous glucocorticoids prescribed for a wide variety of inflammatory, allergic, and neoplastic conditions. The first clinical use of exogenous glucocorticoids, extracted from whole cattle adrenals, was reported in 1930 [2]. Patients with adrenal insufficiency due to Addison’s disease were successfully treated, although transiently, with intravenous infusions of this extract. This was rapidly followed by experiments to isolate individual compounds in the adrenal extract that have biological activity. In 1949, the first clinical use of cortisone was reported [3]. A woman with rheumatoid arthritis (RA) was treated at the Mayo Clinic with cortisone 50 mg intramuscularly (IM) twice daily. RA symptoms improved, but treatment was subsequently discontinued due to development of facial puffiness, hirsutism, acne, and mental disturbances. Soon after, it was reported that orally administered cortisone was as effective as the IM preparation [4]. In 1954, the first analogs of cortisone and hydrocortisone, which were later named prednisone and prednisolone, were used for the treatment of patients with RA [5]. Anti-inflammatory effects were soon enhanced by the development of other glucocorticoids, such as triamcinolone and dexamethasone. However, for use in clinical practice, prednisone and prednisolone emerged as the most commonly used systemic glucocorticoids and remain so in modern times.
Toxic effects of glucocorticoid therapy were easily recognized with the first patient to receive cortisone for RA [3], as they were similar to the effects of endogenous excess seen with Cushing’s syndrome. Adverse skeletal effects of chronic glucocorticoid therapy were described as early as 1954 in a report of vertebral fractures with treatment of 3 men with RA and a boy with juvenile polyarthritis [6]. More patients with glucocorticoid-induced osteoporosis (GIO) and vertebral fractures were reported a few years later [7]. It soon became apparent that osteoporosis and fractures were common in patients receiving long-term glucocorticoid therapy. GIO has been estimated to affect about 50% of patients receiving long-term glucocorticoids and may be the most common form of secondary osteoporosis [8]. Despite the availability of many medications to treat GIO [9], many patients who could benefit are not being treated [10].
Patient Case Report
A 72-year-old woman is a former heavy smoker with severe chronic obstructive pulmonary disease (COPD) . Treatment includes home oxygen and prednisone 7.5 mg daily for past 12 years, with higher doses required for 2–3 weeks several times each year for exacerbations of COPD. She describes have a well-balanced diet with no vitamin or mineral supplements. She has fallen twice in the past 12 months but has no known fracture. She has never had a bone density test and has never received pharmacological therapy to reduce fracture risk. After hearing from a friend that prednisone can be harmful to bones, she asks her physician whether she needs to be evaluated. Although he does not see the need for this, he makes an appointment for consultation with a physician with expertise in osteoporosis. She is found to weigh 102 pounds (46.3 kg); height is 60.0 inches (152.4 cm) with a wall-mounted stadiometer, which is 2.5 inches (6.4 cm) shorter than her historical maximum height. Her gait was unstable and she did poorly on balance testing. She has mild kyphosis. There is no spinal process tenderness to palpation. Dual-energy X-ray absorptiometry (DXA) testing shows lumbar spine T-score = −1.9 with the appearance of degenerative arthritis on the spine image and femoral neck T-score = −2.3. Vertebral fracture assessment (VFA) by DXA shows a severe (grade 3) wedge fracture at T12 and moderate (grade 2) fractures at T9 and T10. On laboratory testing there is a low serum 25-hydroxyvitamin D of 12 ng/mL (30 nmol/L) and elevated serum intact parathyroid (PTH) level of 82 pg/mL (8.7 pmol/L). Serum calcium, albumin, magnesium, phosphorus, alkaline phosphatase, and creatinine are normal. The 24-hour urinary calcium is low at 48 mg. After vitamin D replacement, which corrected the abnormal serum 25-hydroxyvitamin D, PTH, and 24-hour urinary calcium, she is started on alendronate 70 mg weekly. She was referred for physical therapy to improve core strength and balance in an effort to reduce fall risk. Continuing efforts were made to minimize her exposure to system glucocorticoids.
Epidemiology
The prevalence of glucocorticoid use in the general community has been estimated to be between 0.5% and 1% [11,12,13,14]. In a 5-year longitudinal study of 60,000 postmenopausal women conducted in 10 countries, 4.6% were receiving glucocorticoids at the baseline visit [15]. The most common reasons for taking glucocorticoids are chronic rheumatic inflammatory diseases (e.g., RA, lupus) and chronic lung diseases (e.g., COPD, asthma). Other uses include gastrointestinal disorders (e.g., inflammatory bowel diseases, hepatitis), organ transplantation, and treatment of some malignancies. Over 10% of patients on long-term glucocorticoids have been reported to have clinical fractures, with 30–40% having radiographic vertebral fractures [16, 17]. Risk factors for fracture include low-baseline bone mineral density (BMD), the type of glucocorticoid medication used, the dose and duration of treatment, the underlying disease being treated, age and sex of the patient, menopausal status for women, and previous fracture. A large case-control study of subjects on oral glucocorticoids reported a dose-dependent increase in fracture risk with users of prednisolone, with no increase in risk for users of budesonide or hydrocortisone [18]. A large retrospective cohort study in the UK found rapid onset of increased fracture risk with initiating oral glucocorticoid therapy, rapid return of fracture risk toward baseline with discontinuation, and no dose that was “safe” for skeletal health [19]. Even doses of prednisolone less than 2.5 mg daily were associated with an increase in vertebral fracture risk. Inhaled glucocorticoids can be partially absorbed and may have systemic effects [20]. Use of long-term inhaled glucocorticoids in high doses for patients with COPD has been associated with a modest increase in the risk of hip and upper extremity fractures [21]. Most studies of intranasal glucocorticoids in patients with allergic rhinitis have shown no clinically significant systemic effects in usual doses, although more study is needed, particularly in patients receiving combinations of inhaled and intranasal glucocorticoids [22].
Pathophysiology
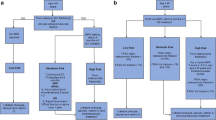
The effects of glucocorticoid therapy on BMD are biphasic. There is an initial rapid phase of 6–12% bone loss in the first year of therapy, followed by subsequent bone loss of about 2–3% per year [23]. Trabecular bone is predominately affected, leading to high risk of fractures in the spine, a skeletal site with a high percentage of trabecular bone [8]. There is marked heterogeneity in the skeletal response of individuals to glucocorticoid therapy, perhaps due to polymorphisms of glucocorticoid receptors or variations of enzymes responsible for metabolizing glucocorticoids. There are many direct and indirect effects of glucocorticoids that lead to skeletal fragility and fractures (Fig. 26.1). The dominant effect of glucocorticoids on bone remodeling is to reduce bone formation by decreasing the number, function, and lifespan of osteoblasts, the bone-forming cells. The activity and lifespan of osteocytes, which act as mechanosensors, are also reduced. The effects of glucocorticoids on osteoclasts are complex and controversial. However, the preponderance of evidence suggests that in the first 3–6 months of glucocorticoid therapy, there is an increase in osteoclastic bone resorption, especially in patients with chronic inflammatory diseases, followed by a subsequent decrease in bone resorption. This might explain, at least in part, the biphasic skeletal response to glucocorticoids. The changing pattern of bone remodeling with long-term glucocorticoids (initial suppression of bone formation and increase in bone resorption, followed by suppression of formation and resorption) raises concern regarding the use of potent antiresorptive medications, such as bisphosphonates and denosumab, for treatment of GIO beyond the first several years [24]. The observation that fracture risk for patients on glucocorticoids rises early in the course of exposure and is greater than expected for the level of BMD is consistent with loss of bone strength due to degradation of bone quality (e.g., bone turnover, bone microarchitecture) and osteocyte apoptosis [25]. Long-term glucocorticoids also have nonskeletal consequences (e.g., sarcopenia, falls) that increase fracture risk [26].
Pathophysiology of GIO [8]. The principal direct skeletal effects of long-term glucocorticoid therapy are due to a decrease in osteoblastic bone formation. Osteocyte function and lifespan is reduced. The effects on osteoclastic bone resorption are biphasic, with an initial transient increase in resorption followed by long-term decrease. Other indirect skeletal effects and nonskeletal effects contribute to high fracture risk with GIO
Glucocorticoids have indirect negative effects on bone cell activity mediated by growth factors than include insulin-like growth factor I (IGF-I) and sex steroids [8]. Glucocorticoids also reduce intestinal calcium absorption and inhibit renal tubular resorption of calcium. In addition, there are important nonskeletal adverse effects of glucocorticoids that influence fracture risk, such as loss of muscle mass and strength (sarcopenia) that can increase the risk of falls [26].
Assessment of Fracture Risk
Clinicians must be vigilant in assessing fracture risk in patients on systemic glucocorticoids. BMD testing by DXA is useful for all patients initiating treatment that is expected to last for more than 3 months. However, fracture risk may increase soon after starting therapy, especially with high doses, even before there has been a major decline in BMD. The fracture risk algorithm, FRAX [27], assumes an average dose of prednisolone (or prednisone) that is 2.5–7.5 mg daily, or equivalent, for more than 3 months. Fracture risk may be underestimated in patients on doses higher than 7.5 mg daily. It has been recommended to adjust FRAX upward for patients on more than 7.5 mg daily by 20% (e.g., from 2.0% to 2.4%) for 10-year probability of hip fracture and upward by 15% (e.g., from 10% to 11.5%) for the 1-year probability of major osteoporotic fracture [28]. There is limited evidence to suggest that trabecular bone score (TBS), a novel grayscale textural analysis of lumbar spine DXA images, might be helpful as an independent predictor of fracture risk in patients on glucocorticoid therapy [29, 30]. TBS score, if available, can be included as a risk factor in the FRAX calculator. Lateral spine imaging by DXA (vertebral fracture assessment - VFA) or conventional radiography is recommended to evaluate for prevalent vertebral fracture in patients who have received glucocorticoid therapy with prednisone 5 mg or more daily for at least 3 months [31]. The finding of a previously unrecognized vertebral fracture may change diagnostic classification, assessment of fracture risk, and treatment strategies [32].
Management
The initial assessment and treatment of patients starting or continuing long-term glucocorticoid therapy is much the same as for osteoporosis of other causes [33]. This includes a thorough skeletal-related medical history and focused physical examination. The patient should be counseled regarding healthy lifestyle and good nutrition, with particular attention to adequacy of calcium and vitamin D intake and prevention of falls. Fracture risk should be assessed and in appropriately selected patients (see Guidelines) pharmacological therapy to reduce fracture risk should be started. Medications approved for prevention and/or treatment of GIO include alendronate, risedronate, zoledronic acid, denosumab, and teriparatide [34]. Each of these agents increases BMD in patients receiving glucocorticoids, and some have been associated with a reduction in fracture risk [9]. Teriparatide, the only anabolic agent approved for treatment of GIO, reduces vertebral fracture risk more than alendronate [35]. Teriparatide is the only approved drug that directly addresses the primary mechanism of bone loss with GIO – impairment of osteoblastic bone formation [36].
Guidelines
The American College of Rheumatology (ACR) conducted a systematic review of the evidence for benefits and harms of options for prevention and treatment of GIO and then used a group consensus process to develop clinical practice guidelines [34]. Patients were stratified according to level of fracture risk (low or moderate/high), age (over 40 years or 40 years and older), and childbearing potential. Recommendations for special populations, such as children and people with organ transplantation, were also included. A summary of selected elements of the ACR guidelines and recommendations for a guideline framework from a working group of the International Osteoporosis Foundation and the European Society of Calcified Tissues [37, 38] is provided in Table 26.1. Guidelines can never accommodate the many variations of clinical circumstances occurring with individual patients and evolving concepts in the management of skeletal diseases. Concerns regarding the 2017 ACR guidelines have been raised [39]. These include the de-emphasis of the use of anabolic therapy compared with the 2010 ACR guidelines, the recommendation to avoid denosumab for renal transplant patients, failure to recommend VFA to evaluate for possible vertebral fracture, and overly stringent criteria for defining treatment failure. As always, treatment decisions in clinical practice should be individualized.
Lessons from the Patient Case Report
Evaluation of skeletal health is mandatory for patients receiving long-term glucocorticoid therapy. Particular attention should be directed to optimizing lifestyle and nutrition, assessing fracture risk, and initiating pharmacological therapy when appropriate. BMD testing by DXA is a useful tool in the assessment of fracture risk , recognizing that these patients may fracture at a higher level of BMD than patients not on glucocorticoids. Vertebral fractures, the most common type of osteoporotic fracture, may not be clinically apparent. Spine imaging by VFA or conventional X-rays may identify previously unrecognized vertebral fractures, which could change diagnostic classification, assessment of fracture risk, and treatment decisions . Vertebral fractures may have adverse effects on pulmonary function, which is especially detrimental to patients with preexisting COPD. While bisphosphonates are the most commonly used medications to treat GIO, there is some evidence suggesting that anabolic therapy is more effective at reducing the risk of vertebral fractures.
Summary
Systemic glucocorticoids are a common cause of drug-induced osteoporosis. Fractures due to GIO can occur early in the course of therapy and may have devastating consequences. Physicians should be vigilant at evaluating patients on glucocorticoids, assessing fracture risk, and initiating countermeasures to reduce fracture risk. Evidence-based guidelines are available to assist physicians in managing patients with GIO. The care of individual patients should be customized according to all available clinical information.
References
Cushing H. The basophil adenomas of the pituitary body and their clinical manifestations (pituitary basophilism). Bull Johns Hopkins Hosp. 1932;50:137–95.
Rowntree LG, Greene CH, Swingle WW, Pfiffner JJ. The treatment of patients with Addison’s disease with the “Cortical Hormone” of Swingle and Pfiffner. Science. 1930;72(1871):482–3.
Hench PS, Kendall EC, et al. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone; compound E) and of pituitary adrenocorticotropic hormone on rheumatoid arthritis. Proc Staff Meet Mayo Clin. 1949;24(8):181–97.
Freyberg RH, Traeger CT, Adams CH, Kuscu T, Wainerdi H, Bonomo I. Effectiveness of cortisone administered orally. Science. 1950;112(2911):429.
Bunim JJ, Pechet MM, Bollet AJ. Studies on metacortandralone and metacortandracin in rheumatoid arthritis; antirheumatic potency, metabolic effects, and hormonal properties. J Am Med Assoc. 1955;157(4):311–8.
Curtiss PH Jr, Clark WS, Herndon CH. Vertebral fractures resulting from prolonged cortisone and corticotropin therapy. JAMA. 1954;156(5):467–9.
Howell DS, Ragan C. The course of rheumatoid arthritis during four years of induced hyperadrenalism (IHA). Medicine (Baltimore). 1956;35(2):83–119.
Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18(10):1319–28.
Whittier X, Saag KG. Glucocorticoid-induced osteoporosis. Rheum Dis Clin N Am. 2016;42(1):177–89.
Curtis JR, Westfall AO, Allison JJ, Becker A, Casebeer L, Freeman A, et al. Longitudinal patterns in the prevention of osteoporosis in glucocorticoid-treated patients. Arthritis Rheum. 2005;52(8):2485–94.
Fardet L, Petersen I, Nazareth I. Monitoring of patients on long-term glucocorticoid therapy: a population-based cohort study. Medicine (Baltimore). 2015;94(15):e647.
Fardet L, Petersen I, Nazareth I. Prevalence of long-term oral glucocorticoid prescriptions in the UK over the past 20 years. Rheumatology (Oxford). 2011;50(11):1982–90.
Soucy E, Bellamy N, Adachi JD, Pope JE, Flynn J, Sutton E, et al. A Canadian survey on the management of corticosteroid induced osteoporosis by rheumatologists. J Rheumatol. 2000;27(6):1506–12.
Overman RA, Yeh JY, Deal CL. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthritis Care Res (Hoboken). 2013;65(2):294–8.
Silverman S, Curtis J, Saag K, Flahive J, Adachi J, Anderson F, et al. International management of bone health in glucocorticoid-exposed individuals in the observational GLOW study. Osteoporos Int. 2015;26(1):419–20.
Curtis JR, Westfall AO, Allison J, Bijlsma JW, Freeman A, George V, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55(3):420–6.
Angeli A, Guglielmi G, Dovio A, Capelli G, de Feo D, Giannini S, et al. High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone. 2006;39(2):253–9.
Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with different types of oral corticosteroids and effect of termination of corticosteroids on the risk of fractures. Calcif Tissue Int. 2008;82(4):249–57.
van Staa TP, Leufkens HGM, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993–1000.
Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy, a systematic review and meta-analysis. Arch Intern Med. 1999;159:941–55.
Gonzalez AV, Coulombe J, Ernst P, Suissa S. Long-term use of inhaled corticosteroids in COPD and the risk of fracture. Chest. 2018;153(2):321–8.
Bensch GW. Safety of intranasal corticosteroids. Ann Allergy Asthma Immunol. 2016;117(6):601–5.
LoCascio V, Bonucci E, Imbimbo B, Ballanti P, Adami S, Milani S, et al. Bone loss in response to long-term glucocorticoid therapy. Bone Miner. 1990;8(1):39–51.
Teitelbaum SL. Glucocorticoids and the osteoclast. Clin Exp Rheumatol. 2015;33(4 Suppl 92):S37–9.
Weinstein RS. Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med. 2011;365(1):62–70.
Klein GL. The effect of glucocorticoids on bone and muscle. Osteoporos Sarcopenia. 2015;1(1):39–45.
University of Sheffield. FRAX fracture risk assessment tool. Available from http://www.shef.ac.uk/FRAX/. Accessed 27 Nov 2017.
Kanis JA, Johansson H, Oden A, McCloskey EV. Guidance for the adjustment of FRAX according to the dose of glucocorticoids. Osteoporos Int. 2011;22(3):809–16.
Harvey NC, Gluer CC, Binkley N, McCloskey EV, Brandi ML, Cooper C, et al. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone. 2015;78:216–24.
Choi YJ, Chung YS, Suh CH, Jung JY, Kim HA. Trabecular bone score as a supplementary tool for the discrimination of osteoporotic fractures in postmenopausal women with rheumatoid arthritis. Medicine (Baltimore). 2017;96(45):e8661.
Shepherd JA, Schousboe JT, Broy SB, Engelke K, Leslie WD. Executive summary of the 2015 ISCD position development conference on advanced measures from DXA and QCT: fracture prediction beyond BMD. J Clin Densitom. 2015;18(3):274–86.
Lewiecki EM, Laster AJ. Clinical applications of vertebral fracture assessment by dual-energy X-ray absorptiometry. J Clin Endocrinol Metab. 2006;91(11):4215–22.
Lewiecki EM. Evaluation of the patient at risk for osteoporosis. In: Marcus R, Feldman D, Dempster DW, Luckey M, Cauley JA, editors. Osteoporosis. 2. Fourth ed. Waltham: Elsevier; 2013. p. 1481–504.
Buckley L, Guyatt G, Fink HA, Cannon M, Grossman J, Hansen KE, et al. 2017 American College of Rheumatology Guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2017;69(8):1521–37.
Saag KG, Shane E, Boonen S, Marin F, Donley DW, Taylor KA, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007;357(20):2028–39.
Amgen. FDA accepts supplemental biologics license application for Prolia® (Denosumab) in glucocorticoid-induced osteoporosis 2017. Available from https://www.amgen.com/media/news-releases/2017/10/fda-accepts-supplemental-biologics-license-application-for-prolia-denosumab-in-glucocorticoidinduced-osteoporosis/. Accessed 26 Nov 2017.
Lekamwasam S, Adachi JD, Agnusdei D, Bilezikian J, Boonen S, Borgstrom F, et al. An appendix to the 2012 IOF-ECTS guidelines for the management of glucocorticoid-induced osteoporosis. Arch Osteoporos. 2012;7:25–30.
Lekamwasam S, Adachi JD, Agnusdei D, Bilezikian J, Boonen S, Borgstrom F, et al. A framework for the development of guidelines for the management of glucocorticoid-induced osteoporosis. Osteoporos Int. 2012;23(9):2257–76.
Maricic M, Deal C, Dore R, Laster A. Comment on 2017 American College of Rheumatology Guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken). 2018;70(6):949–50.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lewiecki, E.M. (2022). Glucocorticoid-Induced Osteoporosis. In: Bandeira, F., Gharib, H., Griz, L., Faria, M. (eds) Endocrinology and Diabetes. Springer, Cham. https://doi.org/10.1007/978-3-030-90684-9_26
Download citation
DOI: https://doi.org/10.1007/978-3-030-90684-9_26
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-90683-2
Online ISBN: 978-3-030-90684-9
eBook Packages: MedicineMedicine (R0)