Abstract
Purpose
The rising proportion of elderly population in high-income societies has resulted in increasing number of subjects with chronic disabling diseases and nutritional deficiency. Elderly’s nutritional status is usually assessed through the mini-nutritional assessment (MNA®). However, its effectiveness may be influenced by weight excess or obesity. We tested the performance of MNA® questionnaire in subjects aged ≥ 65 years from Northern Sardinia, Italy, according to overweight/obesity, and we tried to identify the factors associated with malnutrition.
Methods
A modified version of MNA® (mMNA) test, not including BMI, was compared with the conventional MNA® (cMNA) test, and the overall test performance was assessed by calculating sensitivity, specificity and accuracy. In addition, indexes of cognitive health, disability, comorbidity and polypharmacy were compared between patients with concordant and discordant MNA tests.
Results
cMNA® sensitivity, specificity and accuracy were 67%, 99% and 84% compared with the mMNA test, due to malnourished patients misclassified as normal because of excess weight. Predictors of malnutrition were: depression (p < 0.0001), disability (p < 0.0001) and polypharmacy (p < 0.0001). Interestingly, the average scores of the “global”, “subjective” and “dietary” components of the cMNA®, were significantly lower compared with the corresponding scores of the mMNA.
Conclusions
Excess of weight, a condition progressively rising in the elderly population, may reduce the performance of cMNA® test in detecting malnutrition.
Level of evidence
Level III, case‒control analytic study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The assessment of nutritional status in the elderly is a challenging task requiring the integrated use of disparate sources such as clinical examination, anthropometric measures [1], instrumental procedures [2], laboratory tests [3] and dietary survey [4]. An easy-to-administer questionnaire, the mini-nutritional assessment (MNA®), has been available for decades as initial screening test of nutritional risk [5,6,7,8] and is still considered the “gold standard” tool to detect malnutrition in the geriatric field [9]. Its accuracy has been estimated at around 92% when used by at least two nutritionists, and 98% when integrated with instrumental, biochemical and anthropometric measures [6]. The MNA® test was first proposed in 1989 at the conference of the International Association of Geriatrics and Gerontology in Acapulco, by Bruno Vellas from the Department of Geriatrics, University of Toulouse, France, and Yves Guigoz, researcher at the Nestlé Centre in Switzerland. The declared purpose of the two authors was to provide a fast and reliable tool for the evaluation of nutritional status similar to the Mini-Mental State Examination with a long-time availability for detecting cognitive impairment in the elderly [7]. In 1994 MNA® made its first appearance in a scientific publication [10] and was later validated in a cohort of patients with acute-onset, at the Toulouse hospital [7, 11]. As the test became popular, controversies had raised about its accuracy, especially when compared with alternative nutritional screening tools. Although MNA® sensitivity approaches 100%, its specificity is low, with a positive predictive value of only 66.7%, implying a tendency to overestimate the risk of malnutrition [12]. On the other hand, cross-classification of MNA® and nutritional risk screening (NRS), another popular nutritional assessment tool, resulted in about 30% discrepancy. More specifically, MNA® would tend to classify a smaller number of patients as malnourished or at risk when compared with NRS [13]. To design a faster screening tool, shorter versions of the original MNA® have been developed over time. Among these, the most popular is the MNA®-Short Form (MNA®-SF) developed by Rubenstein et al. in 2001 [5]. This version includes only six items and is administered in a two-step fashion i.e. the full MNA® score is administered only when the MNA®-SF classifies the subject under examination at the risk of malnutrition. However, this shortened test version eliminates many of the subjective items, hence loosing valuable information on the patient’s actual nutritional status.
Among the potential factors able to impact MNA® performance, there are overweight and obesity, which are increasingly detected in the elderly population [14]. In fact, body mass index (BMI), which is a part of the “anthropometric” component of MNA®, can affect the total score up to 10%. However it must be taken into account that in the elderly, weight excess and malnutrition can exist simultaneously. This phenomenon, observed in developed countries and in some populations of emerging countries, was defined as “double burden” of malnutrition [15,16,17].
The main objectives of the present study were: (1) to test the performance of MNA® for nutritional screening in the presence of weight excess; (2) to depict the most sensitive area of the MNA® questionnaire in highlighting malnutrition; and (3) to investigate the relationship between specific MNA® components and the overall functional status of the elderly under examination.
Materials and methods
Study design
This was a retrospective observational single-centre study carried out on sample of elderly subjects from Northern Sardinia. Study participants were outpatients referred by general practitioners or specialists to the Geriatric section of Clinica Medica, a teaching hospital of the University of Sassari, Italy.
Patients eligibility
Study participants were selected from an electronic database of 1344 patients undergoing a geriatric evaluation in a clinical setting. Part of the database was previously utilized for a different purpose [18, 19]. Inclusion criteria were: Sardinian origin of patients (to ensure genetic homogeneity) of age older than 64 years. Exclusion criteria were unavailability of any of the following items: Mini-Nutritional Assessment, body mass index, smoking habits, comorbidity, basic and instrumental activities of daily living, cognitive function and depression status, monthly income, number of drugs taken.
Data collection
Patients were carefully interviewed by a trained geriatrician to collect demographic and clinical information. In case of patients with moderate/severe dementia, family members had been interviewed especially for questions regarding disability.
Clinical records of all study participants reported the information about body height, measured using an electronic scale with an accuracy of up to 0.1 kg, as well as the body weight measured in centimeters using a stadiometer, with the patient’s head aligned according to the Frankfurt horizontal plane [20]. All the measurements had been determined during the outpatient visit. Body mass index (BMI) was calculated as weight/height2 (kg/m2). Nutritional status was evaluated by administering the 18-item (full) version of MNA® questionnaire [10] (Fig. 1). Overall functional state was evaluated through a multidimensional geriatric assessment (MGA) questionnaire [21]. To estimate the comorbidity, all pathological conditions were retrieved from patient clinical records and expressed as cumulative illness rating scale-geriatrics (CIRS-G) [22]. Functional disability in basic activities was measured through the activity of daily living (ADL) scale which assigned a progressive number to each independent function, ranging between 0 (complete dependence) and 6 (complete independence). Autonomy in activities that are physically and cognitively more demanding, but essential for an independent living, were evaluated by the instrumental activities of daily living (IADL) scale. More specifically, because in Latin culture males are rarely involved in household tasks such as cooking, laundry and housekeeping, according to previous studies [23] a shorter version of IADL was used including the following five items: ability to use telephone, handle finances, transportation, shopping and to take medications. A score of 5 corresponds to the ability to be self-sufficient. The mini mental state examination (MMSE) test [24] was used to assess cognitive functions. The test explores consciousness, attention, memory, language, calculation and writing. The overall score, adjusted for age, sex and education ranges from 0 to 30 points [25]. A score lower than 15 indicates a significant impairment of cognitive status; between 15 and 24 mild to moderate dementia, equal or greater than 25 a normal cognitive status. The presence of symptomatic depression was evaluated using a shorter version of the geriatric depression scale (GDS-15) [26]. This scale consists of 15 yes/no items scored as either 0 or 1 . A score between 0 and 5 corresponds to no depression; between 6 and 10 to mild depression; and ≥ 11 to moderate/severe depression [27]. Each examination had been supervised by the same attending physician (P.T.) for all patients during all study periods.
Statistical analysis
The conventional 18-item- MNA® was scored as following: ≥ 24 points: normal nutritional status; between 17 and 23.5 points: risk of malnutrition; and < 17: overt malnutrition. Scores from conventional MNA® (cMNA® including the BMI item) and modified MNA (mMNA, excluding the BMI from the “anthropometric” component) were calculated. The mMNA score could reach at most 27 points instead of the original 30. To assess the impact of overweight/obesity on the performance of MNA®, the scores were compared and the sensitivity, specificity and accuracy of cMNA® were calculated. Body mass index was stratified into four categories: < 18 (kg/m2), 18‒24.9 (kg/m2), 25‒29.9 (kg/m2) and ≥ 30 (kg/m2). Monthly income was used as a proxy for socio-economic status and graded into four categories: < €400, €400‒600, €600‒1000 and more than €1000. According to smoking habit, patients were classified into those who had never smoked and those who were current or former smokers. Marital status was coded into: married, widowed and unmarried or divorced. Education was estimated by the total number of years spent at school. Polypharmacy was grouped according to the number of drugs currently used: 6 drugs or less; from 7 to 9 drugs; 10 drugs or more [28].
Data collected by means of questionnaires were expressed through basic statistical indicators such as mean and standard deviations (SDs) for scalar variables, frequencies and percentages for categorical variables. Comparison between average values or percentages was performed by two-tailed Student’s t test for unpaired samples; in case of m × n contingency tables, the analysis was performed using the χ2 test. The mMNA and cMNA® scores were compared and the true positives (TPs), false positives (FPs), true negatives (TNs), false negatives (FNs), sensitivity, specificity and accuracy were calculated with their 95% confidence intervals (CI). Cohen’s kappa was used to assess the agreement between the two tests, with κ = 1 for perfect agreement and κ = 0 for randomness. Identification of malnutrition predictors was carried out in the whole sample by logistic regression models using a dichotomized mMNA score as dependent variable: 23 to 17 points risk of malnutrition; and less than 17 points overt malnutrition. The independent variables included in the regression analysis, retrieved from MGA, were sex, age, BMI, marital status, monthly income, education, comorbidity and functional capacity. Odds ratios (ORs) and their 95%CIs were calculated by exponentiating the regression coefficients. All statistical analyses were done using SPSS software (version 16.0, Chicago, IL). A threshold value of P < 0.05 was considered to be statistically significant.
Results
Descriptive statistics
Data included in this study were extracted from an electronic database of 1344 geriatric patients evaluated in a teaching hospital of Northern Sardinia between January 2004 and December 2014 and partially used in previous studies [18].
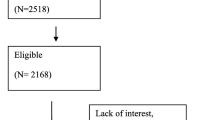
A total of 646 clinical charts from patients aged ≥ 65 years were selected for the study (48.9% females). The majority aged 70‒79 years, without significant differences in the mean age between sexes (75.27 ± 6.89 years versus 76.84 ± 7.11 years). Nearly half (45.7%) of the studied samples consisted of outpatients while the remaining were hospitalized. Table 1 shows the demographic and clinical features of the study patients stratified by sex. Six hundred and ninety-eight patients were excluded because they were younger than 65, or for incomplete data. However, the total MNA® score was available for 646 patients, MNA® partial components were available only for 487 patients (Fig. 2).
Overall, 45% of patients were “at risk of malnutrition” and 23% displayed “overt malnutrition”, significantly higher in women than men (29.7% versus 17.6%; P < 0.0001) (Table 1). Underweight, overweight and frankly obese elderly were 4.8%, 36.4% and 31.1%, respectively, with a marginally significant (P = 0.045) weight excess in women. Mild to moderate dementia (adjusted MMSE score in the range of 15‒24) or moderate to severe dementia (MMSE score ≤ 15) were observed in approximately 45% of patients, with no significant sex differences. Symptomatic depression was detected in 46% of the studied samples, with a significantly higher proportion in women (P < 0.0001). Comorbidity, expressed as CIRS severity, was similar in both sexes (4.2 ± 1.7 in men versus 4.3 ± 1.8 in women).
According to the distribution of ADL and IADL scores, disability was greater in women, whereas no sex difference was found for education (average of 5.9 ± 3.4 years in men, and 5.1 ± 3.7 years in women) (Table 2). As expected, the percentage of former and current smokers, were significantly higher in men (74%) compared to females (18%) (P < 0.0001). The majority of men (71%) were married, while this percentage dropped to 33% in women. The average monthly income exceeded € 1000 in nearly 10% of study participants and ranged € 600‒1000 in 38% of them, with a significant sex difference (P = 0.017) evidencing a women’s disadvantage. Patients taking less than 6 drugs were 37.8%; between 6 and 9 were 50.6% and the 10 or more drugs the remaining 11.6% (Table 1). Clinical and functional characteristics of subjects according to BMI categories are reported in the supplementary material (Table 3). Overall, youngest subjects resulted significantly more obese compared to the oldest ones. Interestingly, among the obese, there were 32 (15.9%) malnourished, and 97 (48.3%) at risk of malnutrition. Moreover, overweight subjects had less disability compared with subjects in the other BMI categories and the trend was statistically significant.
Performance of MNA® test
In 487 patients out of 646 the partial scores of MNA were available in addition to the total score. Table 2 shows the distribution of patients cross-classified according to the cMNA® (including BMI) and the mMNA (excluding BMI). The two scores were concordant in 87.3% and discordant in 12.7% of cases. Cohen’s kappa was 0.805 revealing the substantial agreement. More specifically, 4 patients classified “at risk of malnutrition”by the cMNA® were reclassified as “normal” by the mMNA, and 1 patient with “overt malnutrition” by the cMNA® was reclassified “at risk of malnutrition” by the mMNA. Fifty-seven patients classified “at risk of malnutrition” by the cMNA® were relocated as “overtly malnourished” by the mMNA score. According to the 3 by 3 contingency table there were 188 TPs, at risk of malnutrition; and, 117 TPs, for overt malnutrition. There were 57 patients allocated at risk of malnutrition by the cMNA®, excluding the BMI classified as overt malnourished. The resulting sensitivity, specificity and accuracy of cMNA® were 100%, 97% and 99% for the risk of malnutrition, and 67%, 99% and 84% for overt malnutrition, respectively.
In the supplementary material the data of Table 2 were stratified according to BMI categories. It is evident that the differential scoring of cMNA vs mMNA affects only 5% of patients in the underweight category while it affects 14% of patients in the obesity category. Thus, the cMNA will hardly underestimate the risk of under-weight, the difference being clearly skewed toward the obese patients.
The mean values of the individual components of MNA® for patients with concordant and discordant scores are shown in Table 4. Interestingly, the average scores of the “global”, “subjective” and “dietary” components obtained with cMNA®, were significantly lower when compared with patients reclassified according to the mMNA.
Identification of MNA® critical components to detect malnutrition
The results of multivariate logistic regression models are reported in Table 5. Symptomatic depression, disability and polypharmacy were significant predictors for both risk and overt malnutrition. A low monthly income was associated more to overt malnutrition, but not with the risk. As expected, underweight (BMI < 18 kg/m2) was a significant predictor of overt malnutrition but not of the simple risk. Still, the positive association of overweight/obesity with malnutrition was confirmed.
Discussion
In this retrospective observational study, the performance of MNA® to assess nutrition status was tested in a sample of elderly patients. A striking feature of our sample is the high proportion of individuals with excess of body weight. Mean BMI values were 28.4 kg/m2 in subjects considered “well fed” and 27.8 kg/m2 in those at risk of malnutrition, higher than those previously reported [29, 30]. The proportion of patients with evidence of malnutrition (risk or overt malnutrition) detected by the cMNA® was comparable with data reported in the literature for Western populations [30,31,32] and was greater than Asian countries [33]. The findings observed in our series confirm the coexistence of malnutrition and overweight, the so-called phenomenon of “double burden” [15,16,17] and naturally raised the question of whether this “protective” effect on malnutrition attributable to the BMI is an artefact in overweight patients,due to the inclusion of the BMI value in the anthropometric component of cMNA®. In fact, when the cMNA® (including BMI) and the mMNA (excluding BMI but keeping the same cut-offs) scores were compared, 57 patients resulted falsely well fed, removing the protection of the BMI item. The resulting sensitivity of cMNA® in detecting overt malnutrition was low (67%), whereas it was reasonably high (97%) in detecting the risk of malnutrition.
Additional factors, identified by the multivariable regression analysis, significantly associated with malnutrition in patients with concordant and discordant MNA scores, were low ADL score and symptomatic depression. According to previous studies, the disability was statistically significant in both sexes [34]. Also, the presence of symptomatic depression (GDS-15 score > 5) was identified as an independent predictor of malnutrition, although with different impact in the two sexes: in women it was associated to the simple risk of malnutrition while in men to overt malnutrition. Carpiniello et al. [35] and Cabrera et al. [36] reported a strong association between depression and malnutrition in women, while van’t Veer-Tazelaar et al. [37] found that the effect of sex on depressive symptoms was not significant after adjusting for several confounding factors. However, despite these discordant results, the influence of depression on nutritional status has been extensively demonstrated in the past in different settings: outpatients, institutionalized patients and in the community [29, 36]. Two additional factors identified in the multivariate models showed sex differences: age was a (weak) predictive factor only in women and polypharmacy only in men and for a number of drugs between 5 and 9, similar to previous observation [38]. Another factor associated with the risk of malnutrition was the monthly income, with greater significance for women, and only for average income 400–1000 euros per month, while for men it was a significant risk factor only in subjects with overt malnutrition. As already observed, scarcity of financial resources is one of the causes of insufficient nutrition and/or of poor quality [39].The comparison analysis between the groups of patients classified concordantly or discordantly by the conventional and modified MNAs revealed that the latter patients were more frail than the original group; those reclassified as patients at risk of malnutrition have a higher prevalence of depression, disability and assumed a higher number of drugs; while patients reclassified as malnourished also have disability in the IADL, they are on average more depressed, with a prevalence of the female sex.
The reduced performance of cMNA® in overweight/obese patients might cause underestimation of the percentage of patients truly malnourished, jeopardizing them. It seems that the MNA score that does not take into account the component of overweight/obesity might be more sensitive in detecting malnutrition in the elderly with excess of body weight. In our sample, the subgroup of patients taken as “well fed” according to cMNA and reclassified as malnourished by the mMNA showed a worst overall performance. They were frailer, more depressed, and deficiencies were detected in all MNA® components, not only in the anthropometric ones. This “protection” resulting from being overweight (BMI ≥ 24 kg/m2), artificially relocated patients into a lower nutritional risk category.
A study like the present one has strengths and limitations. Patients selected for the study were quite homogeneous both for socio-cultural aspects (origin from the same geographical area) and for the genetics (the Sardinian population has largely documented homogeneous genetic makeup [40]. A limitation of our study was that polypharmacotherapy was analysed only quantitatively (number of drugs taken daily) rather than qualitatively, without giving prominence to single drugs known, for example, to induce selective nutritional deficits or drugs that alter the taste or decrease appetite.
In conclusion, our results show that excess weight may reduce the performance of cMNA® test in detecting malnutrition. Taken together the results of this study suggest the need for greater attention in identifying obese patients at risk of malnutrition, a condition related to increased morbidity and mortality.
References
Ahmed T, Haboubi N (2010) Assessment and management of nutrition in older people and its importance to health. Clin Interv Aging 5:207–216. https://doi.org/10.2147/CIA.S9664
Rösler A, Lehmann F, Krause T, Wirth R, von Renteln-Kruse W (2010) Nutritional and hydration status in elderly subjects: clinical rating versus bioimpedance analysis. Arch Gerontol Geriatr 50:e81–e85. https://doi.org/10.1016/j.archger.2009.06.007
Bharadwaj S, Ginoya S, Tandon P, Gohel TD, Guirguis J, Vallabh H, Jevenn A, Hanouneh I (2016) Malnutrition: laboratory markers vs nutritional assessment. Gastroenterol Rep (Oxf) 4:272–280. https://doi.org/10.1093/gastro/gow013
Schrader E, Grosch E, Bertsch T, Sieber CC, Volkert D (2016) Nutritional and functional status in geriatric day hospital patients—MNA short form versus full MNA. J Nutr Health Aging 20:918–926. https://doi.org/10.1007/s12603-016-0691-4
Rubenstein LZ, Harker JO, Salvà A, Guigoz Y, Vellas B (2001) Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol A Biol Sci Med Sci 56:M366–372. https://doi.org/10.1093/gerona/56.6.m366
Vellas B, Villars H, Abellan G, Soto ME, Rolland Y, Guigoz Y, Morley JE, Chumlea W, Salva A, Rubenstein LZ, Garry P (2006) Overview of the MNA-Its history and challenges. J Nutr Health Aging 10:456–463
Guigoz Y (2006) The Mini-Nutritional Assessment (MNA®) review of the literature—what does it tell us? J Nutr Health Aging 10:465–487
Belmin J; Expert Panel and Organization Committee (2007) Practical guidelines for the diagnosis and management of weight loss in Alzheimer’s disease: a consensus from appropriateness ratings of a large expert panel. J Nutr Health Aging 11:33–37
Morley JE, Miller DK, Perry HM, Patrick P, Guigoz Y, Vellas B (1999) Anorexia of aging, leptin, and the mini nutritional assessment. In: Vellas B, Garry PJ, Guigoz Y (eds) Mini nutritional assessment (MNA): research and practice in elderly. Nestlé clinical and performance nutrition workshopseries. Lippincott-Raven, Philadelphia, pp 67–76
Guigoz Y, Vellas B, Garry PJ (1994) Mini nutritional assessment: a practical assessment tool for grading the nutritional state of elderly patients. Facts Res Gerontol 4:15–59
Vellas B, Guigoz Y, Baumgartner M, Garry PJ, Lauque S, Albarede JL (2000) Relationships between nutritional markers and the mini nutritional assessment in 155 older persons. J Am Geriatr Soc 48:1300–1309. https://doi.org/10.1111/j.1532-5415.2000.tb02605.x
Baek M-H, Heo Y-R (2015) Evaluation of the efficacy of nutritional screening tools to predict malnutrition in the elderly at a geriatric care hospital. Nutr Res Pract 9:637–643. https://doi.org/10.4162/nrp.2015.9.6.637
Drescher T, Singler K, Ulrich A, Koller M, Keller U, Christ-Crain M, Kressig RW (2010) Comparison of two malnutrition risk screening methods (MNA and NRS 2002) and their association with markers of protein malnutrition in geriatric hospitalized patients. Eur J Clin Nutr 64:887–893. https://doi.org/10.1038/ejcn.2010.64
Samper-Ternent R, Al Snih S (2012) Obesity in older adults: epidemiology and implications for disability and disease. Rev Clin Gerontol 22:10–34
Popkin BM, Lu B, Zhai F (2002) Understanding the nutrition transition: measuring rapid dietary changes in transitional countries. Public Health Nutr 5:947–953. https://doi.org/10.1079/PHN2002370
Doak CM, Adair LS, Bentley M, Monteiro C, Popkin BM (2005) The dual burden household and the nutrition transition paradox. Int J Obes 29:129–136. https://doi.org/10.1038/sj.ijo.0802824
Morley JE (2011) Assessment of malnutrition in older persons: a focus on the mini nutritional assessment. J Nutr Health Aging 15:87–90
Dore MP, Pes GM, Bibbò S, Tedde P, Bassotti G (2018) Constipation in the elderly from Northern Sardinia is positively associated with depression, malnutrition and female gender. Scand J Gastroenterol 53:797–802. https://doi.org/10.1080/00365521.2018.1473485
Pes GM, Licheri G, Soro S, Longo NP, Salis R, Tomassini G, Niolu C, Errigo A, Dore MP. Overweight: a protective factor against comorbidity in the elderly. Environ Res Public Health (in press)
Norton K, Whittingham N, Carter L, Kerr D, Gore C, Marfell-Jones M (1996) Measurement techniques in anthropometry. In: Norton K, Olds T (eds) Anthropometrica. University of New South Wales Press, Sydney, pp 25–76
Volpato S, Cavalieri M, Guerra G, Sioulis F, Ranzini M, Maraldi C, Fellin R, Guralnik JM (2008) Performance-based functional assessment in older hospitalized patients: feasibility and clinical correlates. J Gerontol A Biol Sci Med Sci 63:1393–1398. https://doi.org/10.1093/gerona/63.12.1393
Parmelee PA, Thuras PD, Katz IR, Lawton MP (1995) Validation of the cumulative illness rating scale in a geriatric residential population. J Am Geriatr Soc 43:130–137
Agüero-Torres H, Thomas VS, Winblad B, Fratiglioni L (2002) The impact of somatic and cognitive disorders on the functional status of the elderly. J Clin Epidemiol 55:1007–1012. https://doi.org/10.1016/S0895-4356(02)00461-4
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Molloy DW, Standish TI (1997) A guide to the standardized mini-mental state examination. Int Psychogeriatr 9(Suppl 1):87–94
Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO (1982–1983) Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 17:37–49
Sheikh JI, Yesavage JA (1986) Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clinical Gerontologist 5:165–173
Fulton MM, Allen ER (2005) Polypharmacy in the elderly: a literature review. J Am Acad Nurse Pract 17:123–132. https://doi.org/10.1111/j.1041-2972.2005.0020.x
Saka B, Kaya O, Ozturk GB, Erten N, Karan MA (2010) Malnutrition in the elderly and its relationship with other geriatric syndromes. Clin Nutr 29:745–748. https://doi.org/10.1016/j.clnu.2010.04.006
van Bokhorst-de van der Schueren MA, Lonterman-Monasch S, de Vries OJ, Danner SA, Kramer MH, Muller M (2013) Prevalence and determinants for malnutrition in geriatric outpatients. Clin Nutr 32:1007–1011. https://doi.org/10.1016/j.clnu.2013.05.007
Seiler WO (2001) Clinical picture of malnutrition in ill elderly subject. Nutrition 17:496–498
Jensen GL, Friedmann JM, Coleman CD, Smiciklas-Wright H (2001) Screening for hospitalization and nutritional risks among community-dwelling older persons. Am J Clin Nutr 74:201–205. https://doi.org/10.1093/ajcn/74.2.201
Chang HH, Tsai SL, Chen CY, Liu WJ (2010) Outcomes of hospitalized elderly patients with geriatric syndrome: report of a community hospital reform plan in Taiwan. Arch Gerontol Geriatr 50(Suppl 1):S30–33. https://doi.org/10.1016/s0167-4943(10)70009-1
Jyrkkä J, Enlund H, Lavikainen P, Sulkava R, Hartikainen S (2011) Association of polypharmacy with nutritional status, functional ability and cognitive capacity over a three-year period in an elderly population. Pharmacoepidemiol Drug Saf 20:514–522. https://doi.org/10.1002/pds.2116
Carpiniello B, Carta MG, Rudas N (1989) Depression among elderly people. A psychosocial study of urban and rural populations. Acta Psychiatr Scand 80:445–450
Cabrera MA, Mesas AE, Garcia AR, de Andrade SM (2007) Malnutrition and depression among community-dwelling elderly people. J Am Med Dir Assoc 8:582–584. https://doi.org/10.1016/j.jamda.2007.07.008
van’t Veer-Tazelaar PJ, van Marwijk HW, Jansen AP, Rijmen F, Kostense PJ, van Oppen P, van Hout HP, Stalman WA, Beekman AT (2008) Depression in old age (75 +), the PIKO study. J Affect Disord 106:295–299. https://doi.org/10.1016/j.jad.2007.07.004
Schilp J, Wijnhoven HA, Deeg DJ, Visser M (2011) Early determinants for the development of undernutrition in an older general population: longitudinal Aging Study Amsterdam. Br J Nutr 106:708–717. https://doi.org/10.1017/s0007114511000717
Donini LM, Scardella P, Piombo L, Neri B, Asprino R, Proietti AR, Carcaterra S, Cava E, Cataldi S, Cucinotta D, Di Bella G, Barbagallo M, Morrone A (2013) Malnutrition in elderly: social and economic determinants. J Nutr Health Aging 17:9–15. https://doi.org/10.1007/s12603-012-0374-8
Cavalli-Sforza LL, Menozzi P, Piazza A (1994) The History and Geography of Human Genes. Princeton University Press, New Jersey, p ISBN 0691087504
Acknowledgements
This paper is dedicated to the memory of our dearest colleague and friend, Professor Rinaldo Tedde, geriatrician of great experience and humanity.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All the procedures performed in this study were in accordance with the ethical standards of the Institutional Review Board and Ethics Committee at the University of Sassari (Comitato Etico della Facoltà di Medicina dell’Università degli Studi di Sassari), protocol no. G327/2004, and with the 1964 Helsinki Declaration.
Informed consent
The study protocol was approved by the local Ethics Committee of the Faculty of Medicine (Comitato Etico della Facoltà di Medicina dell’Università degli Studi di Sassari) protocol no. G327/2004, and a written- informed consent was provided by all the participants or their care givers in case of severe dementia.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pes, G.M., Loriga, S., Errigo, A. et al. Is mini-nutritional assessment a reliable tool in detecting malnutrition in elderly with body weight excess?. Eat Weight Disord 25, 1425–1435 (2020). https://doi.org/10.1007/s40519-019-00780-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40519-019-00780-y