Abstract
Purpose
Eating disorders are common in Parkinson’s disease (PD) patients and often class in Impulse control disorders, however, little is known about their phenomenology. Specific symptoms and comorbidities were described in a group of PD patients in this preliminary study.
Methods
Over a period of 6 months, 51 PD patients who experienced significant changes in eating habits following diagnosis of PD and were interviewed during regularly scheduled follow-up visits. We assessed each patient’s height and weight, impulsivity, psychological distress, current eating disorder symptoms, food addiction, food habits and craving.
Results
Among the PD patients who experienced modified dietary habits following diagnosis, few exhibited binge eating disorders (BED) full criteria (3.9%). However, 21.6% of patients experienced episodes of out-of-control eating with a large quantity of food in short time and 39.2% satisfied food addiction (FA) criteria without binge eating disorder. Food cravings more than once a week were experienced in approximately half of the population including all FA patients. Regarding comorbidities, FA PD patients present impulsive features and anxiety.
Conclusions
This study confirms the existence of FA profile in PD patients. Eating disorders even in PD are complex and have a cross-cutting criteria related to out-of-control eating, FA, and BED. The association of anxiety with PD-related food addiction, contrary to L-dopa equivalent daily dose mean score or the presence of dopamine agonists, underline the complex sustainability of the dopaminergic brainstem support. A study on their detailed prevalence in this population could be helpful to better understand unspecified feeding or eating disorder.
Clinical trial number
DR-2012-007.
Name of the registry
French Committee for the Protection of Persons (CPP) & French National Commission on Computing and Liberty (CNIL).
Level of evidence
Level V, descriptive study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Purpose
Impulse control disorders (ICDs) are potentially serious psychiatric complications in patients with Parkinson’s disease (PD) [1]. ICDs consist of compulsive and repetitive behaviors that are excessive and/or ultimately harmful to oneself or others [2] and include compulsive gambling, sexual behaviors, compulsive buying and eating, as well as punding, excessive hobbyism, and overuse of dopamine agents, known as dopamine dysregulation syndrome [1, 3].
Eating disorders (EDs) are common in PD patients [4], however, little is known about the phenomenology of EDs, which are associated with nibbling, overeating, and in some cases, binge eating disorders (BEDs). The new nosological entity of Food Addiction (FA) can be interesting in exploration of these ICDs in PD patients. Some studies has already investigated the relationship between FA and BED, but generally in specific population like obese participants. Results further support the strong relation between the two constructs [5] and question the observation of this phenomenon in PD.
In the Diagnostic and Statistical Manual of Mental Disorders fifth edition (DSM-5), BEDs have been defined as eating an amount of food that is definitely larger than most people would eat during the same period of time under similar circumstances coupled with a perceived lack of control over one’s eating. Patients with BEDs must exhibit marked distress regarding binge eating and may eat too quickly, even if not hungry, and alone because of feeling embarrassed by how much one is eating. Binge eating episodes must occur at least once a week for at least 3 months, and individuals must not engage in inappropriate compensatory behaviors such as purging or fasting. The regulation of food intake is complex and involves multiple levels of control through environmental cues and cognitive, sensory, metabolic, endocrine, and neural pathways [6].
In PD, the definition of EDs differs. Compulsive Eating (CE) described using the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP), based on the Diagnostic and Statistical Manual of Mental Disorders fourth edition (DSM-IV) criterion [7], is a positive response to any two of the five following items: (1) eating too much; (2) thinking too much about eating; (3) having urges or desires for eating behavior; (4) difficulty controlling eating behavior; and (5) engaging in activities specifically to continue eating [1]. The QUIP includes binge eating episodes, but also general symptoms of overeating. It corresponds to recent considerations of subthreshold EDs in the case of individuals who fail to meet all DSM-IV criteria for EDs [8]. There is no clear consensus regarding EDs in PD: are they part of a larger continuum that includes overeating and BEDs, or is there a specific symptomatology? Thus, as a first immediate step, a better understanding of EDs in PD patients is necessary given the current lack of information.
Moreover, we integrate affective outcomes associated with EDs to explore link between pathophysiology of impulsivity, depression and anxiety into this specific ICDs as already demonstrated in a broad range of ICDs [9].
The primary objective of this study was to identify the main characteristics of EDs (e.g., lack of control, compensatory behavior, cravings) in patients with PD. The secondary objective was to assess the relationship between the onset of EDs and other key symptoms of PD, specifically impulsivity and depression.
Methods
PD patients (based on the UK Brain Bank criteria) were screened during regularly scheduled follow-up visits at the Movement Disorders Unit of Clermont-Ferrand French hospital over a 6-month period. Patients reporting to have experienced significantly changed eating habits following PD diagnosis as well as a larger appetite and/or increased snacking during the day and/or night compared with before PD were included. Exclusion criteria were chronic illness (with the exception of PD) that could influence eating habits (e.g., diabetes) and an inability to understand the instructions: language or Mini Mental State Examination < 24.
Socio-demographic and anthropometric parameters (height and weight) and clinical data (features of PD) including medical details were collected. On the same day, PD patients underwent the following assessments details: PD diagnosis, medical history, and medication use were collected through patient interview and confirmed with medical records. Anti-parkinsonian medications were converted into L-DOPA equivalent daily dose (LEDD) [10]. Subjects were then administered portions of the Movement Disorders Society-Unified Parkinson Disease Rating Scale (MDS-UPDRS), which includes an interview and examination by a movement disorder neurologist. Subscores for non-motor experiences of daily living (Part I) and motor examination (Part III) of the MDS-UPDRS were calculated.
We assessed psychological distress with the hospital anxiety and depression scale (HADS). Impulsivity was assessed with the Barratt impulsiveness scale (BIS-11) [11], which is a 30-item questionnaire designed to assess common impulsive behaviors in three separable dispositions: attentional impulsiveness, defined as the (in-)ability to concentrate or focus attention, motor impulsiveness (tendency to act without thinking), and non-planning impulsiveness (lack of future planning and forethought).
Following interview and examination, participants were asked to provide individual information regarding food habits and food addiction (FA) (including DSM-5 criteria and questionnaire on Eating and Weight Patterns (QEWP-R) [12]) and food cravings [13]. QEWP-R focuses primarily on assessing diagnostic criteria for BEDs, but also assesses Night Eating Syndrome (NES) and more generally FA. NES is a circadian delay in the pattern of daily food intake: evening hyperphagia and/or nocturnal ingestions of food.
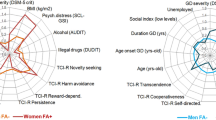
Statistical analysis was performed with SAS 9.3 (SAS Institute, Cary, NC, USA). The tests were two-sided, with a type I error set at α = 0.05. Continuous data were presented as the mean ± standard deviation (SD) with [interquartile range] for body mass index (BMI) (assumption of normality verified using normal probability plots and Shapiro–Wilk’s test). Comparisons between independent groups were analyzed using the Student’s t test for quantitative variables. Comparisons concerning categorical data were performed using the Chi-squared test or Fisher’s exact test. Spider diagram was used as a visual tool to illustrate a comparison between FA and No food addiction (NFA) groups, and represents the multivariate data (categorical, %; quantitative, mean) as a two-dimensional chart with variables displayed on the axes.
Results
In total, 51 patients experienced changes in their dietary habits following PD diagnosis (e.g., larger appetite and/or increased snacking during the day and/or night compared with before PD), and these changes were independent of other illnesses.
Of these patients, 53% were men with an average age of 68 years (SD = 10). The average duration of PD disease was 13 ± 6 years and the average LEDD was 647.5 ± 437.5 mg/day. The mean MDS-UPDRS-1 was 1.9 ± 1.7 and − 3 score was 18 ± 10, which is indicative of a population of PD population with mild disease. These was no evidence of hypoglycemia or hyperthyroidism in any of the patients and approximately 43% of patients were overweight or in obese—class I based on BMI (Table 1).
Among included patients, 3.9% satisfied the BEDs criteria, while 39.2% of patients satisfied FA criteria without BEDs (n = 20). BEDs patients are not included in the FA/NFA group following analyzes. Patterns of food consumption are summarized in Table 2. Approximately 69% of patients ate regularly outside of mealtimes day and/or night. An episode of out-of-control eating, which occurs when an objectively large quantity of food is consumed during a discrete period of time, was described by 12% of patients. In contrast, there were no cases of strictly defined purging or non-purging bulimia nervosa (QEWP-R). Many patients were close, but ultimately did not meet the required criteria for BEDs. Approximately 27% had an episode of out-of-control eating with a large quantity of food in a short time; approximately 45% described insatiable and uncontrollable cravings for food and frequently ate small quantities of food outside of meals. Furthermore, 23.5% of patients ate without a real sensation of hunger. Sleep-related EDs are characterized by abnormal eating patterns during the night, known as NES, which is diagnosed when a person eats during the night with full awareness and may be unable to fall asleep again unless he/she eats. NES was observed in 5.9% of patients (n = 3). All of them have also FA symptoms without BED and are included in the FA group analyze. Additional behaviors were also observed: patients frequently ate alone (47.1%) but not necessarily in secret (hidden attitudes for only 9.8%). 25% of patients (n = 13) had at least one compensatory behavior, but only five patients (9.9%) exhibited these behaviors at least 2–3 times per week and they generally preferred to fast or perform excessive exercise. Additional behaviors were also observed: patients frequently ate alone (47.1%) but not necessarily in secret (hidden attitudes for only 9.8%). 25% of patients (n = 13) had at least one compensatory behavior but only five patients (9.9%) exhibited these behaviors at least 2–3 times per week and they generally preferred to fast or perform excessive exercise (Table 3).
We observed that 43.1% of patients experienced food cravings more than once a week (n = 22). It represents BED patients and FA group analyzed. None of our patients described a craving for fats. Patients with cravings for sweets (n = 13) had food cravings more than once a week, and five (38.5%) experienced cravings at least once a day. All patients that craved food and could not find a way past this episode craved sweets (n = 4).
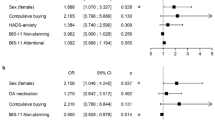
Chronologies of beginning PD-related disease show that 64.7% of patients exhibited EDs early after the diagnosis of PD (1–2 years after), 25.5% approximately 5 years after, and only 9.8% after 10 years or more. One-fourth of patients (n = 13) say their diet modifications were related to their PD treatment: 61.5% underwent L-DOPA therapy and 23.1% were taking pramipexole. There’s no significant difference between FA group without BED (n = 20)/NFA (n = 29) regarding implication of their PD treatment on their diet modifications (respectively 35.0% vs. 20.7%, p = 0.33). In the FA group, significantly more patients were treated with pramipexole than in the NFA group (30.0% vs. 7.0%, p = 0.032) (Fig. 1). MDS-UPDRS-1 and LEDD mean scores and the use of dopamine agonists were not significantly different between FA and NFA groups. The NFA group had a significant lower MDS-UPDRS-3 mean score compared with FA group (14.2, SD = 7.1 vs. 20.2, SD = 10.6, p = 0.032) but all patients experienced only mild to moderate motor disability. Regarding BMI, there’s no significant difference between FA/NFA groups (respectively 23.5 vs. 25.0 p = 0.22). Approximately 7% of NFA patients were overweight or in obese—class I against 10% of FA patients (NS p = 0.97). Results observed for the total BIS-11 score and the scores for each of the subcategories of BIS-11 (attentional, motor, or non-planning impulsiveness) are similar to the scores of the initial group (n = 51). For FA patients (n = 20), 45.0% exhibited impulsive features with a total BIS-11 score > 65. The mean total BIS-11 score was significantly higher in FA group (64.7, SD = 9.6, p = 0.012) compared with the NFA group (58.5, SD = 6.9). One of the two cases of BEDs had a high BIS-11 score (total = 98 and subcategories > 30). Based on the HADS scores, 23.5% of patients experienced anxiety, 5.9% suffered from depression, and 11.8% experienced anxiety and depression. In the phagomania group (n = 20), these percentages rose to 40.0% for anxiety alone and 10.0% for anxiety and depression, although decreased for depression alone (5.0%). Comparison of FA group with NFA group revealed only one significant difference in the proportion of anxious patients (40.0% vs. 13.8%, respectively, p = 0.036).
Spider diagram of characteristic patients in FA and NFA Parkinson’s disease groups. *p < 0.05 and **p < 0.001 (comparison between groups). FA food addiction, NFA no food addiction, NES night eating syndrome, BIS11 > 65: Barratt Impulsiveness Scale score superior to 65′, LEDDl-dopa equivalent daily dose, MDS-UPDRS 1 Movement Disorders Society-Unified Parkinson Disease Rating Scale Part I score, MDS-UPDRS 3 Movement Disorders Society-Unified Parkinson Disease Rating Scale Part III score
Discussion
These results show that EDs in PD are more complex than suspected using QUIP questionnaire. Although not formally recognized by the DSM-5, food addiction (FA) has been well described in the ED and obesity literature, but unexplored in PD. ICDs including EDs and more specially BEDs has already been described in PD [14], although descriptions have been limited. Like in non-PD population [15], EDs in PD present a more disturbed subset than BEDs alone or FA. Their core feature is probably the high-reward dependence and out-of-control eating. Craving and compulsion are an important construct to consider in these PD EDs like already observed in FA in general [16].
The definition of ICDs liked to EDs needs to be modified to include food addiction symptoms, such as diminution of control over consumption, which appears to be a core feature even if whether binge eating represents an addiction remains unclear [17]. We noticed that the cases in this study mimic addiction comportments as has been previously described in humans [18].
Weight is not a sufficient warning sign in this type of ICDs for PD patients or their family. First, weight gain can be seen as a positive evolution of the disease because in popular imagery, it is linked with improvement of health; second, it can be balanced by weight loss, which is a common feature in the 10 years preceding a PD diagnosis [19] and so may go unnoticed. Third, FA can exist without weight gain or obesity [20].
With respect to the association factors in FA group, MDS-UPDRS-1 and LEDD mean scores and the presence of dopamine agonists are not significantly relevant, contrary to other ICDs [21]. Moreover many patients declare that their diet modifications were related to their PD treatment. Several explanations can be hypothesis. First of all, advertising could have influenced what these patients reported. Because of the knowledge gained from the media and medical information, PD patients may be more likely to declare ICDs and easily to suspect the link to their PD treatment [22]. Second, and if the truth of their words is demonstrated, ICDs subtypes might be differentially affected by dopamine degeneration and dopamine suppletion inducing heterogeneity in cases observed [23, 24]. The central mechanism could be that chronic exposure to treatment with dopamine agonists suppresses striatal dopamine D2 receptors (D2R) availability in striatum and in extra-striatal region and may conduct to development of non-motor symptoms in PD [25], but only in some ‘sensitive cases’. Hopefully, future studies will help understand the relevance of extra-striatal D2R system in the development of non-motor symptoms and the associations with exposure to dopaminergic treatment in patients with PD. In obese individuals, a meta-analysis observed that low availability of D2R in the nucleus accumbens reduces activity in the prefrontal cortex, contributing to impulsivity and poor self-control [26]. Recently, Weintraub and Mamikonyan [27] suggest that behavioral changes could be subsequent of an altered imbalance between D1-like and D2-like receptors. They are associated with prefrontal cortex–ventral striatum/amygdala pathways involved in decision making relevant to ICD behaviors. D2-like receptors enable flexible decision making and D1-like receptors promote persistence in choice biases. Another track suggests the role of dopamine transporter (DAT). In PD, it is downregulated to increase available dopamine in the synapse [28]. A greater decrease or lower DAT binding over time increases risk of incident ICDs symptoms, conferring additional risk to those taking DRT [29]. There is a relationship between polymorphisms in the DAT gene and binge eating [30, 31]. The manifestation of BEDs is also linked to changes of dopamine regulation in the ventral striatum and associated alterations to dopamine receptor biology [25, 32]. Furthermore, polymorphisms in the DAT gene are associated with BED, and increased synaptic dopamine and reduced transporter function may result in important changes to mesolimbic biology and appetite stimulation [32]. It seems also important to consider changes outside the dopaminergic system, interaction among different neural networks and particularly role of serotonergic and noradrenergic systems since these are also affected by the PD pathology and implicated in impulse control [30, 33]. A recent work on ICDs behaviors show that they may have a multiple gene interactions and that several neurotransmitter systems may contribute to ICDs pathogenesis [34]. Genetic variants might enhance ICDs risk in the absence of other clinical risk factors (e.g., DA use, higher LEDD), while other variants might increase ICDs risk only under specific treatments (e.g., DA treatment). It will be interesting to study the implication of previous observed candidate selection of single nucleotide polymorphisms in this subgroup of ICDs subjects [35].
Previous studies have exposed ICDs and more precisely compulsive EDs cases during treatment with the dopamine agonist pramipexole [24, 36, 37]. This effect is attributed to excessive activation or sensitization of the mesocorticolimbic dopaminergic pathway, which, under physiological conditions, mediates the response to natural rewards [9, 38]. It has been associated with selective D3 stimulation [37]. It confirms the impact of dopamine neurocircuitry already observed in obese individuals with FA [39]. Younger age or younger age at PD onset appear to be risk factors for EDs, similar to other ICDs [27, 36, 40].
The FA group has a significant higher MDS-UPDRS-3 mean score compared with NFA group. It can be explain by a mediator that can be malnutrition since nutritional status is known to worse PD motor features [41]. It is a supplementary reason to stay vigilant regarding eating habits and more precisely nutritional status in PD to prevent theoretically worsens of the disease process.
Moreover, we confirm that a personality profile characterized by impulsiveness is linked to FA, similar to other ICDs in PD patients [9, 27, 42]. It has already been suggested that increased impulsivity may not only be dependent on medication status, but also on neuroanatomical abnormalities intrinsic to PD [43]. Houeto et al. [44] develop new pathways hypothesis underlying the development of ICDs. One will be dependent on trait impulsivity, exacerbated by nigrostriatal DA denervation, and interacts with DRT to facilitate the development of ICDs in specific PD individuals. Otherwise, impulsivity has been as a core factor of eating pathology including FA [45]. The loss of eating control observed in many EDs and particularly in FA and BED, has also been related to structural and functional changes of regions of the orbitofrontal and anterior cingulate cortices [46], same regions impacted by PD.
EDs have also been linked to a negative emotional state. In the general population, sub-threshold EDs have been described as “emotional eating” [47] and increased “snacking” [48]. In PD, EDs were more likely in patients with mood fluctuations and who were in the “ON” state when they experienced euphoria [49], but not in depressive moods. Our findings also suggest a potential influence of anxiety on EDs, as has been previously observed [42].
In some compulsive disorders, the underpinning neural mechanisms may overlap significantly with those relating to anxiety [50]. A recent meta-analysis on ICDs in PD patients [9] observe that depression and anxiety levels were higher in ICDs PD patients compared to those without ICDs. We do not observe association of EDs and depressive state. Regarding pooled effect size in reported forest plots for depression in this study [9], we see that it is weaker than anxiety and mostly than impulsivity. Our observation on depression outcome may be taken with caution because a weaker effect needs to be compensated by a sample size adjustment. On the other hand, we failed to demonstrate a higher frequency of depression like Srivastava et al. in a specific study in parkin-associated PD [51]. It has also been demonstrated that parkin-associated PD demonstrate a higher frequency and severity of specific ICBs including CE compared to none parkin-associated PD [52]. We do not have explore PD genotype origins in our study’s population, but it seems to be interesting in future works to confirm variants in PD-causing genes linked to specific ICDs development [53, 54].
We confirm that our patients report elevated food craving, particularly, for a half of them, for high sugar foods, mimicking substance-use disorders, as has been previously suggested [55]. Davis and al [56]. suggest that craving mediates the relationship between dopamine signaling and clinically significant food addiction symptoms even after controlling for binge eating and emotional eating. This criterion seems to be interesting in future works particularly in this type of ICDs. In the general population, food addiction has also been associated with increased sweet preference [57] and articles [58, 59] confirm that the most common foods associated with addictive symptoms were those high in refined carbohydrates such as sugar or highly processed foods (added sugars, sweeteners…).
Some limitations of our study must be highlighted. EDs, including FA, are complex disorders influenced by a number of factors. It involves multiple levels of control through environmental cues and cognitive, sensory, metabolic, endocrine, and neural pathways [6, 60]. We did not explore other research paths, such as the hormonal profile of patients [61, 62], satiety signals from previously consumed foods [63], or potential decoherence in brain processes of quantum delaying normal mealtime satiation [64]. We do not assessed FA with the Yale Food Addiction Scale (YFAS) that could have allowed us to quantify the severity of FA [65]. Finally, our study involved a small sample of PD patients, limiting statistical power. Nevertheless, this sample was composed of PD patients of both sexes with a wild range of severities (MDS-UPDRS-3 range [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49]).
Conclusions
Our results support the existence of FA profile in PD patients and more specifically of compulsive eating symptomatology. Food craving seems also to be a very important dimension. Further research to understand the complex mechanisms involved in its pathogenesis are needed. Solely weight or BMI is not sufficient to be a warning sign for FA even in PD. We must be careful on eating behaviors of our patient to prevent addictive eating patterns, especially since it seems to influence motor symptomatology. The association of anxiety with PD-related FA, contrary to L-dopa equivalent daily dose mean score or the presence of dopamine agonists, underline the complex sustainability of the dopaminergic brainstem support. Further research must be encouraged to investigate food addiction symptoms and prevalence of EDs in patients with PD, but also compared with those in general and specific populations.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- EDs:
-
Eating disorders
- BEDs:
-
Binge eating disorders
- BIS-11:
-
Barratt impulsiveness scale
- BMI:
-
Body mass index
- CE:
-
Compulsive eating
- FA:
-
Food addiction
- HADS:
-
Hospital anxiety and depression scale
- ICDs:
-
Impulse control disorders
- PD:
-
Parkinson’s disease
- LEDD:
-
l-DOPA equivalent daily dose
- MDS-UPDRS:
-
Movement Disorders Society-Unified Parkinson Disease Rating Scale
- NES:
-
Night eating syndrome
- NFA:
-
No food addiction
- QEWP-R:
-
Questionnaire on Eating and Weight Patterns
- QUIP:
-
Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease
References
Mestre TA, Strafella AP, Thomsen T, Voon V, Miyasaki J (2013) Diagnosis and treatment of impulse control disorders in patients with movement disorders. Ther Adv Neurol Disord 6:175–188
Schreiber L, Odlaug BL, Grant JE (2011) Impulse control disorders: updated review of clinical characteristics and pharmacological management. Front Psychiatry 2:1
Weintraub D, David AS, Evans AH, Grant JE, Stacy M (2015) Clinical spectrum of impulse control disorders in Parkinson’s disease. Mov Disord 30:121–127
Antonini A, Barone P, Bonuccelli U, Annoni K, Asgharnejad M, Stanzione P (2017) ICARUS study: prevalence and clinical features of impulse control disorders in Parkinson’s disease. J Neurol Neurosurg Psychiatry 88:317–324
Davis C, Curtis C, Levitan RD, Carter JC, Kaplan AS, Kennedy JL (2011) Evidence that ‘food addiction’ is a valid phenotype of obesity. Appetite 57:711–717. https://doi.org/10.1016/j.appet.2011.1008.1017 (Epub 2011 Sep 1013)
Alonso-Alonso M, Woods SC, Pelchat M, Grigson PS, Stice E, Farooqi S, Khoo CS, Mattes RD, Beauchamp GK (2015) Food reward system: current perspectives and future research needs. Nutr Rev 73:296–307
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders. APA, Washington
Preti A, Girolamo G, Vilagut G, Alonso J, Graaf R, Bruffaerts R, Demyttenaere K, Pinto-Meza A, Haro JM, Morosini P (2009) The epidemiology of eating disorders in six European countries: results of the ESEMeD-WMH project. J Psychiatr Res 43:1125–1132
Martini A, Dal Lago D, Edelstyn NMJ, Grange JA, Tamburin S (2018) Impulse control disorder in Parkinson’s Disease: a meta-analysis of cognitive, affective, and motivational correlates. Front Neurol 9:654
Thobois S (2006) Proposed dose equivalence for rapid switch between dopamine receptor agonists in Parkinson’s disease: a review of the literature. Clin Ther 28:1–12
Patton JH, Stanford MS, Barratt ES (1995) Factor structure of the Barratt impulsiveness scale. J Clin Psychol 51:768–774
Yanovski SZ (1993) Binge eating disorder: current knowledge and future directions. Obes Res 1:306–324
Guy-Grand B, Pouillon M, Le Barzic M (1997) Caractérisation des “accés alimentaires impulsifs” chez les sujets obéses”. Cahiers de Nutrition et de Diététique 32:307–312
Voon V, Fox SH (2007) Medication-related impulse control and repetitive behaviors in Parkinson disease. Arch Neurol 64:1089–1096
Wiss DA, Brewerton TD (2017) Incorporating food addiction into disordered eating: the disordered eating food addiction nutrition guide (DEFANG). Eat Weight Disord 22:49–59
Potenza MN, Grilo CM (2014) How relevant is food craving to obesity and its treatment? Front Psychiatry 5:164. https://doi.org/10.3389/fpsyt.2014.00164 (eCollection 02014)
Corwin RL (2011) The face of uncertainty eats. Curr Drug Abuse Rev 4:174–181
Gearhardt AN, White MA, Potenza MN (2011) Binge eating disorder and food addiction. Curr Drug Abuse Rev 4:201–207
Chen H, Zhang SM, Hernan MA, Willett WC, Ascherio A (2003) Weight loss in Parkinson’s disease. Ann Neurol 53:676–679
Eichen DM, Lent MR, Goldbacher E, Foster GD: Exploration of “food addiction” in overweight and obese treatment-seeking adults. Appetite 67:22–24., https://doi.org/10.1016/j.appet.2013.1003.1008 (Epub 2013 Mar 1025., 2013)
Lee JY, Kim JM, Kim JW, Cho J, Lee WY, Kim HJ, Jeon BS (2010) Association between the dose of dopaminergic medication and the behavioral disturbances in Parkinson disease. Parkinsonism Relat Disord 16:202–207
Gendreau KE, Potenza MN (2016) Publicity and reports of behavioral addictions associated with dopamine agonists. J Behav Addict 5:140–143
Voon V, Napier TC, Frank MJ, Sgambato-Faure V, Grace AA, Rodriguez-Oroz M, Obeso J, Bezard E, Fernagut PO (2017) Impulse control disorders and levodopa-induced dyskinesias in Parkinson’s disease: an update. Lancet Neurol 16:238–250. https://doi.org/10.1016/S1474-4422(1017)30004-30002 (Epub 32017 Feb 30015)
Grall-Bronnec M, Victorri-Vigneau C, Donnio Y, Leboucher J, Rousselet M, Thiabaud E, Zreika N, Derkinderen P, Challet-Bouju G (2018) Dopamine agonists and impulse control disorders: a complex association. Drug Saf 41:19–75
Politis M, Wilson H, Wu K, Brooks DJ, Piccini P (2017) Chronic exposure to dopamine agonists affects the integrity of striatal D2 receptors in Parkinson’s patients. NeuroImage: Clin 16:455–460
Benton D, Young HA (2016) A meta-analysis of the relationship between brain dopamine receptors and obesity: a matter of changes in behavior rather than food addiction? Int J Obes Suppl 1:S12–S21
Weintraub D, Mamikonyan E (2019) Impulse control disorders in Parkinson’s disease. Am J Psychiatry 176:5–11
Stoessl AJ, Lehericy S, Strafella AP (2014) Imaging insights into basal ganglia function, Parkinson’s disease, and dystonia. Lancet 384:532–544. https://doi.org/10.1016/S0140-6736(1014)60041-60046 (Epub 62014 Jun 60018)
Smith KM, Xie SX, Weintraub D (2016) Incident impulse control disorder symptoms and dopamine transporter imaging in Parkinson disease. J Neurol Neurosurg Psychiatry 87:864–870
Aracil-Bolanos I, Strafella AP (2016) Molecular imaging and neural networks in impulse control disorders in Parkinson’s disease. Parkinsonism Relat Disord 22(Suppl 1):101–105
Shinohara M, Mizushima H, Hirano M, Shioe K, Nakazawa M, Hiejima Y, Ono Y, Kanba S (2004) Eating disorders with binge-eating behaviour are associated with the s allele of the 3′-UTR VNTR polymorphism of the dopamine transporter gene. J Psychiatry Neurosci 29:134–137
Lopez AM, Weintraub D, Claassen DO (2017) Impulse control disorders and related complications of Parkinson’s disease therapy. Semin Neurol 37:186–192
Vriend C (2018) The neurobiology of impulse control disorders in Parkinson’s disease: from neurotransmitters to neural networks. Cell Tissue Res 373:327–336
Kraemmer J, Smith K, Weintraub D, Guillemot V, Nalls MA, Cormier-Dequaire F, Moszer I, Brice A, Singleton AB, Corvol JC (2016) Clinical-genetic model predicts incident impulse control disorders in Parkinson’s disease. J Neurol Neurosurg Psychiatry 87:1106–1111
Erga AH, Alves G, Larsen JP, Tysnes OB, Pedersen KF (2017) Impulsive and compulsive behaviors in Parkinson’s disease: the Norwegian ParkWest Study. J Parkinsons Dis 7:183–191
Nirenberg MJ, Waters C (2006) Compulsive eating and weight gain related to dopamine agonist use. Mov Disord 21:524–529
Borovac JA (2016) Side effects of a dopamine agonist therapy for Parkinson’s disease: a mini-review of clinical pharmacology. Yale J Biol Med 89:37–47
Evans AH, Lees AJ (2004) Dopamine dysregulation syndrome in Parkinson’s disease. Curr Opin Neurol 17:393–398
Guo J, Simmons WK, Herscovitch P, Martin A, Hall KD (2014) Striatal dopamine D2-like receptor correlation patterns with human obesity and opportunistic eating behavior. Mol Psychiatry 19:1078–1084. https://doi.org/10.1038/mp.2014.1102 (Epub 2014 Sep 1079)
Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, Whetteckey J, Wunderlich GR, Lang AE (2010) Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol 67:589–595
Ongun N (2018) Does nutritional status affect Parkinson’s Disease features and quality of life? PLoS One 13:e0205100
Voon V, Sohr M, Lang AE, Potenza MN, Siderowf AD, Whetteckey J, Weintraub D, Wunderlich GR, Stacy M (2011) Impulse control disorders in Parkinson disease: a multicenter case–control study. Ann Neurol 69:986–996
Weintraub D, Papay K, Siderowf A (2013) Screening for impulse control symptoms in patients with de novo Parkinson disease: a case–control study. Neurology 80:176–180
Houeto JL, Magnard R, Dalley JW, Belin D, Carnicella S (2016) Trait impulsivity and anhedonia: two gateways for the development of impulse control disorders in Parkinson’s disease? Front Psychiatry 7:91
Manasse SM, Espel HM, Schumacher LM, Kerrigan SG, Zhang F, Forman EM, Juarascio AS: Does impulsivity predict outcome in treatment for binge eating disorder? A multimodal investigation. Appetite 105:172–179. https://doi.org/10.1016/j.appet.2016.1005.1026 (Epub 2016 May 1024., 2016)
Tahmasian M, Rochhausen L, Maier F, Williamson KL, Drzezga A, Timmermann L, Van Eimeren T, Eggers C (2015) Impulsivity is associated with increased metabolism in the fronto-insular network in Parkinson’s disease. Front Behav Neurosci 9:317
Konttinen H, Mannisto S, Sarlio-Lahteenkorva S, Silventoinen K, Haukkala A (2010) Emotional eating, depressive symptoms and self-reported food consumption. A population-based study. Appetite 54:473–479
Kawada T, Suzuki S (2011) Depressive state, aging, and prevalence of snacking: a preliminary study. Psychogeriatrics 11:247–248
Miwa H, Kondo T: Alteration of eating behaviors in patients with Parkinson’s disease: possibly overlooked? Neurocase 14:480–484, 2008
Fineberg NA, Apergis-Schoute AM, Vaghi MM, Banca P, Gillan CM, Voon V, Chamberlain SR, Cinosi E, Reid J, Shahper S, Bullmore ET, Sahakian BJ, Robbins TW (2018) Mapping compulsivity in the DSM-5 obsessive compulsive and related disorders: cognitive domains, neural circuitry, and treatment. Int J Neuropsychopharmacol 21:42–58
Srivastava A, Tang MX, Mejia-Santana H, Rosado L, Louis ED, Caccappolo E, Comella C, Colcher A, Siderowf A, Jennings D, Nance M, Bressman S, Scott WK, Tanner C, Mickel S, Andrews H, Waters C, Fahn S, Cote L, Frucht S, Ford B, Alcalay RN, Ross B, Orbe Reilly M, Rezak M, Novak K, Friedman JH, Pfeiffer RD, Marsh L, Hiner B, Merle D, Ottman R, Clark LN, Marder K (2011) The relation between depression and parkin genotype: the CORE-PD study. Parkinsonism Relat Disord 17:740–744. https://doi.org/10.1016/j.parkreldis.2011.1007.1008
Morgante F, Fasano A, Ginevrino M, Petrucci S, Ricciardi L, Bove F, Criscuolo C, Moccia M, De Rosa A, Sorbera C, Bentivoglio AR, Barone P, De Michele G, Pellecchia MT, Valente EM (2016) Impulsive-compulsive behaviors in parkin-associated Parkinson disease. Neurology 87:1436–1441
Napier TC, Corvol JC, Grace AA, Roitman JD, Rowe J, Voon V, Strafella AP (2015) Linking neuroscience with modern concepts of impulse control disorders in Parkinson’s disease. Mov Disord 30:141–149
Obeso JA, Stamelou M, Goetz CG, Poewe W, Lang AE, Weintraub D, Burn D, Halliday GM, Bezard E, Przedborski S, Lehericy S, Brooks DJ, Rothwell JC, Hallett M, DeLong MR, Marras C, Tanner CM, Ross GW, Langston JW, Klein C, Bonifati V, Jankovic J, Lozano AM, Deuschl G, Bergman H, Tolosa E, Rodriguez-Violante M, Fahn S, Postuma RB, Berg D, Marek K, Standaert DG, Surmeier DJ, Olanow CW, Kordower JH, Calabresi P, Schapira AHV, Stoessl AJ (2017) Past, present, and future of Parkinson’s disease: a special essay on the 200th Anniversary of the Shaking Palsy. Mov Disord 32:1264–1310
Schulte EM, Grilo CM, Gearhardt AN (2016) Shared and unique mechanisms underlying binge eating disorder and addictive disorders. Clin Psychol Rev 44:125–139
Davis C, Loxton NJ, Levitan RD, Kaplan AS, Carter JC, Kennedy JL (2013) ‘Food addiction’ and its association with a dopaminergic multilocus genetic profile. Physiol Behav 118:63–69
Rose N, Koperski S, Golomb BA (2010) Mood food: chocolate and depressive symptoms in a cross-sectional analysis. Arch Intern Med 170:699–703
Gordon EL, Ariel-Donges AH, Bauman V, Merlo LJ (2018) What is the evidence for “Food Addiction?”. A systematic review. Nutrients 10(4):477. https://doi.org/10.3390/nu10040477
Schulte EM, Avena NM, Gearhardt AN (2015) Which foods may be addictive? The roles of processing, fat content, and glycemic load. PLoS One 10(2):e0117959. https://doi.org/10.1371/journal.pone.0117959. (eCollection 0112015)
Morton GJ, Meek TH, Schwartz MW (2014) Neurobiology of food intake in health and disease. Nature reviews. Neuroscience 15:367–378
Calvez J, Timofeeva E (2016) Behavioral and hormonal responses to stress in binge-like eating prone female rats. Physiol Behav 157:28–38
Pedram P, Sun G (2014) Hormonal and dietary characteristics in obese human subjects with and without food addiction. Nutrients 7:223–238. https://doi.org/10.3390/nu7010223
Avena NM, Bocarsly ME (2012) Dysregulation of brain reward systems in eating disorders: neurochemical information from animal models of binge eating, bulimia nervosa, and anorexia nervosa. Neuropharmacology 63:87–96
Kurian P, Obisesan TO, Craddock TJA (2017) Oxidative species-induced excitonic transport in tubulin aromatic networks: potential implications for neurodegenerative disease. J Photochem Photobiol B 175:109–124
Gearhardt AN, Corbin WR, Brownell KD (2016) Development of the Yale Food Addiction Scale Version 2.0. Psychol Addict Behav 30:113–121. https://doi.org/10.1037/adb0000136
Funding
The study was funded by a regional public grant (AOI CHU Clermont-Ferrand 2010). The funder had no role in the writing of the study protocol or in the conductance of the study.
Author information
Authors and Affiliations
Contributions
IdC: study design, manuscript preparation and final approval of the version to be submitted. FD: clinical assessment, manuscript preparation and final approval of the version to be submitted. ICB: study design. MLF: clinical assessment. AM: clinical assessment. PD: clinical assessment. BD: clinical assessment. GB: manuscript preparation. YB: manuscript preparation and final approval of the version to be submitted. PML: study design, manuscript preparation and final approval of the version to be submitted. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the French Ethic Committee (Committees of Protection of Persons South-East 6). It has been qualified by Ethics Committee as an observational study. Pursuant to L.1121-1-1 and R.1121-3 of the French Public Health Code in force at the time of the research, written informed consent from participants to conduct this study was not required.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of topical collection on Food and addiction.
Rights and permissions
About this article
Cite this article
de Chazeron, I., Durif, F., Chereau-Boudet, I. et al. Compulsive eating behaviors in Parkinson’s disease. Eat Weight Disord 24, 421–429 (2019). https://doi.org/10.1007/s40519-019-00648-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40519-019-00648-1