Abstract
Parkinson’s disease (PD) is a progressive systemic neurodegenerative disease. At present, there is no cure capable of resolving PD or significantly halting its progression.
Body weight of PD patients tends to vary during the disease. Some patients—especially in the past and, currently, at certain stages—suffer from undernutrition and sarcopenia. Instead, as a result of current pharmacological and surgical therapies, many tend to accumulate fat mass and experience overweight and obesity.
In PD patients treated with dopamine replacement therapy, a frequent, underestimated, and undertreated problem is the development of eating and weight disorders.
In particular, compulsive eating disorders and addiction-like eating disorders—such as compulsive grazing, compulsive overeating, binge eating, and selective cravings—may appear. Like other impulsive-compulsive spectrum disorders (ICSDs), these symptoms develop in the course of PD at the intersection of disease intrinsic dysfunctions, individual predispositions, and treatment effects.
Some historical notes start from the first description of the disease, published by James Parkinson in 1817. The chapter then touches on the following aspects of PD:
-
Epidemiology, course, and mortality
-
Motor and non-motor symptoms
-
Psychiatric comorbidity with particular attention to ICSDs
-
Eating and weight disorders in PD
-
Diagnostic evaluations
-
Medical, surgical, rehabilitative, and palliative therapies
Nazario Melchionda was deceased at the time of publication.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Addiction-like eating behaviors
- Alpha-synuclein
- Anticholinergic agents
- Anxiety
- Apathy
- Artistic activities
- Behavioral addictions
- Binge eating
- Caffeine
- Caregivers
- Cholinesterase inhibitors
- Clozapine
- Cognitive disorders
- Compulsive grazing
- Compulsive overeating
- Creativity
- Deep brain stimulation
- Delusion
- Dementia
- Depression
- Diet
- Dopamine
- Dopamine agonists
- Dyskinesias
- Epidemiology
- Food addiction
- Food craving
- Genetics
- Hallucination
- Hoarding
- Hyposmia
- Impulsive-compulsive spectrum disorders
- Incidence
- Leg restlessness
- Levodopa
- Levodopa-carbidopa
- l-dopa
- Levodopa-carbidopa enteral suspension
- Monoamine oxidase-B inhibitors
- Mortality
- Obesity
- Obsessive-compulsive symptoms
- Palliative care
- Parkinson’s disease
- PD motor symptoms
- PD non-motor symptoms
- PD subtypes
- Physical activity
- Pneumonia ab ingestis
- Prevalence
- Prognosis
- Psychiatric symptoms
- Psychometric tools
- Punding
- Quality of life
- REM sleep behavior disorder
- Rewarding behaviors
- Sarcopenia
- Stigma
- Underweight
- Wearing off periods
31.1 Historical Notes
Parkinson’s disease (PD) is a progressive systemic neurodegenerative disease.
The eponym refers to the English physician James Parkinson (1755–1824) who, in 1817, published the first description of the disease. He called it “shaking palsy” (paralysis agitans) and spoke of a neurological syndrome characterized by:
“involuntary tremulous motion, with lessened muscular power (…) with a propensity to bend the trunk forward, and to pass from a walking to a running pace” [1] (Chap. 1, p. 1)
About nutrition in this disease, Parkinson especially noted the difficulties in chewing and swallowing solid food with possible results in undernutrition. One patient fed very little and almost only milk:
“he is not only no longer able to feed himself, but when the food is conveyed to the mouth, so much are the actions of the muscles of the tongue, pharynx, &c. impeded by impaired action and perpetual agitation, that the food is with difficulty retained in the mouth until masticated; and then as difficultly swallowed.” [1] (Chap. 1, p. 8)
“He took very little nourishment, could chew and swallow no solids, and even found great pain in getting down liquids. Milk was almost his only food” [1] (Chap. IV, p. 40)
The first drugs used in PD were anticholinergic agents active in the central nervous system, starting with belladonna alkaloids. They have been the basic therapy for almost a century.
In the 1960s, studies by Arvid Carlsson and collaborators have linked PD to a dopamine neurotransmitter deficit due to the gradual loss of dopaminergic neurons, mainly within the nigrostriatal pathway [2].
Following that discovery, for over half a century, l-dopa (levodopa or l-3,4-dihydroxyphenylalanine, C9H11NO4) has become the leading pharmacological remedy for Parkinson’s motor symptoms [3]. L-dopa is a molecule capable of overcoming the blood-brain barrier and being converted to dopamine (C8H11NO2) by the dopa-decarboxylase enzyme.
At the end of the twentieth century, another important step was discovering alpha-synuclein, a presynaptic neuronal protein present in PD and other neurological diseases called synucleinopathies [4, 5].
As far as genetics is concerned, numerous genetic factors have been identified that increase the risk of developing the disease, as well as some rare monogenic forms of parkinsonism caused by a mutation in a gene, either dominant or recessive [6].
These monogenic forms are held collectively responsible for 5%–10% of cases of PD [7]. For example, mutations in the LRRK2 gene (also called Park8) are a cause of autosomal dominant PD, a genetic form responsible, in Italy, for 1–2% of sporadic cases of PD and 4–5% of family cases [8].
In recent decades, increasing attention has been paid to PD non-motor symptoms and dopaminergic treatment’s side effects.
This line includes studies on impulse control disorders (ICDs) and, in particular, those on feeding and eating disorders and body weight abnormalities in people with PD. It has been seen that, compared to James Parkinson’s observations on undernutrition, after the introduction of dopaminergic therapy, the onset of eating disorders, overweight, and obesity has become much more frequent. We have recently written:
“The study of ICDs in PD patients offers a heuristic opportunity to explore the role of different dopaminergic projections and dopamine receptors in impulse control difficulties and, in particular, in binge eating, food craving, and food addiction that are still controversial concepts with uncertain implications for etiology and treatment. Many clinical questions arise. Is binge eating disorder (BED) different in PD? Could BED-oriented cognitive-behavioral therapy be useful also in BED with PD? How could we prevent disordered eating behaviors and weight gain in PD?” [9] (p. 384)
31.2 Epidemiology, Course, and Survival
The number of PD patients is continuously growing due to the increased world population and life expectancy. In 1990–2016 the raw prevalence rates increased by 74.3%, those standardized by age by 21.7% [10].
The maximum prevalence is between 85 and 89 years old [10].
The incidence increases with age, and the highest falls in the 70–79 age group [11]. However, there are middle-onset PD (50–69 years) and young-onset PD (before 50 years).
PD affects both sexes, with a slight male prevalence increasing after age 60. The male-to-female ratio is 1.4:1 [10].
The increase in prevalence is due to the increase in older adults, longer illness duration, and probably environmental factors such as pesticides, solvents, metals, and air pollution [10, 12, 13].
The relationship between diet, microbiota, and PD is controversial [14]. For a long time, several studies argue that a healthy diet—for example, the Mediterranean diet—can be a protective factor [15,16,17]. Perhaps abnormalities in gut microbiota may contribute to the pathogenesis of PD [18, 19].
Some reports support a possible protective effect of physical activity, smoking, coffee, and black tea consumption [13, 20, 21].
Plasma caffeine levels were lower in PD subjects versus unaffected controls. Levels were even lower in PD patients carrying the LRRK2 gene mutation predisposing to the disease [22].
Nevertheless, this cross-sectional study is not enough to prove that caffeine consumption decreases the risk of developing PD. Besides, the results may also indicate that people predisposed to PD tend to drink less coffee.
Regarding the prognosis, a subdivision into three subtypes has been proposed [23]. The tripartition is based on the time lapse between diagnosis, worsening of symptoms and disabilities (first milestones: continuous falls, wheelchair dependence, dementia, accommodation in a residential/nursing home), and death. Table 31.1 sums up the division into three possible phenotypes.
This proposal is still under discussion, but it is crucial to keep in mind that PD phenotypic presentation and prognosis are very variable and a mild, slowly progressing course is frequent [24].
Death can be due to disease-related causes such as pneumonia ab ingestis and falls. However, for the most part, people with PD die from the same causes as their peers.
31.3 Motor and Non-motor Symptoms
The typical representation of a PD patient has long been the picture drawn in the nineteenth century by James Parkinson [1] and then by William Richard Gowers [25]: a man, elderly, bent forward, trembling, debilitated, and substantially disabled.
This image must be corrected. It does not consider the numerous female cases, those that begin before the age of 50–60 years, the initial manifestations, and non-motor symptoms. Moreover, this stereotype does not recognize the significant variability in the course of PD and contributes to social stigma and internalized stigma (self-fulfilled prophecies) with increased damage to the quality of life [24].
The main motor symptoms are resting tremor, reduction of facial mimicry, rigidity, bradykinesia, foot dystonia, gait issues, and postural instability.
Non-motor symptoms are hyposmia, hypophonia, fatigue, sleep-wake cycle disturbances with daytime sleepiness and nighttime insomnia, orthostatic hypotension and blood pressure instability, sialorrhea, swallowing disorders, delayed gastric emptying, constipation, frequent and urgent urination, erectile dysfunction, cognitive disorders, and psychopathological symptoms (see Sect. 31.4).
Non-motor symptoms mostly appear as disease and treatment progress. Sometimes, however, they precede the appearance of motor disorders. In all cases, they contribute heavily to the deterioration of quality of life, disability, and disease burden for patients and caregivers.
PD and other neurodegenerative diseases can be associated with REM (rapid eye movement) sleep behavior disorder. REM sleep is normally associated with motor atony. Instead, there are very vivid and unpleasant dreams in REM sleep behavior disorder accompanied by vocal sounds and sudden and violent arm and leg movements. REM sleep behavior disorder can be, together with hyposmia, a prodromal symptom. Late-onset (over 50 years of age) leg restlessness can be an early manifestation of PD, as well [26].
It is unclear which non-motor symptoms depend on dopaminergic deficits, other neurotransmitter systems, or dopamine replacement therapy [27].
In the more advanced stages and malignant and rapidly progressing PD forms, both motor and non-motor symptoms are more severe and disabling, with or without tremor.
31.4 Psychiatric Comorbidity
Cognitive disorders may initially affect attention and executive and visual-spatial functions. They may be mild impairments, but in several cases, dementia is progressively established.
The cumulative probability of dementia after 10 years of PD was 46% [28].
In cases treated with subthalamic nucleus deep brain stimulation (DBS), incidence and prevalence of dementia were not higher than the rates reported in the general PD population [29].
Anxious and depressive symptoms are frequent, especially with advancing age. A study reported anxiety disorders in two-thirds of PD cases and depression in one-third [30].
The presence of obsessive-compulsive symptoms (in particular, hoarding) was also more frequent in elderly patients with PD (31%) than in a control group (21%) [31].
Personality changes and psychotic symptoms, especially visual hallucinations and delusions, can occur. Hallucinations often accompany cognitive decline [32], particularly that of executive functions [33].
Treatment with levodopa and dopamine agonists is implicated in the development of psychotic symptoms in people with PD. However, the interaction between the neurotransmitter dysfunction intrinsic to the disease and drugs is still unknown [34].
For several years, it has been reported that in people with PD taking antiparkinson drugs (dopamine replacement therapy), impulsive-compulsive spectrum disorders (ICSDs) and behavioral addictions (BA) are also often observed [35,36,37,38,39].
ICSDs appear in 40% of people with PD and dopamine replacement therapy [40]. Early-onset of PD seems to be associated with an increased risk of ICSDs and BA [41].
A list of the main ones in alphabetical order is as follows:
-
Binge eating
-
Compulsive buying
-
Compulsive overeating
-
Food addiction
-
Medication abuse (in particular, overuse of dopamine agents or “dopamine dysregulation syndrome”)
-
Obsessive hobby
-
Pathological gambling
-
Punding (the term was coined to describe purposeless, stereotyped behaviors such as repetitive examining and handling objects or compelling attraction for activities such as cleaning) [42]
-
Selective and non-selective food cravings
-
Sex addiction
-
Walkabout (need to wander aimlessly)
The neuro-anatomic circuits involved in ICSDs can be altered by Parkinson’s disease itself and by exogenous influences, including drugs active on the central nervous system.
Treatment with dopaminergic drugs is believed to weaken the ability to control the compulsive repetition of rewarding behaviors.
In PD patients, long-term dopaminergic treatment over-stimulates, in particular, dopamine D2/D3 receptors and induces downregulation [43]. Not only L-DOPA but even more dopamine agonists—such as bromocriptine, pramipexole, rotigotine, and ropinirole—are linked to the development of ICSDs [44].
Many data support the hypothesis that addiction-like behaviors, ICSDs, and compulsive overeating are due to dopamine replacement therapy rather than PD alone [43].
However, not all patients with PD in dopaminergic treatment develop ICSDs.
Predisposition [45] and, in particular, personality play an essential role [46].
Weintraub and collaborators found that about 20% of patients diagnosed with PD but not yet in therapy reported some ICSDs [47]. The percentage was not higher than that found in a control group. Therefore, PD itself does not seem to increase the risk of developing ICSDs significantly.
It is necessary to wonder whether those who have some premorbid impulse control disorders are at greater risk of developing others or worsening when starting dopaminergic therapy.
Some authors have noted in PD patients that dopaminergic therapy increases motivation for artistic activities and creativity. Related behaviors may look like compulsive, but the quality of life appears to have improved [48,49,50,51].
In conclusion, ICSDs in PD patients should be considered multifactorial phenomena involving drug-, patient-, and disease-related factors [52].
31.5 Eating and Weight Disorders
Is obesity a risk factor for the development of PD? The data are discordant.
A Finnish cohort study said yes [53]. However, more recently, a Swedish survey found the opposite [54]. In the same year, South Korean research published data according to which the metabolic syndrome—usually associated with abdominal obesity—increases the risk of developing PD [55].
As for the course, obesity has been associated with a faster progression of motor symptoms and disability, especially in patients with early-onset PD [56].
A balanced diet and healthy body weight are necessary to maintain PD people’s overall health and muscle strength. Cognitive functions are also likely to benefit from a proper diet (e.g., Mediterranean diet), adopted from the early stages of the disease [57].
Eating disorders are frequent in PD patients and body weight tends to vary during the disease.
Decreases in caloric intake and unintentional weight loss have been observed frequently. As James Parkinson had already observed [1], dysphagia and tremor in the upper extremities contribute to undernutrition [58, 59].
A multicenter case-control study found that the mean BMI was significantly lower in PD patients ((22.0 ± 3.4 kg/m2 vs. 25.4 ± 4.3 kg/m2). Visual hallucinations and motor complications (dyskinesia) were associated with low BMI [60].
It has been reported, understandably, the risk of sarcopenia, related to the worsening of functional capacity and nutritional status [61].
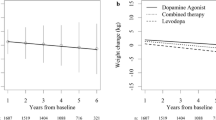
On the other hand, many other PD patients develop obesity. The improvement of motor symptoms obtained with surgical and pharmacological therapies plays a role.
After surgery (subthalamic nucleus deep brain stimulation), many patients (men and women) present an increase in fat mass and become overweight/obese [62, 63].
As for dopaminergic drugs, they contribute to obesity by improving motor symptoms and promoting disinhibition of eating behavior [9, 37].
Disordered eating behaviors are common in PD, but little studied and little known [37].
They are mostly classified as impulse control disorders and occur as compulsive grazing, overeating, selective or non-selective food cravings, and binge eating disorder (BED).
In 2007, a review article reported compulsive eating among the frequent reward-based behaviors observed in PD patients treated with dopaminergic drugs [64].
A few years later, a large multicenter study found that compulsive eating was present in 3.4% of cases, but there was no dose-response relationship between L-dopa or dopamine agonist dose and odds ratio for the onset of the symptom [65].
In a sample of 3090 PD patients, Weintraub et al. found BED in 4.3% of cases [44]. Some researchers found a much higher prevalence for impulse control disorders less strictly defined than BED and called “compulsive eating” (14.3%) [66] and “eating behavior disorder” (7.2%) [36].
Ingrid de Chazeron and collaborators recently studied a sample of PD patients in dopaminergic therapy (age 68 ± 10 years; males 53%) who reported changes in their dietary habits following PD diagnosis [37]. The main results are the following:
-
Anorexia nervosa (AN): no cases
-
Bulimia nervosa (BN): no cases
-
BED full criteria: 4%
-
NES: 6%
-
Addictive-like eating behaviors without BED: 39%
-
Food cravings (sweets) more than once a week: 43%
-
The habit of eating outside meal times and/or during the night: 69%
-
Inappropriate compensatory behaviors:
-
Fasting or excessive exercise (≥ 2 times/week): 10%
-
Laxatives: 6%
-
Self-induced vomiting: 2%
-
Appetite suppressants: 2%
-
Eating disorders began within the first 2 years after the diagnosis of PD in 65% of cases.
In this research—apart from AN, BN, and BED—the other frequencies suffer, of course, from the uncertainty related to the lack of shared diagnostic definitions.
However, more robust studies are lacking and this recent survey is useful because it indicates that disordered eating and weight control behaviors can occur in many patients with PD under dopaminergic treatment.
31.6 Diagnosis and Treatment
Diagnostic evaluation is based on anamnestic data, physical examination, and the possible use of instrumental and psychometric tests.
The doctor looks for prodromal symptoms (hyposmia, REM sleep behavior disorders, etc.), motor symptoms (stiffness, rest tremor, etc.), cognitive decline, and psychiatric symptoms.
In uncertain cases, magnetic resonance imaging can distinguish PD from other parkinsonisms, while dopamine transporter single-photon emission computed tomography permits to distinguish PD from essential tremor [40].
Some psychometric tools can help in the assessment of ICSDs and, in particular, of disordered eating behaviors:
-
Addiction-like Eating Behavior Scale, AEBS [67]. AEBS investigates the presence of addictive-like eating behaviors (according to the food addiction behavioral approach).
-
Ardouin Scale of Behavior in Parkinson’s Disease [68]. The scale is designed for quantifying changes of mood and behavior related to PD and dopaminergic therapy.
-
Barratt Impulsiveness Scale, BIS-11 [69], BIS-R-21 [70]. The questionnaire is the most used measure of self-reported impulsivity.
-
Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease, QUIP [38]. QUIP researches the presence of both overeating and binge eating episodes in PD patients.
-
Questionnaire on Eating and Weight Patterns-5, QEWP-5 [71, 72]. QEWP especially rates the symptoms of BED and night eating syndrome (NES).
-
Yale Food Addiction Scale, version 2.0, YFAS 2.0 [73]. YFAS evaluates eating behaviors related to the current construct of food addiction (according to the food addiction substance approach).
At present, there is no cure capable of resolving the disease or significantly halting its progression: the treatment is symptomatic [5, 40].
The improvement of motor symptoms is entrusted mainly to levodopa-carbidopa, monoamine oxidase-B inhibitors, and dopamine agonists. Typically, combinations of different drugs are used to achieve complementary benefits, reduce doses, and limit dose-dependent side effects. Over time, however, the effect of each dose lasts less and less, and when it runs out, symptoms reappear (wearing off periods). More and more frequent adjustments and administration are necessary, even every 2–3 h [40].
In more severe and advanced cases, surgical approaches can be used:
-
Deep brain stimulation (DBS), with the surgical placement of unilateral or bilateral electrodes in the subthalamic nucleus or the globus pallidus
-
Levodopa-carbidopa enteral suspension (CLES). CLES is delivered into the jejunum via a PEG-J (percutaneous endoscopic gastrostomy jejunal extension) tube implanted surgically.
These severe, advanced, or drug-resistant cases are usually characterized by wearing off (the effect of each dose fades faster and faster) and dyskinesias (involuntary dance-like choreoathetoid movements that generally coincide with the moment of maximum brain concentration of levodopa). In this regard, it should be remembered that, as the disease progresses, the therapeutic window within which dopaminergic drugs improve motor symptoms without causing dyskinesia becomes narrower and narrower.
Subthalamic nucleus DBS relieves motor symptoms but can cause neuropsychiatric disorders and, in particular, loss of motivation. Apathy is probably the most disabling side effect induced by DBS. Apathetic behaviors can be alleviated by D2 and D3 dopaminergic receptor agonists. However, apathy appears to be a direct effect of DBS, not due to post-surgical reduction of dopaminergic treatments [74].
The improvement of motor symptoms with dopaminergic therapy has made non-motor symptoms and their weight on patients’ quality of life more evident: pain, autonomic nervous system disorders, psychiatric disorders including ICSDs, and decline in cognitive abilities.
There is still a lack of high-quality studies that provide robust evidence for the treatment of many non-motor symptoms of PD [40].
For psychotic symptoms—particularly hallucinatory ones—clozapine is helpful but requires careful attention to its side effects.
Cholinesterase inhibitors are used for cognitive deficits.
For anxious and depressive symptoms, mainly selective serotonin reuptake inhibitors are used as drugs along with therapeutic education, counseling, and psychotherapy.
Motor and cognitive rehabilitation programs are helpful.
Exercise and a well-balanced diet are beneficial [75]. Physical activity is considered useful both in the prevention and treatment of PD [20].
A randomized clinical trial recently compared outpatient palliative care with current care standards in patients with PD and related disorders [76]. A neurologist and a primary care practitioner provided standard care. A team composed of a neurologist, social worker, nurse, and chaplain administered integrated palliative care. A palliative medicine specialist offered guidance and selective involvement. Integrated palliative care was associated with greater improvements in patient and/or caregiver outcomes.
Perhaps the team should include a dietician, and the program should also address frequent eating and weight problems.
31.7 Concluding Remarks
Dopaminergic agonists directly activate post-synaptic receptors and resolve the deficit of functioning presynaptic terminals. Motor symptoms improve, but, in several cases, ICSDs occur that complicate and worsen patients’ health and quality of life.
Among the non-motor symptoms and ICSDs are frequent disorders of feeding, eating, and body weight.
People with PD usually do not ask for help with their diet and weight problems, and neurologists, for their part, tend to underestimate them.
References
Parkinson J. An essay on the shaking palsy. London: printed by Whittingham and Rowland for Sherwood, Neely, and Jones; 1817.
Carlsson A. Thirty years of dopamine research. Adv Neurol. 1993;60:1–10.
Lees AJ, Tolosa E, Olanow CW. Four pioneers of L-dopa treatment: Arvid Carlsson, Oleh Hornykiewicz, George Cotzias, and Melvin Yahr. Mov Disord. 2015;30(1):19–36. https://doi.org/10.1002/mds.26120.
Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci U S A. 1998;95(11):6469–73. https://doi.org/10.1073/pnas.95.11.6469.
Braak H, Del Tredici-Braak K, Gasser T. Special issue “Parkinson’s disease”. Cell Tissue Res. 2018;373(1):1–7. https://doi.org/10.1007/s00441-018-2863-5.
Klein C, Westenberger A. Genetics of Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2(1):1–15. https://doi.org/10.1101/cshperspect.a008888.
Cherian A, Divya KP. Genetics of Parkinson’s disease. Acta Neurol Belg. 2020; https://doi.org/10.1007/s13760-020-01473-5.
Sito Italiano della malattia di Parkinson; 2020. Parkinson.it. https://www.parkinson.it/. Accessed 3 Oct 2020.
Melchionda N, Cuzzolaro M. Parkinson’s disease, dopamine, and eating and weight disorders: an illness in the disease? Eat Weight Disord. 2019;24(3):383–4. https://doi.org/10.1007/s40519-019-00684-x.
GBD 2016 Parkinson’s Disease Collaborators. Global, regional, and national burden of Parkinson’s disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):939–53. https://doi.org/10.1016/S1474-4422(18)30295-3.
Hirsch L, Jette N, Frolkis A, Steeves T, Pringsheim T. The incidence of Parkinson’s disease: a systematic review and meta-analysis. Neuroepidemiology. 2016;46(4):292–300. https://doi.org/10.1159/000445751.
Han C, Lu Y, Cheng H, Wang C, Chan P. The impact of long-term exposure to ambient air pollution and second-hand smoke on the onset of Parkinson disease: a review and meta-analysis. Public Health. 2020;179:100–10. https://doi.org/10.1016/j.puhe.2019.09.020.
Belvisi D, Pellicciari R, Fabbrini A, Costanzo M, Pietracupa S, De Lucia M, Modugno N, Magrinelli F, Dallocchio C, Ercoli T, Terravecchia C, Nicoletti A, Solla P, Fabbrini G, Tinazzi M, Berardelli A, Defazio G. Risk factors of Parkinson’s disease: simultaneous assessment, interactions and etiological subtypes. Neurology. 2020; https://doi.org/10.1212/WNL.0000000000010813.
Erro R, Brigo F, Tamburin S, Zamboni M, Antonini A, Tinazzi M. Nutritional habits, risk, and progression of Parkinson disease. J Neurol. 2018;265(1):12–23. https://doi.org/10.1007/s00415-017-8639-0.
Gao X, Chen H, Fung TT, Logroscino G, Schwarzschild MA, Hu FB, Ascherio A. Prospective study of dietary pattern and risk of Parkinson disease. Am J Clin Nutr. 2007;86(5):1486–94. https://doi.org/10.1093/ajcn/86.5.1486.
Molsberry S, Bjornevik K, Hughes KC, Healy B, Schwarzschild M, Ascherio A. Diet pattern and prodromal features of Parkinson’s disease. Neurology. 2020; https://doi.org/10.1212/WNL.0000000000010523.
Paknahad Z, Sheklabadi E, Moravejolahkami AR, Chitsaz A, Hassanzadeh A. The effects of Mediterranean diet on severity of disease and serum Total Antioxidant Capacity (TAC) in patients with Parkinson’s disease: a single center, randomized controlled trial. Nutr Neurosci. 2020:1–8. https://doi.org/10.1080/1028415X.2020.1751509.
Boulos C, Yaghi N, El Hayeck R, Heraoui GN, Fakhoury-Sayegh N. Nutritional risk factors, microbiota and Parkinson’s disease: what is the current evidence? Nutrients. 2019;11(8) https://doi.org/10.3390/nu11081896.
Barichella M, Severgnini M, Cilia R, Cassani E, Bolliri C, Caronni S, Ferri V, Cancello R, Ceccarani C, Faierman S, Pinelli G, De Bellis G, Zecca L, Cereda E, Consolandi C, Pezzoli G. Unraveling gut microbiota in Parkinson’s disease and atypical parkinsonism. Mov Disord. 2019;34(3):396–405. https://doi.org/10.1002/mds.27581.
Fan B, Jabeen R, Bo B, Guo C, Han M, Zhang H, Cen J, Ji X, Wei J. What and how can physical activity prevention function on Parkinson’s disease? Oxid Med Cell Longev. 2020;2020:4293071. https://doi.org/10.1155/2020/4293071.
Hong CT, Chan L, Bai CH. The effect of caffeine on the risk and progression of Parkinson’s disease: a meta-analysis. Nutrients. 2020;12(6) https://doi.org/10.3390/nu12061860.
Crotty GF, Maciuca R, Macklin EA, Wang J, Montalban M, Davis SS, Alkabsh JI, Bakshi R, Chen X, Ascherio A, Astarita G, Huntwork-Rodriguez S, Schwarzschild MA. Association of caffeine and related analytes with resistance to Parkinson’s disease among LRRK2 mutation carriers: a metabolomic study. Neurology. 2020; https://doi.org/10.1212/WNL.0000000000010863.
De Pablo-Fernandez E, Lees AJ, Holton JL, Warner TT. Prognosis and neuropathologic correlation of clinical subtypes of Parkinson disease. JAMA Neurol. 2019;76(4):470–9. https://doi.org/10.1001/jamaneurol.2018.4377.
Armstrong MJ, Okun MS. Time for a new image of parkinson disease. JAMA Neurol. 2020; https://doi.org/10.1001/jamaneurol.2020.2412.
Gowers WR. A Manual of Diseases of the Nervous System, vol. 1. London: J. & A. Churchill; 1886.
Suzuki K, Fujita H, Watanabe Y, Matsubara T, Kadowaki T, Sakuramoto H, Hamaguchi M, Nozawa N, Hirata K. Leg restlessness preceding the onset of motor symptoms of Parkinson disease: a case series of 5 patients. Medicine (Baltimore). 2019;98(33):e16892. https://doi.org/10.1097/MD.0000000000016892.
Marinus J, Zhu K, Marras C, Aarsland D, van Hilten JJ. Risk factors for non-motor symptoms in Parkinson’s disease. Lancet Neurol. 2018;17(6):559–68. https://doi.org/10.1016/S1474-4422(18)30127-3.
Williams-Gray CH, Mason SL, Evans JR, Foltynie T, Brayne C, Robbins TW, Barker RA. The CamPaIGN study of Parkinson’s disease: 10-year outlook in an incident population-based cohort. J Neurol Neurosurg Psychiatry. 2013;84(11):1258–64. https://doi.org/10.1136/jnnp-2013-305277.
Bove F, Fraix V, Cavallieri F, Schmitt E, Lhommée E, Bichon A, Meoni S, Pélissier P, Kistner A, Chevrier E, Ardouin C, Limousin P, Krack P, Benabid AL, Chabardès S, Seigneuret E, Castrioto A, Moro E. Dementia and subthalamic deep brain stimulation in Parkinson disease. A long-term overview. Neurology. 2020;95(4):e384–92. https://doi.org/10.1212/wnl.0000000000009822.
Chuquilin-Arista F, Alvarez-Avellon T, Menendez-Gonzalez M. Prevalence of depression and anxiety in Parkinson disease and impact on quality of life: a community-based study in Spain. J Geriatr Psychiatry Neurol. 2020;33(4):207–13. https://doi.org/10.1177/0891988719874130.
Lo Monaco MR, Di Stasio E, Zuccala G, Petracca M, Genovese D, Fusco D, Silveri MC, Liperoti R, Ricciardi D, Cipriani MC, Laudisio A, Bentivoglio AR. Prevalence of obsessive-compulsive symptoms in elderly Parkinson disease patients: a case-control study. Am J Geriatr Psychiatry. 2020;28(2):167–75. https://doi.org/10.1016/j.jagp.2019.08.022.
Piredda R, Desmarais P, Masellis M, Gasca-Salas C. Cognitive and psychiatric symptoms in genetically determined Parkinson’s disease: a systematic review. Eur J Neurol. 2020;27(2):229–34. https://doi.org/10.1111/ene.14115.
Creese B, Albertyn CP, Dworkin S, Thomas RS, Wan YM, Ballard C. Executive function but not episodic memory decline associated with visual hallucinations in Parkinson’s disease. J Neuropsychol. 2020;14(1):85–97. https://doi.org/10.1111/jnp.12169.
Dave S, Weintraub D, Aarsland D, Ffytche DH. Drug and disease effects in Parkinson’s psychosis: revisiting the role of dopamine. Mov Disord Clin Pract. 2020;7(1):32–6. https://doi.org/10.1002/mdc3.12851.
Weintraub D, Mamikonyan E. Impulse control disorders in Parkinson’s disease. Am J Psychiatry. 2019;176(1):5–11. https://doi.org/10.1176/appi.ajp.2018.18040465.
El Otmani H, Mouni FZ, Abdulhakeem Z, Attar Z, Rashad L, Saali I, El Moutawakil B, Rafai MA, Slassi I, Nadifi S. Impulse control disorders in Parkinson disease: a cross-sectional study in Morocco. Rev Neurol (Paris). 2019;175(4):233–7. https://doi.org/10.1016/j.neurol.2018.07.009.
de Chazeron I, Durif F, Chereau-Boudet I, Fantini ML, Marques A, Derost P, Debilly B, Brousse G, Boirie Y, Llorca PM. Compulsive eating behaviors in Parkinson’s disease. Eat Weight Disord. 2019;24(3):421–9. https://doi.org/10.1007/s40519-019-00648-1.
Weintraub D, Hoops S, Shea JA, Lyons KE, Pahwa R, Driver-Dunckley ED, Adler CH, Potenza MN, Miyasaki J, Siderowf AD, Duda JE, Hurtig HI, Colcher A, Horn SS, Stern MB, Voon V. Validation of the questionnaire for impulsive-compulsive disorders in Parkinson’s disease. Mov Disord. 2009;24(10):1461–7. https://doi.org/10.1002/mds.22571.
Gatto EM, Aldinio V. Impulse control disorders in Parkinson’s disease. a brief and comprehensive review. Front Neurol. 2019;10:351. https://doi.org/10.3389/fneur.2019.00351.
Armstrong MJ, Okun MS. Diagnosis and treatment of parkinson disease: a review. JAMA. 2020;323(6):548–60. https://doi.org/10.1001/jama.2019.22360.
Castro-Martinez XH, Garcia-Ruiz PJ, Martinez-Garcia C, Martinez-Castrillo JC, Vela L, Mata M, Martinez-Torres I, Feliz-Feliz C, Palau F, Hoenicka J. Behavioral addictions in early-onset Parkinson disease are associated with DRD3 variants. Parkinsonism Relat Disord. 2018;49:100–3. https://doi.org/10.1016/j.parkreldis.2018.01.010.
Friedman JH. Punding on levodopa. Biol Psychiatry. 1994;36(5):350–1. https://doi.org/10.1016/0006-3223(94)90636-x.
Napier TC, Kirby A, Persons AL. The role of dopamine pharmacotherapy and addiction-like behaviors in Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2020;109942:102. https://doi.org/10.1016/j.pnpbp.2020.109942.
Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, Whetteckey J, Wunderlich GR, Lang AE. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589–95. https://doi.org/10.1001/archneurol.2010.65.
Latella D, Maggio MG, Maresca G, Saporoso AF, Le Cause M, Manuli A, Milardi D, Bramanti P, De Luca R, Calabro RS. Impulse control disorders in Parkinson’s disease: a systematic review on risk factors and pathophysiology. J Neurol Sci. 2019;398:101–6. https://doi.org/10.1016/j.jns.2019.01.034.
Farnikova K, Obereigneru R, Kanovsky P, Prasko J. Comparison of personality characteristics in Parkinson disease patients with and without impulse control disorders and in healthy volunteers. Cogn Behav Neurol. 2012;25(1):25–33. https://doi.org/10.1097/WNN.0b013e31824b4103.
Weintraub D, Papay K, Siderowf A, Parkinson’s progression markers Initiative. Screening for impulse control symptoms in patients with de novo Parkinson disease: a case-control study. Neurology. 2013;80(2):176–80. https://doi.org/10.1212/WNL.0b013e31827b915c.
Joutsa J, Martikainen K, Kaasinen V. Parallel appearance of compulsive behaviors and artistic creativity in Parkinson’s disease. Case Rep Neurol. 2012;4(1):77–83. https://doi.org/10.1159/000338759.
Inzelberg R. The awakening of artistic creativity and Parkinson’s disease. Behav Neurosci. 2013;127(2):256–61. https://doi.org/10.1037/a0031052.
Walker RH, Warwick R, Cercy SP. Augmentation of artistic productivity in Parkinson’s disease. Mov Disord. 2006;21(2):285–6. https://doi.org/10.1002/mds.20758.
Canesi M, Rusconi ML, Isaias IU, Pezzoli G. Artistic productivity and creative thinking in Parkinson’s disease. Eur J Neurol. 2012;19(3):468–72. https://doi.org/10.1111/j.1468-1331.2011.03546.x.
Grall-Bronnec M, Victorri-Vigneau C, Donnio Y, Leboucher J, Rousselet M, Thiabaud E, Zreika N, Derkinderen P, Challet-Bouju G. Dopamine agonists and impulse control disorders: a complex association. Drug Saf. 2018;41(1):19–75. https://doi.org/10.1007/s40264-017-0590-6.
Hu G, Jousilahti P, Nissinen A, Antikainen R, Kivipelto M, Tuomilehto J. Body mass index and the risk of Parkinson disease. Neurology. 2006;67(11):1955–9. https://doi.org/10.1212/01.wnl.0000247052.18422.e5.
Roos E, Grotta A, Yang F, Bellocco R, Ye W, Adami HO, Wirdefeldt K, Trolle Lagerros Y. Body mass index, sitting time, and risk of Parkinson disease. Neurology. 2018;90(16):e1413–7. https://doi.org/10.1212/WNL.0000000000005328.
Nam GE, Kim SM, Han K, Kim NH, Chung HS, Kim JW, Han B, Cho SJ, Yu JH, Park YG, Choi KM. Metabolic syndrome and risk of Parkinson disease: a nationwide cohort study. PLoS Med. 2018;15(8):e1002640. https://doi.org/10.1371/journal.pmed.1002640.
Kim R, Jun JS. Impact of overweight and obesity on functional and clinical outcomes of early Parkinson’s disease. J Am Med Dir Assoc. 2020;21(5):697–700. https://doi.org/10.1016/j.jamda.2019.11.019.
Goldman JG, Vernaleo BA, Camicioli R, Dahodwala N, Dobkin RD, Ellis T, Galvin JE, Marras C, Edwards J, Fields J, Golden R, Karlawish J, Levin B, Shulman L, Smith G, Tangney C, Thomas CA, Troster AI, Uc EY, Coyan N, Ellman C, Ellman M, Hoffman C, Hoffman S, Simmonds D. Cognitive impairment in Parkinson’s disease: a report from a multidisciplinary symposium on unmet needs and future directions to maintain cognitive health. NPJ Parkinsons Dis. 2018;4(19) https://doi.org/10.1038/s41531-018-0055-3.
Miller N, Allcock L, Hildreth AJ, Jones D, Noble E, Burn DJ. Swallowing problems in Parkinson disease: frequency and clinical correlates. J Neurol Neurosurg Psychiatry. 2009;80(9):1047–9. https://doi.org/10.1136/jnnp.2008.157701.
Fagerberg P, Klingelhoefer L, Bottai M, Langlet B, Kyritsis K, Rotter E, Reichmann H, Falkenburger B, Delopoulos A, Ioakimidis I. Lower energy intake among advanced vs. early Parkinson’s disease patients and healthy controls in a clinical lunch setting: a cross-sectional study. Nutrients. 2020;12(7) https://doi.org/10.3390/nu12072109.
Suzuki K, Okuma Y, Uchiyama T, Miyamoto M, Haruyama Y, Kobashi G, Sakakibara R, Shimo Y, Hatano T, Hattori N, Yamamoto T, Hirano S, Yamamoto T, Kuwabara S, Kaji Y, Fujita H, Kadowaki T, Hirata K. Determinants of low body mass index in patients with Parkinson’s disease: a multicenter case-control study. J Park Dis. 2020;10(1):213–21. https://doi.org/10.3233/JPD-191741.
da Luz MCL, Bezerra GKA, Asano AGC, Chaves de Lemos MDC, Cabral PC. Determinant factors of sarcopenia in individuals with Parkinson’s disease. Neurol Sci. 2020; https://doi.org/10.1007/s10072-020-04601-4.
Bannier S, Montaurier C, Derost PP, Ulla M, Lemaire JJ, Boirie Y, Morio B, Durif F. Overweight after deep brain stimulation of the subthalamic nucleus in Parkinson disease: long term follow-up. J Neurol Neurosurg Psychiatry. 2009;80(5):484–8. https://doi.org/10.1136/jnnp.2008.158576.
Locke MC, Wu SS, Foote KD, Sassi M, Jacobson CE, Rodriguez RL, Fernandez HH, Okun MS. Weight changes in subthalamic nucleus vs globus pallidus internus deep brain stimulation: results from the COMPARE Parkinson disease deep brain stimulation cohort. Neurosurgery. 2011;68(5):1233–7; discussion 1237-1238. https://doi.org/10.1227/NEU.0b013e31820b52c5.
Voon V, Fox SH. Medication-related impulse control and repetitive behaviors in Parkinson disease. Arch Neurol. 2007;64(8):1089–96. https://doi.org/10.1001/archneur.64.8.1089.
Lee JY, Kim JM, Kim JW, Cho J, Lee WY, Kim HJ, Jeon BS. Association between the dose of dopaminergic medication and the behavioral disturbances in Parkinson disease. Parkinsonism Relat Disord. 2010;16(3):202–7. https://doi.org/10.1016/j.parkreldis.2009.12.002.
Perez-Lloret S, Rey MV, Fabre N, Ory F, Spampinato U, Brefel-Courbon C, Montastruc JL, Rascol O. Prevalence and pharmacological factors associated with impulse-control disorder symptoms in patients with Parkinson disease. Clin Neuropharmacol. 2012;35(6):261–5. https://doi.org/10.1097/WNF.0b013e31826e6e6d.
Ruddock HK, Christiansen P, Halford JCG, Hardman CA. The development and validation of the Addiction-like Eating Behaviour Scale. Int J Obes (Lond). 2017;41(11):1710–7. https://doi.org/10.1038/ijo.2017.158.
Rieu I, Martinez-Martin P, Pereira B, De Chazeron I, Verhagen Metman L, Jahanshahi M, Ardouin C, Chereau I, Brefel-Courbon C, Ory-Magne F, Klinger H, Peyrol F, Schupbach M, Dujardin K, Tison F, Houeto JL, Krack P, Durif F. International validation of a behavioral scale in Parkinson’s disease without dementia. Mov Disord. 2015;30(5):705–13. https://doi.org/10.1002/mds.26223.
Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51(6):768–74. https://doi.org/10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1.
Kapitany-Foveny M, Urban R, Varga G, Potenza MN, Griffiths MD, Szekely A, Paksi B, Kun B, Farkas J, Kokonyei G, Demetrovics Z. The 21-item Barratt Impulsiveness Scale Revised (BIS-R-21): an alternative three-factor model. J Behav Addict. 2020;9(2):225–46. https://doi.org/10.1556/2006.2020.00030.
Spitzer R, Yanovski S, Marcus M. Questionnaire on eating and weight patterns, revised. Pittsburgh, PA: Behavioral Measurement Database Services; 1994.
Yanovski SZ, Marcus MD, Wadden TA, Walsh BT. The Questionnaire on Eating and Weight Patterns-5: an updated screening instrument for binge eating disorder. Int J Eat Disord. 2015;48(3):259–61. https://doi.org/10.1002/eat.22372.
Gearhardt AN, Corbin WR, Brownell KD. Development of the Yale food addiction scale version 2.0. Psychol Addict Behav. 2016;30(1):113–21. https://doi.org/10.1037/adb0000136.
Vachez Y, Carcenac C, Magnard R, Kerkerian-Le Goff L, Salin P, Savasta M, Carnicella S, Boulet S. Subthalamic nucleus stimulation impairs motivation: implication for apathy in Parkinson’s disease. Mov Disord. 2020;35(4):616–28. https://doi.org/10.1002/mds.27953.
Ray S, Agarwal P. Depression and anxiety in Parkinson disease. Clin Geriatr Med. 2020;36(1):93–104. https://doi.org/10.1016/j.cger.2019.09.012.
Kluger BM, Miyasaki J, Katz M, Galifianakis N, Hall K, Pantilat S, Khan R, Friedman C, Cernik W, Goto Y, Long J, Fairclough D, Sillau S, Kutner JS. Comparison of integrated outpatient palliative care with standard care in patients with Parkinson disease and related disorders: a randomized clinical trial. JAMA Neurol. 2020;77(5):551–60. https://doi.org/10.1001/jamaneurol.2019.4992.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Additional information
The chapter is dedicated to the memory of Nazario Melchionda (1939–2020), dear colleague and friend.
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Cuzzolaro, M., Melchionda, N. (2022). Parkinson’s Disease and Eating and Weight Disorders. In: Manzato, E., Cuzzolaro, M., Donini, L.M. (eds) Hidden and Lesser-known Disordered Eating Behaviors in Medical and Psychiatric Conditions . Springer, Cham. https://doi.org/10.1007/978-3-030-81174-7_31
Download citation
DOI: https://doi.org/10.1007/978-3-030-81174-7_31
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-81173-0
Online ISBN: 978-3-030-81174-7
eBook Packages: MedicineMedicine (R0)