Abstract
Ghrelin is a gastric hormone circulating in acylated (AG) and unacylated (UnAG) forms. This narrative review aims at presenting current emerging knowledge on the impact of ghrelin forms on energy balance and metabolism. AG represents ~ 10% of total plasma ghrelin, has an appetite-stimulating effect and is the only form for which a receptor has been identified. Moreover, other metabolic AG-induced effects have been reported, including the modulation of glucose homeostasis with stimulation of liver gluconeogenesis, the increase of fat mass and the improvement of skeletal muscle mitochondrial function. On the other hand, UnAG has no orexigenic effects, however recent reports have shown that it is directly involved in the modulation of skeletal muscle energy metabolism by improving a cluster of interlinked functions including mitochondrial redox activities, tissue inflammation and insulin signalling and action. These findings are in agreement with human studies which show that UnAG circulating levels are positively associated with insulin sensitivity both in metabolic syndrome patients and in a large cohort from the general population. Moreover, ghrelin acylation is regulated by a nutrient sensor mechanism, specifically set on fatty acids availability. These recent findings consistently point towards a novel independent role of UnAG as a regulator of muscle metabolic pathways maintaining energy status and tissue anabolism. While a specific receptor for UnAG still needs to be identified, recent evidence strongly supports the hypothesis that the modulation of ghrelin-related molecular pathways, including those involved in its acylation, may be a potential novel target in the treatment of metabolic derangements in disease states characterized by metabolic and nutritional complications.
Level of evidence Level V, narrative review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The coordinated regulation of food intake, energy expenditure and adiposity is dependent on a complex signalling network involving both peripheral signals and central nervous system. Growing evidence has shown that the gut plays a key role in this homeostatic process and may, therefore, be considered as the body’s largest endocrine organ [1]. The gastrointestinal tract is, in fact, able to release more than 20 different hormones, mostly in relation to the quality and quantity of nutrients in the tract. These have so far been characterized as able to produce a large and widespread set of effects, including, at local level, the regulation of gut motility, the modulation of glucose homeostasis and of peripheral insulin sensitivity as well as the stimulation of hunger or satiety feelings at central level [2]. Among these peptides, increasing interest is growing for ghrelin, the only gut hormone known to stimulate appetite [1, 2]. Since 1996, 9716 papers are currently (October 2018) recorded in Pubmed, as detected by performing a general search using the keyword “ghrelin”. This narrative review aims at presenting and discussing the most significant advancements in the understanding of the complex biology of this hormone and of its effects on the modulation of energy balance and metabolism.
Ghrelin: cell biology
Ghrelin is a gastric hormone, first identified in 1996 by Kojima et al. in rat stomach [3]. In the previous years, several small synthetic molecules had been discovered for their ability to induce growth hormone (GH) release by acting at hypothamical level independently from GH releasing hormone pathways [4] and were, therefore, named growth hormone secretagogues (GHS) [5, 6]. However, while a specific receptor for GHS (GHSR) had been identified in 1996 [7], its endogenous ligand was unknown until Kojima et al. finally identified a novel hormone that was able to simulate GH secretion through GHSR [3] and named it “ghrelin” after the Proto-Indo-European word root (“ghre”) meaning “grow” [8]. Almost contemporarily, Tomasetto and others identified the same hormone for its regulatory role in gastrointestinal motility and named it motilin-related peptide [9].
Ghrelin is mainly secreted by endocrine cells (P/D1 in humans and X/A-like in rats) located in the gastric fundus, and gastrectomy reduces ghrelin plasma concentrations by 65% [10], but its expression has also been described in duodenum, jejuna, ileum, colon and at lower concentrations in the pancreas, adipose tissue, kidneys, testes, placenta, hypophysis and nucleus arcuatus in the hypothalamus, an important region for appetite regulation [11,12,13,14,15,16,17,18,19,20].
Transcriptional regulation and polymorphisms
The gene coding for the ghrelin peptide, GHRL, is highly conserved in mammals [8] and spans 5 kb on chromosome 3p 25–36 in humans. The sequence includes four exons encoding a precursor 117 aa protein, preproghrelin [21], while two further exons with regulatory function were discovered later [22]. Preproghrelin undergoes splicing and editing ultimately resulting in the bioactive peptides obestatin and ghrelin (Fig. 1).
Interestingly, obestatin, a 23 amino acid peptide discovered by Zhang et al. [25], is also involved in the complex-regulation of the gut–brain network, with initial reports showing its ability to counteract ghrelin’s effects [25], potentially suggesting GHRL as an important effector in the maintenance of energy homeostasis. However, while pleiotropic metabolic effects have been reported, other groups did not confirm the inhibitory effect on food intake, making of obestatin a controversial peptide whose effects are currently largely undefined [26,27,28,29,30].
Genomic variation of the ghrelin gene has been associated with obesity development in humans. Two polymorphisms have been reported in humans: Leu72Met and Arg51Gln [31,32,33]. Individuals presenting Leu72Met allele are reportedly protected against fat accumulation and associated metabolic comorbidities [34]. The Arg51Gln polymorphism changes the processing site of ghrelin within its precursor protein, preventing normal ghrelin editing. Importantly, its prevalence was shown to be 6.3% in obese subject, while it was not detectable among non obese individuals, showing a clear link with obesity development [33].
Post-translational modifications
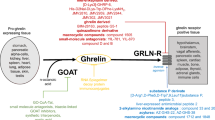
It is important to note that the hormone described by Kojima et al. as an endogenous ligand for GHSR was an acylated peptide. The ghrelin peptide, in fact, undergoes post-translational modifications, the main being acylation on S3 by the membrane bound enzyme ghrelin-O-acyl-transferase (GOAT) [35] (Fig. 1). This enzyme, highly conserved across species, has a tissue expression profile similar to ghrelin, with highest expression in stomach, pancreas and intestine and ghrelin acylation is completely prevented in GOAT knock-out mice [36,37,38]. While fatty acids derived from acetic (C2) to tetradecanoic acid (C14:0) are all possible ligands, octanoic acid (C8:0) is the principal fatty acid involved in this reaction, with decanoic (C10:0) and likely decenoic (C10:1) acids also being reported as optimal ligands [37, 39,40,41]. No differential effects between these identified acylated forms on receptor binding and GH secretion activity in vitro has been found [40].
Ghrelin phosphorylation has also been reported, with induced protein structural changes which affect both acylation and membrane binding in vitro [42], but its potential importance in vivo is currently unclear.
Ghrelin secretion and circulating forms
Ghrelin release from the stomach has been reported to involve sympathetic nerves [43] and recent evidence shows that ghrelin secretion in gastric endocrine cells is mediated by a series of G-protein coupled receptors (GPCRs), allowing for its release to be integrated in a network of modulatory signals [44].
Both acylated (AG) and unacylated (UnAG) ghrelin forms are detectable in human and animal plasma. Interestingly, most circulating ghrelin is unacylated, whereas the acylated hormone is generally considered to only account for approximately 10% [35, 45,46,47] of total ghrelin, with possible variations depending also on the detection technique used. Mizutani et al. have shown that, while unacylated ghrelin is localized in both gastric open-type cells and closed-type round cells, the acylated form is present only in the latter [48, 49]. Both cell types are able to release hormone forms, with enhanced unacylated but not acylated ghrelin secretion at lower gastric pH [49], suggesting a potentially different physiological role for the two forms.
Circulating ghrelin is subject to de-acylation and cleavage, with a half-life of respectively 240 min in humans and 30 min in rats, depending on the mediation of different enzymatic systems across species [50].
Ghrelin acylation influences its transport across compartments, in particular across the blood–brain barrier (BBB). While octanoylated ghrelin crosses the mouse BBB mainly from brain to blood, passage for the unacylated peptide was observed only in the opposite direction [51]. Interestingly, later studies showed that whole body energy balance impacts on ghrelin transport at BBB level, with obese mice showing reduced permeability compared to lean animals. Moreover, triglyceride co-administration increased ghrelin transport [52], suggesting a role for nutrients in modulating ghrelin action at central level.
Ghrelin release modulation and feedback regulation
Regulation of ghrelin secretion is still partly unknown but it is well established that ghrelin mRNA and plasma concentrations are increased during fasting [53, 54], and in humans circulating ghrelin is characterized by a peak just before meals, suggesting a potential role in meal initiation [55, 56]. On the contrary, ghrelin expression and plasma concentrations are decreased by food intake [56, 57] and in relation to food composition, with maximum inhibitory effect observed after carbohydrate ingestion, compared to proteins and lipids [58, 59].
In the long term, plasma ghrelin levels are known to be related to body weight and composition, with lower levels in obese patients and higher concentration in anorexia and in negative energy balance conditions including cachexia [8, 60, 61]. Importantly, among selected obese individuals, lower ghrelin levels were specifically related to a decrease in UnAG with no change in AG levels compared to non-obese [62]. Interestingly, the same study also showed that AG levels in obese individuals which did not meet the diagnostic criteria for metabolic syndrome diagnosis were comparable to both lean and obese subjects with the metabolic syndrome [62] and in obese patients undergoing bariatric surgery, the obesity-associated altered AG/UnAG ratio was found to be maintained even at 12 months after surgery despite weight loss [63]. This evidence suggests that the modulation of ghrelin acylation and its kinetics may be a potentially interesting target for further research not just in the treatment of obesity and its related complications, but also in the mechanisms underlying the pathogenesis of obesity per se.
At molecular level, ingested fatty acids are directly used for AG acylation and GOAT activation is reported to be modulated by ingestion and availability of medium chain fatty acids and triglycerides [8, 64,65,66]. GOAT is also potently inhibited by octanoylated ghrelin end-products, suggesting the existence of a negative feedback regulation in AG synthesis [37]. Moreover, recent evidence shows that GOAT expression levels decrease during prolonged fasting, leading to an increase in UnAG rather than AG in that condition. Interestingly, GOAT-null mice, while not expressing AG, present a marked increase in UnAG levels in association with lower body weight and fat mass, and opposite effects are observed in transgenic mice overexpressing GOAT [37, 64]. This body of evidence strongly supports the hypothesis that the GOAT-ghrelin system acts as a nutrient sensor providing information on the presence of nutrients, potentially leading to the optimization of nutrient partitioning and growth signals [64, 65, 67].
Ghrelin receptor
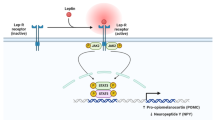
Acylated ghrelin’s receptor is a G-protein coupled receptor produced in two isoforms by alternative splicing of an mRNA transcript of a single gene, located on chromosome 3 q26-27 [8]. AG binding sequence has been identified in the first four residues at the N-terminus of ghrelin, which include the octanoylation site in Ser3 [68], and its interaction with ghrelin receptor (GHSR) leads to a Gq-mediated activation of phospholipase C and subsequent production of inositol 3 phosphate and diacylglycerol. In turn, this leads to Ca2+ release from the sarcoplasmic reticulum and ultimately to GH secretion.
GHSR1a is highly expressed in the pituitary and hypothalamus but also at lower levels in other brain areas including hippocampus, ventral tegmental area, nucleus tractus solitarius and substantia nigra. Interestingly, also numerous peripheral tissues express GHSR including intestine, pancreas, heart, lung, kidney and adipose tissue [69,70,71,72,73]. Evidence by several authors is consistent in failing to detect GHSR1a expression in both skeletal muscle and liver [69, 74,75,76,77,78]. Interestingly the expression of both ghrelin and GHS-R1b has been reported also in tissues not expressing the active receptor form, including liver [69, 77], suggesting that ghrelin may anyway produce tissue-specific effects by activating different pathways [69, 79].
Supported by observations showing differential biological activities between ghrelin forms, as well as by evidence from activation experiments of GHS-R1 variants in different cell types, some authors have proposed the existence of a novel class of receptors specifically binding UnAG [80, 81]. However, no receptor for UnAG has been currently identified [67, 80].
Ghrelin effects on energy metabolism, body mass and composition
Since its discovery, ghrelin has been progressively characterized as a hormone involved in energy balance homeostasis as well as in GH secretion, as its functions span from central regulation of feeding to the modulation of whole body and tissue-specific metabolism [67]. With regard to ghrelin acylation, AG has long been considered the active form of the hormone for its interaction with GHSR and for its impact on GH secretion and on appetite stimulation, while UnAG was regarded as a precursor/degraded form without specific biological activities. As a consequence, until recent years most studies were focused on AG, or did not differentiate the two forms [82].
Food intake and energy balance
Ghrelin has a modulatory role in the regulation of energy homeostasis, including appetite stimulation [83, 84]. Both peripheral and central treatment with AG increase food intake and body weight in experimental models [85,86,87]. In agreement with the described low permeability of BBB to AG in the blood-to-brain direction [51], one study reports that ghrelin signalling from the stomach to the central nervous system (CNS) is principally mediated by afferent vagal nerve, and ghrelin-induced stimulation of appetite and GH secretion are prevented by blocking vagal fibres [20]. However, this point remains controversial since other studies show that vagal afferents are not necessary for AG effects on appetite stimulation and ghrelin analogues are effective also after gastrectomy and related vagotomy [88,89,90]. Importantly, effects of AG in appetite stimulation are preserved in GH-deficient rats, showing its independence from GH release [85].
At CNS level AG-induced effect on appetite stimulation is mediated by hypothalamic neuropeptide Y (NPY) secretion but also by interaction with other known appetite regulators at this level, including AgRP, orexin, endocannabinoids and leptin [8, 85, 91, 92]. NPY-producing cells largely express ghrelin receptors, and ghrelin i.v. administration in mice largely stimulates hypothalamic activity in the same neurons [93, 94].
Nucelus caudatus and mesolimbic centres are also involved in long term energy homeostasis regulation by ghrelin [55] and effects on appetite possibly involve hedonic appetite regulation pathways [95]. In agreement, using functional magnetic resonance imaging, Davis et al. have shown that ghrelin administration increases activity in food-reward brain regions in humans [96].
UnAG effects on food intake poorly understood and likely marginal. While some authors report that in rodent models peripheral UnAG treatment decreases food intake in association with slower gastric transit [97], others do not confirm this effect but describe an inhibitory effect of UnAG on AG-induced increase in food intake when both forms are administered simultaneously [98]. This effect appears to be independent of GHSR1a modulation and at least in part mediated by UnAG-induced release of nesfatin-1, an inhibitor of NPY. Central administration of UnAG, on the contrary, is reportedly also orexigenic [99], indicating that further investigation on UnAG effects and receptor interaction is needed.
Whole body glucose homeostasis
Ghrelin also causes several direct effects on systemic and tissue metabolism, independently of food intake. At whole body level, ghrelin has an important impact on glucose homeostasis, as an important player in the pathophysiology of obesity-related metabolic complications [100]. Not long after ghrelin’s discovery, Broglio et al. reported that AG increases blood glucose levels and reduces insulin secretion [101]. Later studies showed that AG reduces glucose-stimulated insulin secretion, rather than fasting insulin levels [102]. Consistently, GHSR null mice have lower fasting glycaemia compared to control [103]. Underlying mechanisms were later investigated, showing that AG inhibits insulin release at pancreatic level by acting on voltage-dependent K channels (Kv) in β-cells. In fact, AG interaction with GHSR activates Kv channels through the receptor coupled G-protein αi, thus preventing Ca2+ signalling and limiting insulin exocytosis [104].
Ghrelin also modulates insulin sensitivity. In humans, total circulating ghrelin levels are positively associated with insulin sensitivity both in the general population [105] and in insulin-resistant diseases, including chronic renal disease [106] and obesity [107]. In addition, epidemiological data clearly shows that total plasma ghrelin levels are also inversely associated to the risk of developing type 2 diabetes and to several cardiovascular risk factors [105, 108].
With specific regard to the unacylated form, in 2004 Broglio et al. reported that UnAG coadministration with AG in humans counteracted the decrease in insulin levels induced by AG alone [109]. Later evidence showed that, at variance with AG, UnAG potently rises insulin release in glucose-stimulated conditions in rats [110], suggesting a potential independent role for UnAG in regulating glucose and lipid metabolism. Studies performed in a cohort of 45 metabolic syndrome patients clearly showed different associations of ghrelin forms with insulin resistance. AG ghrelin levels were, in fact, positively correlated with insulin resistance (HOMA index), while UnAG levels were markedly inversely correlated with the same parameter [62].
Importantly, in a population cohort from the Mo.Ma epidemiological study [111], UnAG was independently positively associated with insulin sensitivity, and lower UnAG plasma levels predicted 5-year insulin resistance [112]. Although it has been reported that UnAG may show a positive modulatory effect on insulin release in vitro [113, 114], in vivo studies collectively strongly suggest that UnAG metabolic effects are mainly related to the modulation of insulin action at tissue level. In excellent agreement, other studies show that acute administration of UnAG does not impact basal or stimulated insulin secretion in β-cells in humans [115].
However, further investigation on the molecular mechanisms involved in ghrelin forms’ metabolic actions is required, as several studies show that ghrelin signalling transduction is interlinked with other pathways, and may be modulated by different expression of receptor forms and by interactions among tissues [116,117,118,119].
Ghrelin and liver metabolism
Ghrelin reportedly modulates hepatic gluconeogenesis, and therefore, glucose release from the liver. Moreover, AG and UnAG have differential effects on glucose release in cultured hepatocytes, with AG stimulating gluconeogenesis and UnAG suppressing it [120]. It should be pointed out that the underlying mechanisms need further investigation as the same effects were not replied with the GHSR1a agonist hexarelin, in agreement with reports of no expression of GHSR in hepatocytes [120] and with the fact that hexarelin administration in humans does not increase plasma glucose levels [120].
However, consistently with in vitro experiments, the expression of PGC1α, a gluconeogenesis inducer, is increased in the liver of AG-treated rats [121], and mice studies with radiolabeled glucose showed that AG partially antagonizes insulin-induced suppression of gluconeogenesis [116]. Moreover, AG reduces insulin signalling in rodents, and this effect is not associated with changes in mitochondrial function [117, 121]. The same authors showed that sustained AG treatment also causes modulation of liver lipid metabolism by inducing a pro-lipogenic gene expression pattern, increasing tissue triglyceride content and reducing the activation of the stimulator of fatty acid oxidation AMP-activated protein kinase (AMPK) [117].
Both antioxidant and anti-inflammatory effects of AG have also been reported in the liver. In in vivo experiments of liver injury in rodent models, as well as in in vitro experiments on primary human stellate cells exposed to chemical damage, AG blunted liver pro-oxidant and pro-inflammatory changes and this result was associated with reduced fibrosis [76, 122].
AG was also reported to improve liver redox state in association with improved inflammation markers in high fat diet-fed rats [123, 124]. However, studies in high fat fed rats show that the beneficial impact of AG on liver redox state and inflammation is not paralleled, expect in one study [123], by improved hepatic insulin signalling, but rather by decreased activating phosphorylation at AKT and GSK-3β levels [121, 124, 125]. This finding, which is also in agreement with in vitro studies in hepatoma cells [125], is consistent with reports showing that in rodent high-fat feeding models, liver AKT activation may directly contribute to hepatic lipogenesis, oxidative stress and inflammation [126].
Fewer reports on UnAG effects on liver metabolism are available. Recent evidence has shown that 4-day UnAG administration does not modify liver redox state, mitochondrial function, inflammation and insulin signalling in young healthy rats, and that these findings are tissue-specific [127]. These results have also been confirmed in transgenic mice with UnAG expression upregulation [128]. However, recent evidence both in vivo and in vitro suggests that in tissue metabolic stress conditions, such as during ischemia/reperfusion, UnAG may improve liver mitochondrial function and protect against apoptosis [129].
Ghrelin and adipose tissue
Appetite stimulating effects of ghrelin were very soon associated with increased body weight, and particularly with fat mass [87]. Further studies in animal models have shown that ghrelin-induced effects are mainly observed in retroperitoneal fat mass and only to a lesser extent in subcutaneous adipose tissue [130]. In a model of daily peripheral ghrelin administration these effects were found to be independent of appetite-induced increased food intake but instead related to reduced fat utilization [87], and in vitro experiments confirmed that ghrelin inhibits lipolysis in adipocytes [131]. Also, AG administration did not impact on food intake in high fat diet feeding but increased adipose tissue mass and favoured the expression of lipogenesis markers [132]. Consistently, ghrelin- or GHRS-null mice were protected from high fat diet induced obesity [133, 134].
Ghrelin promotes adipocyte differentiation [135] and ghrelin’s proadipogenic effect are at least in part mediated by peroxisome proliferator-activated receptor γ (PPARγ2), a transcription factor which favours triglyceride synthesis and downregulates lipolysis [136].
Interestingly, in white adipose tissue, ghrelin enhances the expression of the uncoupling protein 2 (UCP-2), a protein involved in the regulation of mitochondrial reactive oxygen species (ROS) generation, and in UCP-2 null mice, ghrelin enhances its lipogenic effects, suggesting a possible feedback regulation mechanisms involving mitochondrial function [137].
UnAG impact on adipose tissue has been less investigated. While in vitro reports suggest that it may induce at least in part superimposable effects to those produced by AG on adipogenesis upregulation and lipolysis inhibition [131, 138], in vivo studies in rodents show that UnAG peripheral administration may reduce fat mass [139]. Although further studies are needed on the potential role of UnAG on adipose tissue regulation, reported evidence suggests that UnAG is an active hormone with modulatory functions in the complex context of lipid homeostasis.
Ghrelin and skeletal muscle
Skeletal muscle metabolism is characterized by a cluster of interlinked metabolic functional pathways, including mitochondrial function, redox state regulation, inflammation and insulin signalling and action [84, 140,141,142,143,144]. Increased muscle ROS production and inflammation are linked at the level of IκB/NF-κB activation, and may cause insulin resistance by inhibition of insulin signalling downstream of insulin receptor [145,146,147,148]. Interestingly, ghrelin has been reported to be an important modulator of these factors at several levels.
Mitochondrial function
Mitochondrial respiration may be modulated by several mechanisms including UCPs, which selectively reduce mitochondrial ROS generation by inducing mild uncoupling [149,150,151]. In skeletal muscle, both UCP2 mRNA and protein levels are increased after 4-day AG treatment at non orexigenic doses in healthy rats, and this finding is importantly associated with enhanced mitochondrial enzyme activities [117]. Moreover, the same AG treatment improved altered mitochondrial oxidative capacity and transcription of mitochondrial regulatory genes in both uremic rats and in mice with chronic heart failure [152, 153], and was associated with preserved muscle triglyceride accumulation in high fat diet-fed rodents [121].
On the contrary, recent evidence has shown that UnAG treatment is associated with reduced ATP synthesis in healthy rats and that this finding is also present in obese mice with UnAG overexpression [127].
Redox state
In addition, a role for ghrelin in blunting oxidative stress is supported by several studies at whole body level and in several tissues. In obese patients ghrelin levels negatively correlate with systemic oxidative stress marker 8-epi-prostaglandin F2α (8-epi-PGF2α) [154] and in normobaric hypoxia, ghrelin administration attenuates hypoxia-induced increase in plasma levels of malondialdehyde (MDA), another marker of oxidative stress [155]. Moreover, evidence supporting ghrelin as a negative modulator for oxidative stress has been reported also tissues. In experimental models of ischemic or alendronate-induced gastric injury, intravenous ghrelin treatment lowered tissue damage in association with lower ROS production [156, 157], and reperfusion with ghrelin in a rat model of cardiac cachexia decreased myocardial lipid peroxidation [158].
Underlying mechanisms may involve a negative modulation in ROS generation. While AG effect on ROS production by inducing mild uncoupling in mitochondria has already been described, some observations suggest that AG may also act by increasing antioxidant mechanisms [159,160,161].
Few data are available on ghrelin effects on muscle oxidative stress. AG potential role in skeletal muscle redox state modulation has been investigated in a rodent model of diet-induced obesity. In 1-month high fat diet-fed rats, sustained 4-days AG treatment did not modify obesity-induced increase in muscle glutathione peroxidase (GPx) or glutathione oxidation status [162].
On the contrary, some interesting in vitro evidence shows that UnAG, differently from AG, reduces mitochondrial ROS generation in neonatal ventricular myocytes [163]. Moreover, Togliatto et al. have shown that increased skeletal muscle ROS imbalance in a mouse model of limb ischemia was counteracted by UnAG but not AG treatment via an increase in SOD-2 expression [164]. The same authors also observed similar protection of skeletal muscle from ROS in a mouse model of glucose intolerance with peripheral artery disease [165]. A potential role for UnAg in ROS modulation in skeletal muscle was further confirmed and defined by recent evidence, which showed both in vivo and in vitro that UnAG lowers mitochondrial ROS generation, thus improving overall redox state and that this finding is unrelated to changes in antioxidant systems [127, 166]. Improved redox state was also observed in the gastrocnemius muscle of diet-induced obese mice overexpressing UnAG, with levels comparable to wild-type control [127].
Inflammation
AG has been reported to lower inflammation in different experimental settings [167]. GHSR is expressed in both human T lymphocytes and monocytes, and in these cells ghrelin inhibits the expression of the pro-inflammatory cytokines IL-1β, IL-6 e TNF-α [168]. Accordingly, ghrelin levels are increased in septic dogs [169] and ghrelin is among the first increasing hormones responding to endotoxic shock in humans [170], further supporting its potential anti-inflammatory role. Moreover, AG-induced reduction of pro-inflammatory cytokines is paralleled by increased levels of the anti-inflammatory cytokine IL-10 in several cell types [171, 172]. This consistent evidence on the systemic and tissue anti-inflammatory effects of ghrelin has suggested its potential use as a therapeutic agent in clinical settings characterized by high inflammation. Clinical trials have so far shown that ghrelin treatment suppresses airway neutrophil-dominant inflammation in patients with chronic respiratory infection [173] and that postoperative ghrelin administration in patients with oesophageal cancer inhibited inflammatory mediators and ameliorated their clinical course [174].
The role of ghrelin in skeletal muscle inflammation is largely to be investigated. However, sustained administration of AG markedly lowered tissue NF-κB nuclear translocation and tissue TNFα expression in a rodent model of diet-induced obesity, independently from changes in redox state [162]. Interestingly, AG effects in lowering inflammation are also associated with improved redox state in different models [156, 157], strongly suggesting an interplay between ROS production or scavenging and inflammation modulation. Since in skeletal muscle high TNFα levels may reduce mitochondrial function [175], AG might improve mitochondrial function with a mechanism at least in part involving the reduction of TNF-α levels.
UnAG effects on muscle inflammation have only been recently investigated, with reports showing that hormone-induced improvements in muscle redox state are paralleled by the development of an anti-inflammatory cytokine pattern at tissue level both in vivo and in vitro [127, 166].
Tissue insulin signalling and action
Available data globally suggests that AG enhances mitochondrial function, with reports showing also associated improvements in redox state and inflammation. In several models and experimental settings, it has been shown that these effects, alone or combined, are associated with increased tissue insulin sensitivity and action [146,147,148, 153], indicating that AG may potentially improve insulin sensitivity at least partly through these pathways.
In several tissues AG has in fact been reported to activate protein kinase B (AKT), a main mediator of insulin signalling pathway, in association with beneficial effects in different experimental settings [176,177,178,179,180]. Acute AG infusion in humans [181, 182] or experimental models [116] has provided conflicting results in term of systemic or muscle insulin sensitivity changes, with reports of enhanced [116, 121, 168], unchanged [182] or reduced [181] insulin action.
Fewer reports are available on UnAG effects on insulin signalling. Interestingly, one study by Lear et al. showed that in HL-1 cardiac cells and in primary cultures of neonatal rat cardiomyocytes, while both ghrelin forms do not activate AKT, UnAG but not AG increases insulin-induced GLUT4 activation [183]. Recent evidence shows that in young adult rats as well as in vitro, UnAG increases activating phosphorylation at AKT level and downstream, with activation of GSK-3β, and of the protein anabolic mediators PRAS40 and P70S6K [127, 144, 166]. Importantly, UnAG treatment was able to counteract muscular mass wasting in a rodent model of chronic kidney disease [166].
Autophagy
Autophagy is an intracellular selective auto-degradation process that contributes to amino acid recycling for essential proteins synthesis, but may also eliminate dysfunctional mitochondria, thus removing inefficient energy consumption and also excess ROS generation [184, 185].
In 2012 Słupecka et al. showed that enteral AG administration was able to favour small intestine mucosa renewal in new-born piglets in association with enhanced autophagy [186], demonstrating for the first time a link between ghrelin and autophagy. These findings were followed by other studies in different tissues, experimental models and conditions, and mostly confirmed ghrelin as an autophagy inducer.
Interestingly, experiments in an in vitro model of cardiac hypoxic injury showed that AG stimulated autophagy with parallel reduction of ROS generation [187]. In skeletal muscle cells, both AG and UG reportedly enhanced autophagy markers, while blunting apoptosis. Moreover, in a mouse model of gene-induced insulin resistance, UnAG was also able to improve muscle insulin signalling and GLUT4 activation in association with increased autophagy [188]. Recent evidence both in vivo and in vitro further shows that UnAG-induced beneficial effects on skeletal muscle metabolism, with lower mitochondrial ROS production, lower inflammation and enhanced insulin signalling and action in rat muscle are at least in part be mediated through upregulation of autophagy [127, 166] (Fig. 2). In vitro experiments with prolonged hormone incubation of C2C12 myotubes confirmed UnAG-relate findings, while only highest AG doses selectively induced a moderate increase of GSK-3βS9 phosphorylation but failed to reduce ROS production and to enhance downstream insulin signalling [127], suggesting that AG may be a weaker autophagy inducer than UnAG.
Proposed interactions between UnAG and clustered metabolic alterations in skeletal muscle: chronic UnAG over-exposure lowers mitochondrial production of reactive oxygen species (ROS), inflammation and insulin signalling activation levels. Our findings further indicate UnAG to directly lower mitochondrial ROS generation through autophagy stimulation
Ghrelin in obesity-induced insulin resistance
Despite the fact that ghrelin was at first characterized for its effects on GH secretion, polymorphism studies in ghrl gene have also established a link between ghrelin, obesity and obesity-correlated comorbidities development [33, 34]. Moreover, effects of ghrelin on appetite stimulation and therefore on body weight and fat mass increase were soon identified [67]. Also, patients with Prader–Willy Syndrome (PWS), a complex genetic disease characterized by hypomentia, hormonal impairments and early obesity development, present high levels of circulating AG but not UnAG both in fasting and in postprandial conditions [189,190,191,192]. These findings soon led to the hypothesis that ghrelin agonists could be potentially used as anti-obesity drugs. Some studies further supported this hypothesis showing that engineered mice lacking GHSR expression, as well as mice with induced expression of an inactive form of GHSR, did not develop obesity under high fat diet treatment [133, 134, 193]. However, enthusiasm for treating obesity by counteracting ghrelin-mediated effects on appetite was replaced by scepticisms, as data from other studies showed that decreased ghrelin action does not always result in hypophagia and loss of body mass [194, 195], and that the ablation of ghrelin cells in adult mice does not decrease response to HFD [196].
However, observations suggesting that ghrelin could regulate glucose homeostasis and energy metabolism also independently of food intake, led to further investigations of its potential role in obesity and obesity-related co-morbidities, given their complex metabolic pathophysiology [197,198,199]. The first evidence linking ghrelin with human obesity and insulin resistance was reported soon after the discovery of the hormone. In 2001, Tschöp et al. clearly showed that plasma ghrelin levels were decreased in obese humans [61] and a few years later McLaughlin et al. showed that among obese individuals, ghrelin levels were lower in insulin-resistant subjects [107]. This finding was further investigated in studies in selected patients with the metabolic syndrome which demonstrated that obesity-associated reduction in total plasma ghrelin levels was related to a decrease of the unacylated form, while absolute AG levels were not modified compared to non-obese [62]. The same study also showed that in metabolic syndrome patients, among markers [200], while AG levels were positively associated with insulin resistance, waist circumference and BMI, UnAG levels strongly negatively correlated with HOMA [62]. More recently, data analysis from a large cohort has confirmed these findings both in the general population and more specifically in overweight subjects. In addition, UnAG levels were also found to be independently negatively associated with the development of insulin resistance after 5 years [201].
At tissue level, ghrelin has shown to produce a pattern of tissue-specific effects in obese models. In rats fed with high fat diet (HFD) (60% of calories from fat) diet for 4 weeks, 4 day sustained AG administration improved liver oxidative stress and inflammation [124] and similar results were obtained also by other authors after 8 weeks HFD and AG treatment throughout the whole period [123]. These findings were not paralleled, except in one study [123], by improved hepatic insulin signalling, but rather associated with decreased activating phosphorylation at AKT and GSK-3β levels [121, 124, 125]. Decreased hepatic insulin sensitivity in obese rats is also reportedly associated with a decrease in liver expression of insulin receptor substrate 1 (IRS-1), an important mediator and modulator of insulin signalling transmission from insulin receptor to AKT [202]. Interestingly, this finding was reversed by UnAG administration [203]. Collectively, these observations are consistent with reports showing that liver AKT activation may directly contribute to hepatic lipogenesis, oxidative stress and inflammation in rodent models of obesity [126]. Mechanisms involved in AG modulation of liver metabolism in diet-induced obesity are unknown, but a recent report has shown that in fat-induced obese rodents, and in in vitro hepatocytes incubated with saturated fatty acids, AG treatment reduced lipotoxicity via autophagy induction [204].
With regard to adipose tissue, Perez-Tilve et al. have shown that chronic central AG administration in HFD-fed rats, while not increasing food intake, was however associated with enhanced lipogenesis and increased body fat mass, indicating that AG modulation of adiposity is independent of orexigenic effects [132].
In skeletal muscle, sustained 4-day AG treatment was associated with the prevention of obese-related tissue triglyceride content after 4 weeks of HFD [121, 205]. Moreover, although in a similar study by the same group no effect on obesity-induced increase in muscle oxidized glutathione was observed, sustained administration of AG markedly lowered tissue NF-κB nuclear translocation and tissue TNFα expression [162]. This finding was not however associated with significant increases in muscle insulin signalling activation at AKT and GSK-3β levels compared to non-treated obese rats [162].
On the contrary, recent evidence shows for UnAG more clear results. The beneficial effects of UnAG on muscle mitochondrial function, redox state, inflammation and insulin signalling observed in lean rats were in fact confirmed in transgenic mice, in which UnAG overexpression was able to normalize obesity-induced insulin resistance [127].
Conclusions
Collectively, the literature shows that ghrelin forms may play a major role in energy homeostasis and in the regulation of energy metabolism with complex interactions at several levels. While AG is a main appetite modulator at CNS level that also induces a complex set of tissue-specific metabolic effects at tissue level, UnAG is emerging as a novel independent hormone, which is directly able to reduce skeletal muscle ROS generation also by increasing autophagy, with associated improved tissue inflammation and insulin activity. Moreover, reports of beneficial effects induced by ghrelin forms in different models of pathological conditions, including obesity, suggest that further research is strongly needed to investigate the potential use of ghrelin forms in clinical practice. Further study of the molecular mechanisms involved might also lead to the discovery of important new therapeutic targets.
References
Murphy KG, Bloom SR (2006) Gut hormones and the regulation of energy homeostasis. Nature 444(7121):854–859. https://doi.org/10.1038/nature05484
Badman MK, Flier JS (2005) The gut and energy balance: visceral allies in the obesity wars. Science 307(5717):1909–1914. https://doi.org/10.1126/science.1109951
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402(6762):656–660. https://doi.org/10.1038/45230
Inui A, Asakawa A, Bowers CY, Mantovani G, Laviano A, Meguid MM, Fujimiya M (2004) Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. FASEB J 18(3):439–456. https://doi.org/10.1096/fj.03-0641rev
Bowers CY, Momany F, Reynolds GA, Chang D, Hong A, Chang K (1980) Structure–activity relationships of a synthetic pentapeptide that specifically releases growth hormone in vitro. Endocrinology 106(3):663–667. https://doi.org/10.1210/endo-106-3-663
Bowers CY, Momany FA, Reynolds GA, Hong A (1984) On the in vitro and in vivo activity of a new synthetic hexapeptide that acts on the pituitary to specifically release growth hormone. Endocrinology 114(5):1537–1545. https://doi.org/10.1210/endo-114-5-1537
Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, Chou M, Liu KK, McKee KK, Pong SS, Chaung LY, Elbrecht A, Dashkevicz M, Heavens R, Rigby M, Sirinathsinghji DJ, Dean DC, Melillo DG, Patchett AA, Nargund R, Griffin PR, DeMartino JA, Gupta SK, Schaeffer JM, Smith RG, Van der Ploeg LH (1996) A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 273(5277):974–977
Kojima M, Kangawa K (2005) Ghrelin: structure and function. Physiol Rev 85(2):495–522. https://doi.org/10.1152/physrev.00012.2004
Tomasetto C, Karam SM, Ribieras S, Masson R, Lefebvre O, Staub A, Alexander G, Chenard MP, Rio MC (2000) Identification and characterization of a novel gastric peptide hormone: the motilin-related peptide. Gastroenterology 119(2):395–405
Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K (2001) Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab 86(10):4753–4758. https://doi.org/10.1210/jcem.86.10.7885
Sakata I, Nakamura K, Yamazaki M, Matsubara M, Hayashi Y, Kangawa K, Sakai T (2002) Ghrelin-producing cells exist as two types of cells, closed- and opened-type cells, in the rat gastrointestinal tract. Peptides 23(3):531–536
Knerr I, Herzog D, Rauh M, Rascher W, Horbach T (2006) Leptin and ghrelin expression in adipose tissues and serum levels in gastric banding patients. Eur J Clin Investig 36(6):389–394. https://doi.org/10.1111/j.1365-2362.2006.01642.x
Korbonits M, Bustin SA, Kojima M, Jordan S, Adams EF, Lowe DG, Kangawa K, Grossman AB (2001) The expression of the growth hormone secretagogue receptor ligand ghrelin in normal and abnormal human pituitary and other neuroendocrine tumors. J Clin Endocrinol Metab 86(2):881–887. https://doi.org/10.1210/jcem.86.2.7190
Volante M, Allia E, Gugliotta P, Funaro A, Broglio F, Deghenghi R, Muccioli G, Ghigo E, Papotti M (2002) Expression of ghrelin and of the GH secretagogue receptor by pancreatic islet cells and related endocrine tumors. J Clin Endocrinol Metab 87(3):1300–1308. https://doi.org/10.1210/jcem.87.3.8279
Wierup N, Svensson H, Mulder H, Sundler F (2002) The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regul Pept 107(1–3):63–69
Mori K, Yoshimoto A, Takaya K, Hosoda K, Ariyasu H, Yahata K, Mukoyama M, Sugawara A, Hosoda H, Kojima M, Kangawa K, Nakao K (2000) Kidney produces a novel acylated peptide, ghrelin. FEBS Lett 486(3):213–216
Tena-Sempere M, Barreiro ML, Gonzalez LC, Gaytan F, Zhang FP, Caminos JE, Pinilla L, Casanueva FF, Dieguez C, Aguilar E (2002) Novel expression and functional role of ghrelin in rat testis. Endocrinology 143(2):717–725. https://doi.org/10.1210/endo.143.2.8646
Gualillo O, Caminos J, Blanco M, Garcia-Caballero T, Kojima M, Kangawa K, Dieguez C, Casanueva F (2001) Ghrelin, a novel placental-derived hormone. Endocrinology 142(2):788–794. https://doi.org/10.1210/endo.142.2.7987
Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M (2000) Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141(11):4255–4261. https://doi.org/10.1210/endo.141.11.7757
Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M (2002) The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 123(4):1120–1128
Wajnrajch MP, Ten I, Gertner JM, Leibel RL (2000) Genomic organization of the human GHRELIN gene. Int J Disabil Hum Dev 1(4):231–234
Seim I, Collet C, Herington AC, Chopin LK (2007) Revised genomic structure of the human ghrelin gene and identification of novel exons, alternative splice variants and natural antisense transcripts. BMC Genom 8:298. https://doi.org/10.1186/1471-2164-8-298
Sato T, Nakamura Y, Shiimura Y, Ohgusu H, Kangawa K, Kojima M (2012) Structure, regulation and function of ghrelin. J Biochem 151(2):119–128. https://doi.org/10.1093/jb/mvr134
Liu B, Garcia EA, Korbonits M (2011) Genetic studies on the ghrelin, growth hormone secretagogue receptor (GHSR) and ghrelin O-acyl transferase (GOAT) genes. Peptides 32(11):2191–2207. https://doi.org/10.1016/j.peptides.2011.09.006
Zhang JV, Ren PG, Avsian-Kretchmer O, Luo CW, Rauch R, Klein C, Hsueh AJ (2005) Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science 310(5750):996–999. https://doi.org/10.1126/science.1117255
Depoortere I (2012) GI functions of GPR39: novel biology. Curr Opin Pharmacol 12(6):647–652. https://doi.org/10.1016/j.coph.2012.07.019
Gargantini E, Grande C, Trovato L, Ghigo E, Granata R (2013) The role of obestatin in glucose and lipid metabolism. Horm Metab Res 45(13):1002–1008. https://doi.org/10.1055/s-0033-1351325
Baragli A, Lanfranco F, Allasia S, Granata R, Ghigo E (2011) Neuroendocrine and metabolic activities of ghrelin gene products. Peptides 32(11):2323–2332. https://doi.org/10.1016/j.peptides.2011.10.024
Gesmundo I, Gallo D, Favaro E, Ghigo E, Granata R (2013) Obestatin: a new metabolic player in the pancreas and white adipose tissue. IUBMB Life 65(12):976–982. https://doi.org/10.1002/iub.1226
Granata R, Gallo D, Luque RM, Baragli A, Scarlatti F, Grande C, Gesmundo I, Cordoba-Chacon J, Bergandi L, Settanni F, Togliatto G, Volante M, Garetto S, Annunziata M, Chanclon B, Gargantini E, Rocchietto S, Matera L, Datta G, Morino M, Brizzi MF, Ong H, Camussi G, Castano JP, Papotti M, Ghigo E (2012) Obestatin regulates adipocyte function and protects against diet-induced insulin resistance and inflammation. FASEB J 26(8):3393–3411. https://doi.org/10.1096/fj.11-201343
Miraglia del Giudice E, Santoro N, Cirillo G, Raimondo P, Grandone A, D’Aniello A, Di Nardo M, Perrone L (2004) Molecular screening of the ghrelin gene in Italian obese children: the Leu72Met variant is associated with an earlier onset of obesity. Int J Obes Relat Metab Disord 28(3):447–450. https://doi.org/10.1038/sj.ijo.0802572
Poykko S, Ukkola O, Kauma H, Savolainen MJ, Kesaniemi YA (2003) Ghrelin Arg51Gln mutation is a risk factor for Type 2 diabetes and hypertension in a random sample of middle-aged subjects. Diabetologia 46(4):455–458. https://doi.org/10.1007/s00125-003-1058-z
Ukkola O, Ravussin E, Jacobson P, Snyder EE, Chagnon M, Sjostrom L, Bouchard C (2001) Mutations in the preproghrelin/ghrelin gene associated with obesity in humans. J Clin Endocrinol Metab 86(8):3996–3999. https://doi.org/10.1210/jcem.86.8.7914
Ukkola O, Ravussin E, Jacobson P, Perusse L, Rankinen T, Tschop M, Heiman ML, Leon AS, Rao DC, Skinner JS, Wilmore JH, Sjostrom L, Bouchard C (2002) Role of ghrelin polymorphisms in obesity based on three different studies. Obes Res 10(8):782–791. https://doi.org/10.1038/oby.2002.106
Hosoda H, Kojima M, Matsuo H, Kangawa K (2000) Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun 279(3):909–913. https://doi.org/10.1006/bbrc.2000.4039
Sakata I, Yang J, Lee CE, Osborne-Lawrence S, Rovinsky SA, Elmquist JK, Zigman JM (2009) Colocalization of ghrelin O-acyltransferase and ghrelin in gastric mucosal cells. Am J Physiol Endocrinol Metab 297(1):E134–E141. https://doi.org/10.1152/ajpendo.90859.2008
Yang J, Zhao TJ, Goldstein JL, Brown MS (2008) Inhibition of ghrelin O-acyltransferase (GOAT) by octanoylated pentapeptides. Proc Natl Acad Sci USA 105(31):10750–10755. https://doi.org/10.1073/pnas.0805353105
Lim CT, Kola B, Grossman A, Korbonits M (2011) The expression of ghrelin O-acyltransferase (GOAT) in human tissues. Endocr J 58(8):707–710
Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, Witcher DR, Luo S, Onyia JE, Hale JE (2008) Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA 105(17):6320–6325. https://doi.org/10.1073/pnas.0800708105
Hosoda H, Kojima M, Mizushima T, Shimizu S, Kangawa K (2003) Structural divergence of human ghrelin. Identification of multiple ghrelin-derived molecules produced by post-translational processing. J Biol Chem 278(1):64–70. https://doi.org/10.1074/jbc.M205366200
Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL (2008) Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 132(3):387–396. https://doi.org/10.1016/j.cell.2008.01.017
Dehlin E, Liu J, Yun SH, Fox E, Snyder S, Gineste C, Willingham L, Geysen M, Gaylinn BD, Sando JJ (2008) Regulation of ghrelin structure and membrane binding by phosphorylation. Peptides 29(6):904–911. https://doi.org/10.1016/j.peptides.2008.02.001
Mundinger TO, Cummings DE, Taborsky GJ Jr (2006) Direct stimulation of ghrelin secretion by sympathetic nerves. Endocrinology 147(6):2893–2901. https://doi.org/10.1210/en.2005-1182
Engelstoft MS, Park WM, Sakata I, Kristensen LV, Husted AS, Osborne-Lawrence S, Piper PK, Walker AK, Pedersen MH, Nohr MK, Pan J, Sinz CJ, Carrington PE, Akiyama TE, Jones RM, Tang C, Ahmed K, Offermanns S, Egerod KL, Zigman JM, Schwartz TW (2013) Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol Metab 2(4):376–392. https://doi.org/10.1016/j.molmet.2013.08.006
Tong J, Dave N, Mugundu GM, Davis HW, Gaylinn BD, Thorner MO, Tschop MH, D’Alessio D, Desai PB (2013) The pharmacokinetics of acyl, des-acyl, and total ghrelin in healthy human subjects. Eur J Endocrinol 168(6):821–828. https://doi.org/10.1530/EJE-13-0072
Liu J, Prudom CE, Nass R, Pezzoli SS, Oliveri MC, Johnson ML, Veldhuis P, Gordon DA, Howard AD, Witcher DR, Geysen HM, Gaylinn BD, Thorner MO (2008) Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J Clin Endocrinol Metab 93(5):1980–1987. https://doi.org/10.1210/jc.2007-2235
Akamizu T, Shinomiya T, Irako T, Fukunaga M, Nakai Y, Nakai Y, Kangawa K (2005) Separate measurement of plasma levels of acylated and desacyl ghrelin in healthy subjects using a new direct ELISA assay. J Clin Endocrinol Metab 90(1):6–9. https://doi.org/10.1210/jc.2004-1640
Janssen S, Laermans J, Verhulst PJ, Thijs T, Tack J, Depoortere I (2011) Bitter taste receptors and alpha-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc Natl Acad Sci USA 108(5):2094–2099. https://doi.org/10.1073/pnas.1011508108
Mizutani M, Atsuchi K, Asakawa A, Matsuda N, Fujimura M, Inui A, Kato I, Fujimiya M (2009) Localization of acyl ghrelin- and des-acyl ghrelin-immunoreactive cells in the rat stomach and their responses to intragastric pH. Am J Physiol Gastrointest Liver Physiol 297(5):G974–G980
De Vriese C, Gregoire F, Lema-Kisoka R, Waelbroeck M, Robberecht P, Delporte C (2004) Ghrelin degradation by serum and tissue homogenates: identification of the cleavage sites. Endocrinology 145(11):4997–5005. https://doi.org/10.1210/en.2004-0569
Banks WA, Tschop M, Robinson SM, Heiman ML (2002) Extent and direction of ghrelin transport across the blood–brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther 302(2):822–827. https://doi.org/10.1124/jpet.102.034827
Banks WA, Burney BO, Robinson SM (2008) Effects of triglycerides, obesity, and starvation on ghrelin transport across the blood–brain barrier. Peptides 29(11):2061–2065. https://doi.org/10.1016/j.peptides.2008.07.001
Barazzoni R, Zanetti M, Stebel M, Biolo G, Cattin L, Guarnieri G (2003) Hyperleptinemia prevents increased plasma ghrelin concentration during short-term moderate caloric restriction in rats. Gastroenterology 124(5):1188–1192
Gualillo O, Caminos JE, Nogueiras R, Seoane LM, Arvat E, Ghigo E, Casanueva FF, Dieguez C (2002) Effect of food restriction on ghrelin in normal-cycling female rats and in pregnancy. Obes Res 10(7):682–687. https://doi.org/10.1038/oby.2002.92
Cummings DE, Overduin J (2007) Gastrointestinal regulation of food intake. J Clin Invest 117(1):13–23. https://doi.org/10.1172/JCI30227
Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS (2001) A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50(8):1714–1719
Tschop M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, Landgraf R, Folwaczny C (2001) Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest 24(6):RC19–R21. https://doi.org/10.1007/BF03351037
Cummings DE, Foster-Schubert KE, Overduin J (2005) Ghrelin and energy balance: focus on current controversies. Curr Drug Targets 6(2):153–169
Sanchez J, Oliver P, Palou A, Pico C (2004) The inhibition of gastric ghrelin production by food intake in rats is dependent on the type of macronutrient. Endocrinology 145(11):5049–5055. https://doi.org/10.1210/en.2004-0493
Otto B, Cuntz U, Fruehauf E, Wawarta R, Folwaczny C, Riepl RL, Heiman ML, Lehnert P, Fichter M, Tschop M (2001) Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur J Endocrinol 145(5):669–673
Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML (2001) Circulating ghrelin levels are decreased in human obesity. Diabetes 50(4):707–709
Barazzoni R, Zanetti M, Ferreira C, Vinci P, Pirulli A, Mucci M, Dore F, Fonda M, Ciocchi B, Cattin L, Guarnieri G (2007) Relationships between desacylated and acylated ghrelin and insulin sensitivity in the metabolic syndrome. J Clin Endocrinol Metab 92(10):3935–3940. https://doi.org/10.1210/jc.2006-2527
Barazzoni R, Zanetti M, Nagliati C, Cattin MR, Ferreira C, Giuricin M, Palmisano S, Edalucci E, Dore F, Guarnieri G, de Manzini N (2013) Gastric bypass does not normalize obesity-related changes in ghrelin profile and leads to higher acylated ghrelin fraction. Obesity (Silver Spring) 21(4):718–722. https://doi.org/10.1002/oby.20272
Kirchner H, Gutierrez JA, Solenberg PJ, Pfluger PT, Czyzyk TA, Willency JA, Schurmann A, Joost HG, Jandacek RJ, Hale JE, Heiman ML, Tschop MH (2009) GOAT links dietary lipids with the endocrine control of energy balance. Nat Med 15(7):741–745. https://doi.org/10.1038/nm.1997
Nishi Y, Hiejima H, Hosoda H, Kaiya H, Mori K, Fukue Y, Yanase T, Nawata H, Kangawa K, Kojima M (2005) Ingested medium-chain fatty acids are directly utilized for the acyl modification of ghrelin. Endocrinology 146(5):2255–2264. https://doi.org/10.1210/en.2004-0695
Barazzoni R, Gortan Cappellari G, Semolic A, Ius M, Dore F, Giacca M, Zanetti M, Vinci P, Guarnieri G (2017) Intravenous lipid infusion and total plasma fatty acids positively modulate plasma acylated ghrelin in vivo. Clin Nutr 36(3):775–781. https://doi.org/10.1016/j.clnu.2016.05.017
Muller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, Batterham RL, Benoit SC, Bowers CY, Broglio F, Casanueva FF, D’Alessio D, Depoortere I, Geliebter A, Ghigo E, Cole PA, Cowley M, Cummings DE, Dagher A, Diano S, Dickson SL, Dieguez C, Granata R, Grill HJ, Grove K, Habegger KM, Heppner K, Heiman ML, Holsen L, Holst B, Inui A, Jansson JO, Kirchner H, Korbonits M, Laferrere B, LeRoux CW, Lopez M, Morin S, Nakazato M, Nass R, Perez-Tilve D, Pfluger PT, Schwartz TW, Seeley RJ, Sleeman M, Sun Y, Sussel L, Tong J, Thorner MO, van der Lely AJ, van der Ploeg LH, Zigman JM, Kojima M, Kangawa K, Smith RG, Horvath T, Tschop MH (2015) Ghrelin. Mol Metab 4(6):437–460. https://doi.org/10.1016/j.molmet.2015.03.005
Bednarek MA, Feighner SD, Pong SS, McKee KK, Hreniuk DL, Silva MV, Warren VA, Howard AD, Van Der Ploeg LH, Heck JV (2000) Structure–function studies on the new growth hormone-releasing peptide, ghrelin: minimal sequence of ghrelin necessary for activation of growth hormone secretagogue receptor 1a. J Med Chem 43(23):4370–4376
Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB, Korbonits M (2002) The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab 87(6):2988. https://doi.org/10.1210/jcem.87.6.8739
Hattori N, Saito T, Yagyu T, Jiang BH, Kitagawa K, Inagaki C (2001) GH, GH receptor, GH secretagogue receptor, and ghrelin expression in human T cells, B cells, and neutrophils. J Clin Endocrinol Metab 86(9):4284–4291. https://doi.org/10.1210/jcem.86.9.7866
Shuto Y, Shibasaki T, Wada K, Parhar I, Kamegai J, Sugihara H, Oikawa S, Wakabayashi I (2001) Generation of polyclonal antiserum against the growth hormone secretagogue receptor (GHS-R): evidence that the GHS-R exists in the hypothalamus, pituitary and stomach of rats. Life Sci 68(9):991–996
Papotti M, Ghe C, Cassoni P, Catapano F, Deghenghi R, Ghigo E, Muccioli G (2000) Growth hormone secretagogue binding sites in peripheral human tissues. J Clin Endocrinol Metab 85(10):3803–3807. https://doi.org/10.1210/jcem.85.10.6846
Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD (1997) Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res 48(1):23–29
Gershon E, Vale WW (2014) CRF type 2 receptors mediate the metabolic effects of ghrelin in C2C12 cells. Obesity (Silver Spring) 22(2):380–389. https://doi.org/10.1002/oby.20535
McGirr R, McFarland MS, McTavish J, Luyt LG, Dhanvantari S (2011) Design and characterization of a fluorescent ghrelin analog for imaging the growth hormone secretagogue receptor 1a. Regul Pept 172(1–3):69–76. https://doi.org/10.1016/j.regpep.2011.08.011
Moreno M, Chaves JF, Sancho-Bru P, Ramalho F, Ramalho LN, Mansego ML, Ivorra C, Dominguez M, Conde L, Millan C, Mari M, Colmenero J, Lozano JJ, Jares P, Vidal J, Forns X, Arroyo V, Caballeria J, Gines P, Bataller R (2010) Ghrelin attenuates hepatocellular injury and liver fibrogenesis in rodents and influences fibrosis progression in humans. Hepatology 51(3):974–985. https://doi.org/10.1002/hep.23421
Ueberberg B, Unger N, Saeger W, Mann K, Petersenn S (2009) Expression of ghrelin and its receptor in human tissues. Horm Metab Res 41(11):814–821. https://doi.org/10.1055/s-0029-1233462
Filigheddu N, Gnocchi VF, Coscia M, Cappelli M, Porporato PE, Taulli R, Traini S, Baldanzi G, Chianale F, Cutrupi S, Arnoletti E, Ghe C, Fubini A, Surico N, Sinigaglia F, Ponzetto C, Muccioli G, Crepaldi T, Graziani A (2007) Ghrelin and des-acyl ghrelin promote differentiation and fusion of C2C12 skeletal muscle cells. Mol Biol Cell 18(3):986–994. https://doi.org/10.1091/mbc.E06-05-0402
Sun Y, Garcia JM, Smith RG (2007) Ghrelin and growth hormone secretagogue receptor expression in mice during aging. Endocrinology 148(3):1323–1329. https://doi.org/10.1210/en.2006-0782
Callaghan B, Furness JB (2014) Novel and conventional receptors for ghrelin, desacyl-ghrelin, and pharmacologically related compounds. Pharmacol Rev 66(4):984–1001. https://doi.org/10.1124/pr.113.008433
Muccioli G, Baragli A, Granata R, Papotti M, Ghigo E (2007) Heterogeneity of ghrelin/growth hormone secretagogue receptors. Toward the understanding of the molecular identity of novel ghrelin/GHS receptors. Neuroendocrinology 86(3):147–164. https://doi.org/10.1159/000105141
Dardzinska JA, Malgorzewicz S, Kaska L, Proczko M, Stefaniak T, Stankiewicz M, Sledzinski Z (2014) Fasting and postprandial acyl and desacyl ghrelin levels in obese and non-obese subjects. Endokrynol Pol 65(5):377–381. https://doi.org/10.5603/EP.2014.0052
Gil-Campos M, Aguilera CM, Canete R, Gil A (2006) Ghrelin: a hormone regulating food intake and energy homeostasis. Br J Nutr 96(2):201–226
Barazzoni R, Gortan Cappellari G, Zanetti M, Guarnieri G (2012) Ghrelin and muscle metabolism in chronic uremia. J Ren Nutr 22(1):171–175. https://doi.org/10.1053/j.jrn.2011.10.017
Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S (2001) A role for ghrelin in the central regulation of feeding. Nature 409(6817):194–198. https://doi.org/10.1038/35051587
Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, Batterham RL, Taheri S, Stanley SA, Ghatei MA, Bloom SR (2001) Ghrelin causes hyperphagia and obesity in rats. Diabetes 50(11):2540–2547
Tschop M, Smiley DL, Heiman ML (2000) Ghrelin induces adiposity in rodents. Nature 407(6806):908–913. https://doi.org/10.1038/35038090
Dornonville de la Cour C, Lindqvist A, Egecioglu E, Tung YC, Surve V, Ohlsson C, Jansson JO, Erlanson-Albertsson C, Dickson SL, Hakanson R (2005) Ghrelin treatment reverses the reduction in weight gain and body fat in gastrectomised mice. Gut 54(7):907–913. https://doi.org/10.1136/gut.2004.058578
Adachi S, Takiguchi S, Okada K, Yamamoto K, Yamasaki M, Miyata H, Nakajima K, Fujiwara Y, Hosoda H, Kangawa K, Mori M, Doki Y (2010) Effects of ghrelin administration after total gastrectomy: a prospective, randomized, placebo-controlled phase II study. Gastroenterology 138(4):1312–1320. https://doi.org/10.1053/j.gastro.2009.12.058
Arnold M, Mura A, Langhans W, Geary N (2006) Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci 26(43):11052–11060. https://doi.org/10.1523/JNEUROSCI.2606-06.2006
Kola B, Farkas I, Christ-Crain M, Wittmann G, Lolli F, Amin F, Harvey-White J, Liposits Z, Kunos G, Grossman AB, Fekete C, Korbonits M (2008) The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PloS One 3(3):e1797. https://doi.org/10.1371/journal.pone.0001797
Bagnasco M, Dube MG, Kalra PS, Kalra SP (2002) Evidence for the existence of distinct central appetite, energy expenditure, and ghrelin stimulation pathways as revealed by hypothalamic site-specific leptin gene therapy. Endocrinology 143(11):4409–4421
Wang L, Saint-Pierre DH, Tache Y (2002) Peripheral ghrelin selectively increases Fos expression in neuropeptide Y-synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci Lett 325(1):47–51
Willesen MG, Kristensen P, Romer J (1999) Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology 70(5):306–316
Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK (2006) Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol 494(3):528–548. https://doi.org/10.1002/cne.20823
Malik S, McGlone F, Bedrossian D, Dagher A (2008) Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab 7(5):400–409. https://doi.org/10.1016/j.cmet.2008.03.007
Asakawa A, Inui A, Fujimiya M, Sakamaki R, Shinfuku N, Ueta Y, Meguid MM, Kasuga M (2005) Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut 54(1):18–24. https://doi.org/10.1136/gut.2004.038737
Inhoff T, Monnikes H, Noetzel S, Stengel A, Goebel M, Dinh QT, Riedl A, Bannert N, Wisser AS, Wiedenmann B, Klapp BF, Tache Y, Kobelt P (2008) Desacyl ghrelin inhibits the orexigenic effect of peripherally injected ghrelin in rats. Peptides 29(12):2159–2168. https://doi.org/10.1016/j.peptides.2008.09.014
Toshinai K, Yamaguchi H, Sun Y, Smith RG, Yamanaka A, Sakurai T, Date Y, Mondal MS, Shimbara T, Kawagoe T, Murakami N, Miyazato M, Kangawa K, Nakazato M (2006) Des-acyl ghrelin induces food intake by a mechanism independent of the growth hormone secretagogue receptor. Endocrinology 147(5):2306–2314. https://doi.org/10.1210/en.2005-1357
Barazzoni R, Deutz NEP, Biolo G, Bischoff S, Boirie Y, Cederholm T, Cuerda C, Delzenne N, Leon Sanz M, Ljungqvist O, Muscaritoli M, Pichard C, Preiser JC, Sbraccia P, Singer P, Tappy L, Thorens B, Van Gossum A, Vettor R, Calder PC (2017) Carbohydrates and insulin resistance in clinical nutrition: recommendations from the ESPEN expert group. Clin Nutr 36(2):355–363. https://doi.org/10.1016/j.clnu.2016.09.010
Broglio F, Arvat E, Benso A, Gottero C, Muccioli G, Papotti M, van der Lely AJ, Deghenghi R, Ghigo E (2001) Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab 86(10):5083–5086. https://doi.org/10.1210/jcem.86.10.8098
Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Kahn SE, Cummings DE, Tschop MH, D’Alessio D (2010) Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes 59(9):2145–2151. https://doi.org/10.2337/db10-0504
Sun Y, Butte NF, Garcia JM, Smith RG (2008) Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology 149(2):843–850. https://doi.org/10.1210/en.2007-0271
Dezaki K, Kakei M, Yada T (2007) Ghrelin uses Galphai2 and activates voltage-dependent K+ channels to attenuate glucose-induced Ca2+ signaling and insulin release in islet beta-cells: novel signal transduction of ghrelin. Diabetes 56(9):2319–2327. https://doi.org/10.2337/db07-0345
Poykko SM, Kellokoski E, Horkko S, Kauma H, Kesaniemi YA, Ukkola O (2003) Low plasma ghrelin is associated with insulin resistance, hypertension, and the prevalence of type 2 diabetes. Diabetes 52(10):2546–2553
Barazzoni R, Zanetti M, Stulle M, Mucci MP, Pirulli A, Dore F, Panzetta G, Vasile A, Biolo G, Guarnieri G (2008) Higher total ghrelin levels are associated with higher insulin-mediated glucose disposal in non-diabetic maintenance hemodialysis patients. Clin Nutr 27(1):142–149. https://doi.org/10.1016/j.clnu.2007.06.013
McLaughlin T, Abbasi F, Lamendola C, Frayo RS, Cummings DE (2004) Plasma ghrelin concentrations are decreased in insulin-resistant obese adults relative to equally obese insulin-sensitive controls. J Clin Endocrinol Metab 89(4):1630–1635. https://doi.org/10.1210/jc.2003-031572
Zanetti M, Gortan Cappellari G, Semolic A, Burekovic I, Fonda M, Cattin L, Barazzoni R (2017) Gender-specific association of desacylated ghrelin with subclinical atherosclerosis in the metabolic syndrome. Arch Med Res. https://doi.org/10.1016/j.arcmed.2017.09.002
Broglio F, Gottero C, Prodam F, Gauna C, Muccioli G, Papotti M, Abribat T, Van Der Lely AJ, Ghigo E (2004) Non-acylated ghrelin counteracts the metabolic but not the neuroendocrine response to acylated ghrelin in humans. J Clin Endocrinol Metab 89(6):3062–3065. https://doi.org/10.1210/jc.2003-031964
Gauna C, Kiewiet RM, Janssen JA, van de Zande B, Delhanty PJ, Ghigo E, Hofland LJ, Themmen AP, van der Lely AJ (2007) Unacylated ghrelin acts as a potent insulin secretagogue in glucose-stimulated conditions. Am J Physiol Endocrinol Metab 293(3):E697–E704. https://doi.org/10.1152/ajpendo.00219.2007
Barazzoni R, Gortan Cappellari G, Semolic A, Chendi E, Ius M, Situlin R, Zanetti M, Vinci P, Guarnieri G (2014) The association between hematological parameters and insulin resistance is modified by body mass index—results from the North-East Italy MoMa population study. PloS One 9(7):e101590. https://doi.org/10.1371/journal.pone.0101590
Barazzoni R, Gortan Cappellari G, Semolic A, Ius M, Mamolo L, Dore F, Giacca M, Zanetti M, Vinci P, Guarnieri G (2016) Plasma total and unacylated ghrelin predict 5-year changes in insulin resistance. Clin Nutr 35(5):1168–1173. https://doi.org/10.1016/j.clnu.2015.10.002
Granata R, Settanni F, Biancone L, Trovato L, Nano R, Bertuzzi F, Destefanis S, Annunziata M, Martinetti M, Catapano F, Ghe C, Isgaard J, Papotti M, Ghigo E, Muccioli G (2007) Acylated and unacylated ghrelin promote proliferation and inhibit apoptosis of pancreatic beta-cells and human islets: involvement of 3′,5′-cyclic adenosine monophosphate/protein kinase A, extracellular signal-regulated kinase 1/2, and phosphatidyl inositol 3-kinase/Akt signaling. Endocrinology 148(2):512–529. https://doi.org/10.1210/en.2006-0266
Gauna C, Delhanty PJ, van Aken MO, Janssen JA, Themmen AP, Hofland LJ, Culler M, Broglio F, Ghigo E, van der Lely AJ (2006) Unacylated ghrelin is active on the INS-1E rat insulinoma cell line independently of the growth hormone secretagogue receptor type 1a and the corticotropin releasing factor 2 receptor. Mol Cell Endocrinol 251(1–2):103–111. https://doi.org/10.1016/j.mce.2006.03.040
Tong J, Davis HW, Summer S, Benoit SC, Haque A, Bidlingmaier M, Tschop MH, D’Alessio D (2014) Acute administration of unacylated ghrelin has no effect on Basal or stimulated insulin secretion in healthy humans. Diabetes 63(7):2309–2319. https://doi.org/10.2337/db13-1598
Heijboer AC, van den Hoek AM, Parlevliet ET, Havekes LM, Romijn JA, Pijl H, Corssmit EP (2006) Ghrelin differentially affects hepatic and peripheral insulin sensitivity in mice. Diabetologia 49(4):732–738. https://doi.org/10.1007/s00125-006-0138-2
Barazzoni R, Bosutti A, Stebel M, Cattin MR, Roder E, Visintin L, Cattin L, Biolo G, Zanetti M, Guarnieri G (2005) Ghrelin regulates mitochondrial-lipid metabolism gene expression and tissue fat distribution in liver and skeletal muscle. Am J Physiol Endocrinol Metab 288(1):E228–E235. https://doi.org/10.1152/ajpendo.00115.2004
Pardo M, Roca-Rivada A, Al-Massadi O, Seoane LM, Camina JP, Casanueva FF (2010) Peripheral leptin and ghrelin receptors are regulated in a tissue-specific manner in activity-based anorexia. Peptides 31(10):1912–1919. https://doi.org/10.1016/j.peptides.2010.06.022
van Thuijl H, Kola B, Korbonits M (2008) Appetite and metabolic effects of ghrelin and cannabinoids: involvement of AMP-activated protein kinase. Vitam Horm 77:121–148. https://doi.org/10.1016/S0083-6729(06)77006-6
Gauna C, Delhanty PJ, Hofland LJ, Janssen JA, Broglio F, Ross RJ, Ghigo E, van der Lely AJ (2005) Ghrelin stimulates, whereas des-octanoyl ghrelin inhibits, glucose output by primary hepatocytes. J Clin Endocrinol Metab 90(2):1055–1060. https://doi.org/10.1210/jc.2004-1069
Barazzoni R, Zanetti M, Cattin MR, Visintin L, Vinci P, Cattin L, Stebel M, Guarnieri G (2007) Ghrelin enhances in vivo skeletal muscle but not liver AKT signaling in rats. Obesity 15(11):2614–2623. https://doi.org/10.1038/oby.2007.313
Obay BD, Tasdemir E, Tumer C, Bilgin H, Atmaca M (2008) Dose dependent effects of ghrelin on pentylenetetrazole-induced oxidative stress in a rat seizure model. Peptides 29(3):448–455. https://doi.org/10.1016/j.peptides.2007.11.020
Li Y, Hai J, Li L, Chen X, Peng H, Cao M, Zhang Q (2013) Administration of ghrelin improves inflammation, oxidative stress, and apoptosis during and after non-alcoholic fatty liver disease development. Endocrine 43(2):376–386. https://doi.org/10.1007/s12020-012-9761-5
Barazzoni R, Semolic A, Cattin MR, Zanetti M, Guarnieri G (2014) Acylated ghrelin limits fat accumulation and improves redox state and inflammation markers in the liver of high-fat-fed rats. Obesity (Silver Spring) 22(1):170–177. https://doi.org/10.1002/oby.20454
Murata M, Okimura Y, Iida K, Matsumoto M, Sowa H, Kaji H, Kojima M, Kangawa K, Chihara K (2002) Ghrelin modulates the downstream molecules of insulin signaling in hepatoma cells. J Biol Chem 277(7):5667–5674. https://doi.org/10.1074/jbc.M103898200
Liu HY, Hong T, Wen GB, Han J, Zuo D, Liu Z, Cao W (2009) Increased basal level of Akt-dependent insulin signaling may be responsible for the development of insulin resistance. Am J Physiol Endocrinol Metab 297(4):E898–E906. https://doi.org/10.1152/ajpendo.00374.2009
Gortan Cappellari G, Zanetti M, Semolic A, Vinci P, Ruozi G, Falcione A, Filigheddu N, Guarnieri G, Graziani A, Giacca M, Barazzoni R (2016) Unacylated ghrelin reduces skeletal muscle reactive oxygen species generation and inflammation and prevents high-fat diet-induced hyperglycemia and whole-body insulin resistance in rodents. Diabetes 65(4):874–886. https://doi.org/10.2337/db15-1019
Gortan Cappellari G, Zanetti M, Semolic A, Vinci P, Ruozi G, De Nardo M, Filigheddu N, Guarnieri G, Giacca M, Graziani A (2015) Unacylated ghrelin does not alter mitochondrial function, redox state and triglyceride content in rat liver in vivo. Clin Nutr Exp 4:1–7
Rossetti A, Togliatto G, Rolo AP, Teodoro JS, Granata R, Ghigo E, Columbano A, Palmeira CM, Brizzi MF (2017) Unacylated ghrelin prevents mitochondrial dysfunction in a model of ischemia/reperfusion liver injury. Cell Death Discov 3:17077. https://doi.org/10.1038/cddiscovery.2017.77
Davies JS, Kotokorpi P, Eccles SR, Barnes SK, Tokarczuk PF, Allen SK, Whitworth HS, Guschina IA, Evans BA, Mode A, Zigman JM, Wells T (2009) Ghrelin induces abdominal obesity via GHS-R-dependent lipid retention. Mol Endocrinol 23(6):914–924. https://doi.org/10.1210/me.2008-0432
Muccioli G, Pons N, Ghe C, Catapano F, Granata R, Ghigo E (2004) Ghrelin and des-acyl ghrelin both inhibit isoproterenol-induced lipolysis in rat adipocytes via a non-type 1a growth hormone secretagogue receptor. Eur J Pharmacol 498(1–3):27–35. https://doi.org/10.1016/j.ejphar.2004.07.066
Perez-Tilve D, Heppner K, Kirchner H, Lockie SH, Woods SC, Smiley DL, Tschop M, Pfluger P (2011) Ghrelin-induced adiposity is independent of orexigenic effects. FASEB J 25(8):2814–2822. https://doi.org/10.1096/fj.11-183632
Wortley KE, del Rincon JP, Murray JD, Garcia K, Iida K, Thorner MO, Sleeman MW (2005) Absence of ghrelin protects against early-onset obesity. J Clin Invest 115(12):3573–3578. https://doi.org/10.1172/JCI26003
Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, Elmquist JK (2005) Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest 115(12):3564–3572. https://doi.org/10.1172/JCI26002
Kim MS, Yoon CY, Jang PG, Park YJ, Shin CS, Park HS, Ryu JW, Pak YK, Park JY, Lee KU, Kim SY, Lee HK, Kim YB, Park KS (2004) The mitogenic and antiapoptotic actions of ghrelin in 3T3-L1 adipocytes. Mol Endocrinol 18(9):2291–2301. https://doi.org/10.1210/me.2003-0459
Choi K, Roh SG, Hong YH, Shrestha YB, Hishikawa D, Chen C, Kojima M, Kangawa K, Sasaki S (2003) The role of ghrelin and growth hormone secretagogues receptor on rat adipogenesis. Endocrinology 144(3):754–759. https://doi.org/10.1210/en.2002-220783
Andrews ZB, Erion DM, Beiler R, Choi CS, Shulman GI, Horvath TL (2010) Uncoupling protein-2 decreases the lipogenic actions of ghrelin. Endocrinology 151(5):2078–2086. https://doi.org/10.1210/en.2009-0850
Miegueu P, St Pierre D, Broglio F, Cianflone K (2011) Effect of desacyl ghrelin, obestatin and related peptides on triglyceride storage, metabolism and GHSR signaling in 3T3-L1 adipocytes. J Cell Biochem 112(2):704–714. https://doi.org/10.1002/jcb.22983
Zhang W, Chai B, Li JY, Wang H, Mulholland MW (2008) Effect of des-acyl ghrelin on adiposity and glucose metabolism. Endocrinology 149(9):4710–4716. https://doi.org/10.1210/en.2008-0263
Barazzoni R, Zanetti M, Gortan Cappellari G, Semolic A, Boschelle M, Codarin E, Pirulli A, Cattin L, Guarnieri G (2012) Fatty acids acutely enhance insulin-induced oxidative stress and cause insulin resistance by increasing mitochondrial reactive oxygen species (ROS) generation and nuclear factor-kappaB inhibitor (IkappaB)-nuclear factor-kappaB (NFkappaB) activation in rat muscle, in the absence of mitochondrial dysfunction. Diabetologia 55(3):773–782. https://doi.org/10.1007/s00125-011-2396-x
Zanetti M, Barazzoni R, Guarnieri G (2008) Inflammation and insulin resistance in uremia. J Ren Nutr 18(1):70–75. https://doi.org/10.1053/j.jrn.2007.10.015
Barazzoni R (2004) Skeletal muscle mitochondrial protein metabolism and function in ageing and type 2 diabetes. Curr Opin Clin Nutr Metab Care 7(1):97–102
Guarnieri G, Zanetti M, Vinci P, Cattin MR, Barazzoni R (2009) Insulin resistance in chronic uremia. J Ren Nutr 19(1):20–24. https://doi.org/10.1053/j.jrn.2008.11.014
Gortan Cappellari G, Zanetti M, Vinci P, Guarnieri G, Barazzoni R (2017) Unacylated ghrelin: a novel regulator of muscle intermediate metabolism with potential beneficial effects in chronic kidney disease. J Ren Nutr 27(6):474–477. https://doi.org/10.1053/j.jrn.2017.05.005
Wei Y, Sowers JR, Clark SE, Li W, Ferrario CM, Stump CS (2008) Angiotensin II-induced skeletal muscle insulin resistance mediated by NF-kappaB activation via NADPH oxidase. Am J Physiol Endocrinol Metab 294(2):E345–E351. https://doi.org/10.1152/ajpendo.00456.2007
Cai D, Frantz JD, Tawa NE Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE (2004) IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 119(2):285–298. https://doi.org/10.1016/j.cell.2004.09.027
Schenk S, Saberi M, Olefsky JM (2008) Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest 118(9):2992–3002. https://doi.org/10.1172/JCI34260
Morino K, Petersen KF, Shulman GI (2006) Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 55 Suppl 2:S9–S15. https://doi.org/10.2337/diabetes
Saleh MC, Wheeler MB, Chan CB (2002) Uncoupling protein-2: evidence for its function as a metabolic regulator. Diabetologia 45(2):174–187. https://doi.org/10.1007/s00125-001-0737-x
Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD (2002) Superoxide activates mitochondrial uncoupling proteins. Nature 415(6867):96–99. https://doi.org/10.1038/415096a
Barazzoni R, Zanetti M, Bosutti A, Biolo G, Vitali-Serdoz L, Stebel M, Guarnieri G (2005) Moderate caloric restriction, but not physiological hyperleptinemia per se, enhances mitochondrial oxidative capacity in rat liver and skeletal muscle–tissue-specific impact on tissue triglyceride content and AKT activation. Endocrinology 146(4):2098–2106. https://doi.org/10.1210/en.2004-1396
Barazzoni R, Zhu X, Deboer M, Datta R, Culler MD, Zanetti M, Guarnieri G, Marks DL (2010) Combined effects of ghrelin and higher food intake enhance skeletal muscle mitochondrial oxidative capacity and AKT phosphorylation in rats with chronic kidney disease. Kidney Int 77(1):23–28. https://doi.org/10.1038/ki.2009.411
Barazzoni R, Gortan Cappellari G, Palus S, Vinci P, Ruozi G, Zanetti M, Semolic A, Ebner N, von Heahling S, Sinagra G, Giacca M, Springer J (2017) Acylated ghrelin treatment normalizes skeletal muscle mitochondrial oxidative capacity and AKT phosphorylation in rat chronic heart failure. J Cachex Sarcopenia Muscle. https://doi.org/10.1002/jcsm.12254
Suematsu M, Katsuki A, Sumida Y, Gabazza EC, Murashima S, Matsumoto K, Kitagawa N, Akatsuka H, Hori Y, Nakatani K, Togashi K, Yano Y, Adachi Y (2005) Decreased circulating levels of active ghrelin are associated with increased oxidative stress in obese subjects. Eur J Endocrinol 153(3):403–407. https://doi.org/10.1530/eje.1.01977
Omrani H, Alipour MR, Mohaddes G (2015) Ghrelin improves antioxidant defense in blood and brain in normobaric hypoxia in adult male rats. Adv Pharm Bull 5(2):283–288. https://doi.org/10.15171/apb.2015.039
El Eter E, Al Tuwaijiri A, Hagar H, Arafa M (2007) In vivo and in vitro antioxidant activity of ghrelin: attenuation of gastric ischemic injury in the rat. J Gastroenterol Hepatol 22(11):1791–1799. https://doi.org/10.1111/j.1440-1746.2006.04696.x
Iseri SO, Sener G, Yuksel M, Contuk G, Cetinel S, Gedik N, Yegen BC (2005) Ghrelin against alendronate-induced gastric damage in rats. J Endocrinol 187(3):399–406. https://doi.org/10.1677/joe.1.06432
Chang L, Ren Y, Liu X, Li WG, Yang J, Geng B, Weintraub NL, Tang C (2004) Protective effects of ghrelin on ischemia/reperfusion injury in the isolated rat heart. J Cardiovasc Pharmacol 43(2):165–170
Kheradmand A, Alirezaei M, Birjandi M (2010) Ghrelin promotes antioxidant enzyme activity and reduces lipid peroxidation in the rat ovary. Regul Pept 162(1–3):84–89. https://doi.org/10.1016/j.regpep.2010.02.008
Zwirska-Korczala K, Adamczyk-Sowa M, Sowa P, Pilc K, Suchanek R, Pierzchala K, Namyslowski G, Misiolek M, Sodowski K, Kato I, Kuwahara A, Zabielski R (2007) Role of leptin, ghrelin, angiotensin II and orexins in 3T3 L1 preadipocyte cells proliferation and oxidative metabolism. J Physiol Pharmacol 58(Suppl 1):53–64
Neamati S, Alirezaei M, Kheradmand A (2011) Ghrelin acts as an antioxidant agent in the rat kidney. Int J Pept Res Ther 17(3):239–245
Barazzoni R, Zanetti M, Semolic A, Cattin MR, Pirulli A, Cattin L, Guarnieri G (2011) High-fat diet with acyl-ghrelin treatment leads to weight gain with low inflammation, high oxidative capacity and normal triglycerides in rat muscle. PLoS One 6(10):e26224. https://doi.org/10.1371/journal.pone.0026224
Ruozi G, Bortolotti F, Falcione A, Dal Ferro M, Ukovich L, Macedo A, Zentilin L, Filigheddu N, Gortan Cappellari G, Baldini G, Zweyer M, Barazzoni R, Graziani A, Zacchigna S, Giacca M (2015) AAV-mediated in vivo functional selection of tissue-protective factors against ischaemia. Nat Commun 6:7388. https://doi.org/10.1038/ncomms8388
Togliatto G, Trombetta A, Dentelli P, Cotogni P, Rosso A, Tschop MH, Granata R, Ghigo E, Brizzi MF (2013) Unacylated ghrelin promotes skeletal muscle regeneration following hindlimb ischemia via SOD-2-mediated miR-221/222 expression. J Am Heart Assoc 2(6):e000376. https://doi.org/10.1161/JAHA.113.000376
Togliatto G, Trombetta A, Dentelli P, Gallo S, Rosso A, Cotogni P, Granata R, Falcioni R, Delale T, Ghigo E, Brizzi MF (2015) Unacylated ghrelin induces oxidative stress resistance in a glucose intolerance and peripheral artery disease mouse model by restoring endothelial cell miR-126 expression. Diabetes 64(4):1370–1382. https://doi.org/10.2337/db14-0991
Gortan Cappellari G, Semolic A, Ruozi G, Vinci P, Guarnieri G, Bortolotti F, Barbetta D, Zanetti M, Giacca M, Barazzoni R (2017) Unacylated ghrelin normalizes skeletal muscle oxidative stress and prevents muscle catabolism by enhancing tissue mitophagy in experimental chronic kidney disease. FASEB J. https://doi.org/10.1096/fj.201700126R
Baatar D, Patel K, Taub DD (2011) The effects of ghrelin on inflammation and the immune system. Mol Cell Endocrinol 340(1):44–58. https://doi.org/10.1016/j.mce.2011.04.019
Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, Lillard JW Jr, Taub DD (2004) Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Investig 114(1):57–66. https://doi.org/10.1172/JCI21134
Yilmaz Z, Ilcol YO, Ulus IH (2008) Endotoxin increases plasma leptin and ghrelin levels in dogs. Crit Care Med 36(3):828–833. https://doi.org/10.1097/01.CCM.0B013E3181611F5AA
Vila G, Maier C, Riedl M, Nowotny P, Ludvik B, Luger A, Clodi M (2007) Bacterial endotoxin induces biphasic changes in plasma ghrelin in healthy humans. J Clin Endocrinol Metab 92(10):3930–3934. https://doi.org/10.1210/jc.2007-1194
Waseem T, Duxbury M, Ito H, Ashley SW, Robinson MK (2008) Exogenous ghrelin modulates release of pro-inflammatory and anti-inflammatory cytokines in LPS-stimulated macrophages through distinct signaling pathways. Surgery 143(3):334–342. https://doi.org/10.1016/j.surg.2007.09.039
Gonzalez-Rey E, Chorny A, Delgado M (2006) Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology 130(6):1707–1720. https://doi.org/10.1053/j.gastro.2006.01.041
Kodama T, Ashitani J, Matsumoto N, Kangawa K, Nakazato M (2008) Ghrelin treatment suppresses neutrophil-dominant inflammation in airways of patients with chronic respiratory infection. Pulm Pharmacol Ther 21(5):774–779. https://doi.org/10.1016/j.pupt.2008.05.001
Takata A, Takiguchi S, Miyazaki Y, Miyata H, Takahashi T, Kurokawa Y, Yamasaki M, Nakajima K, Mori M, Kangawa K, Doki Y (2015) Randomized phase II study of the anti-inflammatory effect of ghrelin during the postoperative period of esophagectomy. Ann Surg 262(2):230–236. https://doi.org/10.1097/SLA.0000000000000986
Valerio A, Cardile A, Cozzi V, Bracale R, Tedesco L, Pisconti A, Palomba L, Cantoni O, Clementi E, Moncada S, Carruba MO, Nisoli E (2006) TNF-alpha downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J Clin Investig 116(10):2791–2798. https://doi.org/10.1172/JCI28570
Han D, Huang W, Ma S, Chen J, Gao L, Liu T, Zhang R, Li X, Li C, Fan M, Chen Y, Cao F (2015) Ghrelin improves functional survival of engrafted adipose-derived mesenchymal stem cells in ischemic heart through PI3K/Akt signaling pathway. BioMed Res Int 2015:858349. https://doi.org/10.1155/2015/858349
Hao XK, Wu W, Wang CX, Xie GB, Li T, Wu HM, Huang LT, Zhou ML, Hang CH, Shi JX (2014) Ghrelin alleviates early brain injury after subarachnoid hemorrhage via the PI3K/Akt signaling pathway. Brain Res 1587:15–22. https://doi.org/10.1016/j.brainres.2014.08.069
Waseem T, Duxbury M, Ashley SW, Robinson MK (2014) Ghrelin promotes intestinal epithelial cell proliferation through PI3K/Akt pathway and EGFR trans-activation both converging to ERK 1/2 phosphorylation. Peptides 52:113–121. https://doi.org/10.1016/j.peptides.2013.11.021
Yang D, Liu Z, Zhang H, Luo Q (2013) Ghrelin protects human pulmonary artery endothelial cells against hypoxia-induced injury via PI3-kinase/Akt. Peptides 42:112–117. https://doi.org/10.1016/j.peptides.2013.01.012
Chen X, Chen Q, Wang L, Li G (2013) Ghrelin induces cell migration through GHSR1a-mediated PI3K/Akt/eNOS/NO signaling pathway in endothelial progenitor cells. Metab Clin Exp 62(5):743–752. https://doi.org/10.1016/j.metabol.2012.09.014
Vestergaard ET, Djurhuus CB, Gjedsted J, Nielsen S, Moller N, Holst JJ, Jorgensen JO, Schmitz O (2008) Acute effects of ghrelin administration on glucose and lipid metabolism. J Clin Endocrinol Metab 93(2):438–444. https://doi.org/10.1210/jc.2007-2018
Vestergaard ET, Buhl M, Gjedsted J, Madsen M, Jessen N, Nielsen S, Gaylinn BD, Liu J, Thorner MO, Moller N, Jorgensen JO (2011) Acute peripheral metabolic effects of intraarterial ghrelin infusion in healthy young men. J Clin Endocrinol Metab 96(2):468–477. https://doi.org/10.1210/jc.2010-1995
Lear PV, Iglesias MJ, Feijoo-Bandin S, Rodriguez-Penas D, Mosquera-Leal A, Garcia-Rua V, Gualillo O, Ghe C, Arnoletti E, Muccioli G, Dieguez C, Gonzalez-Juanatey JR, Lago F (2010) Des-acyl ghrelin has specific binding sites and different metabolic effects from ghrelin in cardiomyocytes. Endocrinology 151(7):3286–3298. https://doi.org/10.1210/en.2009-1205
Kim I, Rodriguez-Enriquez S, Lemasters JJ (2007) Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys 462(2):245–253. https://doi.org/10.1016/j.abb.2007.03.034
Klionsky DJ, Emr SD (2000) Autophagy as a regulated pathway of cellular degradation. Science 290(5497):1717–1721
Slupecka M, Wolinski J, Pierzynowski SG (2012) The effects of enteral ghrelin administration on the remodeling of the small intestinal mucosa in neonatal piglets. Regul Pept 174(1–3):38–45. https://doi.org/10.1016/j.regpep.2011.11.007
Tong XX, Wu D, Wang X, Chen HL, Chen JX, Wang XX, Wang XL, Gan L, Guo ZY, Shi GX, Zhang YZ, Jiang W (2012) Ghrelin protects against cobalt chloride-induced hypoxic injury in cardiac H9c2 cells by inhibiting oxidative stress and inducing autophagy. Peptides 38(2):217–227. https://doi.org/10.1016/j.peptides.2012.06.020
Tam BT, Pei XM, Yung BY, Yip SP, Chan LW, Wong CS, Siu PM (2015) Unacylated ghrelin restores insulin and autophagic signaling in skeletal muscle of diabetic mice. Pflug Arch 467(12):2555–2569. https://doi.org/10.1007/s00424-015-1721-5
Kuppens RJ, Delhanty PJ, Huisman TM, van der Lely AJ, Hokken-Koelega AC (2016) Acylated and unacylated ghrelin during OGTT in Prader–Willi syndrome: support for normal response to food intake. Clin Endocrinol. https://doi.org/10.1111/cen.13036
Choe YH, Song SY, Paik KH, Oh YJ, Chu SH, Yeo SH, Kwon EK, Kim EM, Rha MY, Jin DK (2005) Increased density of ghrelin-expressing cells in the gastric fundus and body in Prader–Willi syndrome. J Clin Endocrinol Metab 90(9):5441–5445. https://doi.org/10.1210/jc.2004-1935
Cummings DE, Clement K, Purnell JQ, Vaisse C, Foster KE, Frayo RS, Schwartz MW, Basdevant A, Weigle DS (2002) Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med 8(7):643–644. https://doi.org/10.1038/nm0702-643
Nicholls RD, Knepper JL (2001) Genome organization, function, and imprinting in Prader–Willi and Angelman syndromes. Annu Rev Genom Hum Genet 2:153–175. https://doi.org/10.1146/annurev.genom.2.1.153
Gagnon J, Zhu L, Anini Y, Wang Q (2015) Neutralizing circulating ghrelin by expressing a growth hormone secretagogue receptor-based protein protects against high-fat diet-induced obesity in mice. Gene Ther 22(9):750–757. https://doi.org/10.1038/gt.2015.38
Wiedmer P, Nogueiras R, Broglio F, D’Alessio D, Tschop MH (2007) Ghrelin, obesity and diabetes. Nat Clin Pract Endocrinol Metab 3(10):705–712. https://doi.org/10.1038/ncpendmet0625
Wortley KE, Anderson KD, Garcia K, Murray JD, Malinova L, Liu R, Moncrieffe M, Thabet K, Cox HJ, Yancopoulos GD, Wiegand SJ, Sleeman MW (2004) Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc Natl Acad Sci USA 101(21):8227–8232. https://doi.org/10.1073/pnas.0402763101
McFarlane MR, Brown MS, Goldstein JL, Zhao TJ (2014) Induced ablation of ghrelin cells in adult mice does not decrease food intake, body weight, or response to high-fat diet. Cell Metab 20(1):54–60. https://doi.org/10.1016/j.cmet.2014.04.007
Sbraccia P, Nisoli E, Barazzoni R (2018) Obesity: focus on ongoing multidisciplinary and comprehensive research. Eat Weight Disord 23(1):1. https://doi.org/10.1007/s40519-017-0462-1
Barazzoni R, Gortan Cappellari G, Ragni M, Nisoli E (2018) Insulin resistance in obesity: an overview of fundamental alterations. Eat Weight Disord 23(2):149–157. https://doi.org/10.1007/s40519-018-0481-6
Barazzoni R, Bischoff SC, Boirie Y, Busetto L, Cederholm T, Dicker D, Toplak H, Van Gossum A, Yumuk V, Vettor R (2018) Sarcopenic obesity: time to meet the challenge. Clin Nutr. https://doi.org/10.1016/j.clnu.2018.04.018
Barazzoni R, Gortan Cappellari G, Semolic A, Ius M, Zanetti M, Gabrielli A, Vinci P, Guarnieri G, Simon G (2018) Central adiposity markers, plasma lipid profile and cardiometabolic risk prediction in overweight-obese individuals. Clin Nutr. https://doi.org/10.1016/j.clnu.2018.04.014
Barazzoni R, Gortan Cappellari G, Semolic A, Ius M, Mamolo L, Dore F, Giacca M, Zanetti M, Vinci P, Guarnieri G (2015) Plasma total and unacylated ghrelin predict 5-year changes in insulin resistance. Clin Nutr. https://doi.org/10.1016/j.clnu.2015.10.002
Gual P, Le Marchand-Brustel Y, Tanti JF (2005) Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie 87(1):99–109. https://doi.org/10.1016/j.biochi.2004.10.019
Delhanty PJ, Huisman M, Baldeon-Rojas LY, van den Berge I, Grefhorst A, Abribat T, Leenen PJ, Themmen AP, van der Lely AJ (2013) Des-acyl ghrelin analogs prevent high-fat-diet-induced dysregulation of glucose homeostasis. FASEB J 27(4):1690–1700. https://doi.org/10.1096/fj.12-221143
Mao Y, Cheng J, Yu F, Li H, Guo C, Fan X (2015) Ghrelin attenuated lipotoxicity via autophagy induction and nuclear factor-kappab inhibition. Cell Physiol Biochem 37(2):563–576. https://doi.org/10.1159/000430377
Bischoff SC, Boirie Y, Cederholm T, Chourdakis M, Cuerda C, Delzenne NM, Deutz NE, Fouque D, Genton L, Gil C, Koletzko B, Leon-Sanz M, Shamir R, Singer J, Singer P, Stroebele-Benschop N, Thorell A, Weimann A, Barazzoni R (2017) Towards a multidisciplinary approach to understand and manage obesity and related diseases. Clin Nutr 36(4):917–938. https://doi.org/10.1016/j.clnu.2016.11.007
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
As this review does not provide original results, formal consent is not required.
Additional information
This article is part of the topical collection on Italian Society of Obesity’s Reviews.
Rights and permissions
About this article
Cite this article
Gortan Cappellari, G., Barazzoni, R. Ghrelin forms in the modulation of energy balance and metabolism. Eat Weight Disord 24, 997–1013 (2019). https://doi.org/10.1007/s40519-018-0599-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40519-018-0599-6