Abstract
Purpose of review
Carbapenem-producing organisms (CPO) have become important clinical pathogens in nosocomial infections causing increases in hospital stay, mortality, and costs. We sought to summarize recent findings related to the commonly encountered types of carbapenemases and their detection and review recent findings related to therapeutic options.
Recent findings
Many new rapid and reliable methods of identifying carbapenem resistance have become available, underscoring the necessity to have the laboratory capacity for phenotypic and/or genotypic detection of carbapenemases to facilitate optimal therapy according to the characteristics of the isolate. The existing literature summaries on the use of combination therapy vs. monotherapy for CPO infections are based on predominantly observational studies and although they suggest a more favorable outcome in overall mortality with combination therapy, but there is significant heterogeneity within the studies, supporting the need for more randomized trials. Several novel therapeutic agents which hold promise for CPOs are in research and development stages and include zidebactam and nacubactam (β-lactamase inhibitors) in combination with cefepime and cefiderocol. In addition, clinical trials are either complete or ongoing with ceftazidime-avibactam, meropenem-vaborbactam, imipenem-relebactam, aztreonam-avibactam, plazomicin, and eravacycline which offer promise for the future.
Summary
The management of CPO infections requires access to the relevant laboratory diagnostic tools to aid in the diagnosis and to be aware of the availability of both older combination therapies and novel therapeutic options which are currently undergoing clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The World Health Organization (WHO) has acknowledged the rapid global emergence and spread of highly resistant bacteria as one of the greatest single threats to medicine and global population health, jeopardizing many of the transformative health practices of the last century [1]. In recent years, the increased incidence of carbapenem-producing organisms (CPOs) has become a major concern, particularly in specific regions of the world [2, 3]. The WHO recently identified a list of global priority pathogens for which research and development are required and the top three critical organisms identified were carbapenem-resistant (CR) Acinetobacter baumannii, CR Pseudomonas aeruginosa, and CR Enterobacteriaceae (CRE) [4]. The focus of this review will be resistance due to carbapenemase production. The terms CPO, CRE (carbapenem-resistant Enterobacteriaceae), and CPE (carbapenemase-producing Enterobacteriaceae) appear frequently in the literature, but they should not be regarded as interchangeable terms. CRE refers to phenotypic resistance which may be due to any number of mechanisms, some of which may not be due to carbapenemase production. The terms CPO and CPE refer to the mechanism of phenotypic resistance related to the production of carbapenemase enzymes.
Carbapenemases are enzymes that hydrolyze carbapenems. The carbapenemases belong to a broad family of β-lactamase enzymes and may be classified on a functional or molecular basis [5,6,7,8,9]. Based on molecular structure, there are four major classes of β-lactamases with three of them acting on a serine-based (serine-β-lactamase) mechanism and one by a zinc-based (metallo-β-lactamase) mechanism [6,7,8,9]. In the presence of metal chelators, there is inhibition of the activity of metallo-β-lactamases but no inhibition of serine-β-lactamases [6,7,8,9]. According to Ambler’s classification, which is based upon the amino acid sequences of the β-lactamases, the four classes are designated as A, B, C, and D. The commonly encountered class A (serine-dependent enzymes) carbapenemases include the Klebsiella pneumoniae carbapenemase (KPC) family. The major class B (metallo-β-lactamases) carbapenemases include VIM (Verona integron-encoded), IMP (imipenemase), and NDM (New Delhi metallo-β-lactamase). The class D carbapenemases (also serine enzymes) include the OXA-type enzymes. An Ambler class C β-lactamase has been demonstrated to hydrolyze imipenem but is considered rare [10].
The metallo-β-lactamases have significant carbapenemase activity and exhibit resistance to β-lactamase inhibitors such as clavulanic acid, but are not active against monobactams such as aztreonam [6,7,8,9].
Knowledge of the existence of metallo-β-lactamase carbapenemases in certain bacteria dates back to the 1960s, so it is not a recent phenomenon [8]. The metallo-β-lactamase carbapenemases had been found in “resident” genes within the chromosomes of certain Bacillus species, Stenotrophomonas maltophilia, Aeromonas spp., certain environmental pseudomonads and flavobacteria [8]. Initially, these bacteria were not considered to be of major clinical significance, and hence, not much attention was directed to these observations. However, the finding of an IMP-type “non-resident” metallo-β-lactamase carbapenemase on a highly mobile plasmid within a strain of Pseudomonas aeruginosa in Japan in 1987 was significant and heralded a new challenge from a clinical perspective [11]. Multiple reports of additional strains of bacteria, including other Pseudomonas aeruginosa strains, Serratia marcescens, Achromobacter species, and Klebsiella pneumoniae, carrying this carbapenemase on mobile plasmids appeared in Japan, Europe, and Canada over the next few years [12,13,14,15].
Another major type of mobile metallo-β-lactamase carbapenemase was first described in Verona, Italy, in 1999 and accordingly was named a VIM-type carbapenemase [16]. Since its original description, this mobile VIM-type carbapenemase has been reported widely throughout Europe, South America, parts of Asia, and the USA [17].
A more recently recognized type of mobile metallo-β-lactamase carbapenemase is the New Delhi (NDM) type. The original description of NDM-1 was in 2009 involving a patient receiving treatment for a K. pneumoniae urinary tract infection in a Swedish hospital, but who had been hospitalized in New Delhi, India, previously [18]. A major paper from 2010 by Kumarasamy identified 37 isolates of members of the Enterobacteriaceae family carrying the NDM-1 gene in 29 patients in the UK [19]. Since then, multiple NDM variants have been described and they are endemic throughout the Indian subcontinent and since then outbreaks of CPOs with NDM carbapenemases have been reported in every continent [2, 3].
One of the most frequent globally encountered mobile carbapenemases is a serine-β-lactamase found in Klebsiella pneumoniae, hence the term KPC, which was originally described in 1996 in North Carolina [20]. This mechanism of resistance has been most commonly encountered in strains of Klebsiella pneumoniae but also has been encountered in other members of the Enterobacteriaceae family including Escherichia coli, Proteus spp., Enterobacter spp., Citrobacter spp., and Serratia spp. [2, 3, 21]. Outbreaks of carbapenemase-producing Klebsiella pneumoniae have been reported in the USA, and it is endemic in countries such as Greece, India, Pakistan, and Italy.
Class D OXA-type carbapenemases have been mainly described in Acinetobacter baumannii from Greece, Turkey, India, Spain, Belgium, Mexico, Brazil, Argentina, Colombia, several European countries, and some regions of Africa [2, 3]. According to the European Center for Disease Prevention and Control, in 2016, up to 66% and 33% of the K. pneumoniae isolates in Greece and Italy, respectively, were resistant to carbapenems, while the proportion of isolates of E. coli resistant to carbapenems was much lower [22].
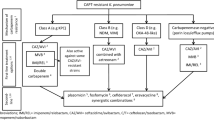
Diagnostic tools
The detection and identification of resistance to carbapenems can be complex for the following reasons: (1) a bacterial strain may have more than one mechanism of resistance, (2) the phenotypic tests have different sensitivity and specificity dependent on the type of carbapenemase, and (3) not all laboratories have the same resources for the identification and detection of resistance.
A microorganism may be a producer of a carbapenemase if it exhibits intermediate or resistant minimal inhibitory concentrations (MICs) to any carbapenem, considering the appropriate breakpoints, or if it has a positive phenotypic test [23•, 24]. There are several methods that are used to detect the presence of carbapenem resistance which were the subject of a recent review and hence only will be briefly reviewed [23•].
Automated methods
Most laboratories use automated identification systems and provide antibiotic susceptibility results according to the breakpoint for the MICs recommended by the Clinical and Laboratory Standard Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [24, 25]. These platforms require updating of their breakpoint points every year, and the most recent updates were in early 2019. There are differences between CLSI and EUCAST including which organisms constitute members of the Enterobacteriaceae and Enterobacterales families, respectively.
There are also discrepancies between breakpoints used for specific organism susceptibilities and how they are assessed, as the testing utilizes different carbapenems and carbapenem β-lactamase inhibitor combinations [24, 25]. In addition, the use of epidemiological cutoff (ECOFF) values as opposed to MIC values may display greater sensitivity than traditional breakpoints with carbapenemase-producing Enterobacteriaceae [26]. The Vitek-2®, MicroScan®, and Phoenix® systems were evaluated in a large 11,000-isolate study [27], and a high sensitivity for the detection of carbapenemase-producing E. coli and K. pneumoniae was found (KPC and MBL with the exception of IMP). The Phoenix® system had a sensitivity of ~ 90%, MicroScan® ~ 85%, and Vitek-2® ~ 74% using the CLSI 2014–2015 breakpoints. In general, the detection of the OXA-group carbapenemase-producing organisms was less accurate, although the performance seemed to improve for the Phoenix® system in more recent studies. However, the sensitivity to detect IMI-1 (class A) was just 43%, and this latter observation may be related to the high false negative rates with imipenem susceptibility found in some strains [28].
Phenotypic assays
Phenotypic tests are performed directly on isolates from pure cultures in the microbiology laboratory. Of the existing tests, CLSI currently recommends the use of Carba-NP and mCIM/eCIM (modified EDTA-modified carbapenem inactivation method, respectively) and no longer considers the modified Hodge test to be reliable [24, 29].
The Carba-NP is a colorimetric test that uses reagents on a liquid medium, including phenol red as a dye; the test is based on the change of color from red to yellow/orange due to the pH variation by the imipenem enzymatic hydrolysis in the presence of carbapenemases. It has a sensitivity of 96% in KPC- and MBL-type carbapenemase-producing organisms, and 60% in OXA-type carbapenemases. It has shown false positives with AmpC-producing organisms. The main advantage of this test is its ability to obtain rapid results in 2 h. There are some commercial tests available such as Rapidec® and Rapid Carba Screen®, which have a sensitivity equivalent to the original test [30]. For this test, a 10-mg meropenem disc impregnated with 10 mL of solution with the study strain is used and subsequently placed on a Muller-Hinton agar with a carbapenem-susceptible E. coli strain. After incubation for 18–24 h, the lack of inhibition halo reflex suggests the inactivation of meropenem by the carbapenemase-producing strain. The eCIM (addition of EDTA) is only performed after a positive mCIM; the inhibition halo increases ≥ 5 mm with respect to the mCIM which translates to the presence of MBL. The sensitivity of these methods is 93 to 97% with specificity close to 100% for class A and B carbapenemases; for class D, the sensitivity is less than 80% [24, 30].
Multiple protocols have studied the MALDI-TOF MS (matrix-assisted laser desorption/ionization time of flight mass spectrometry) platform. Some studies have found a sensitivity of 100%, for strains of carbapenem-producing Pseudomonas aeruginosa and Acinetobacter baumannii complex; however, there is still no standardization of this method [31].
There are several commercially available E-tests for detection of carbapenemases and inactivation methods with EDTA and phenylboronic acid (PBA) that have a sensitivity greater than 90%, even with class D carbapenemases.
Molecular assays
The molecular identification of the resistance genes is the best tool that a laboratory can utilize; however, equipment costs are high and are not always available. Most molecular identification techniques are based on polymerase chain reaction (PCR). Some laboratories have developed in-house PCR assays, especially for the detection of blaKPC genes. There are also other commercially available assays for the identification of the main genes that confer resistance to carbapenemases including the BioFire FilmArray® blood culture identification panel, the Nanosphere Verigene® GN panel, Check Direct® CPE and BD MAX® CRE assays, the Xpert® Carba-R, Check MDR®, and Acuitas® Resistome Test, and these have been reviewed recently [23•]. At this time, not all of these assays have Food and Drug Administration (FDA) approval [32].
Therapeutic options
Therapeutic options are significantly reduced in cases of infection due to microorganisms that exhibit resistance to carbapenems, and these infections have significant clinical implications. Nosocomial infections caused by carbapenem-resistant Enterobacteriaceae (CRE) have been reported to increase mortality. In a case-control study in Beijing, China, a 30-day overall mortality rate of 35.1% was found among patients with CRE infections vs. 11.8% among patients with carbapenem-susceptible strains (p = 0.008) and in-hospital mortality was 57.4% vs. 16.1%, respectively (p < 0.001) [33]. In a systematic review and meta-analysis exploring 12 studies, the reported mortality was significantly higher in patients with severe CRE infections compared with those with severe non-CRE infections (OR 3.39; 95% CI, 2.35–4.89) and similarly in monotherapy-treated compared with combination therapy–treated patients (OR, 2.19; 95% CI, 1.00–4.80). However, there was moderate-to-high heterogeneity in both analyses with I2 scores of 45.5% and 84.2%, respectively [34•]. In a recent publication by Tamma et al., among 83 episodes of bacteremia (92% associated with blaKPC Enterobacteriaceae), mortality in patients with CRE infection was 4 times higher than in patients with non-CRE infections (adjusted OR 4.92; 95% CI, 1.01–24.81) [35].
Therapeutic options for CPO infections are not optimal, and we herein review the use of existing therapies alone and in combination and discuss new therapeutic agents that have recently become available or are undergoing clinical trials.
Existing “older” monotherapy and combination antibiotic options for CPO infections
Carbapenem, polymyxin, fosfomycin, tigecycline monotherapy and combination therapy
Multiple antibiotic combinations have been studied in CPO infections with the aim of improving their therapeutic efficacy and reducing the selection of resistance in other microorganisms. Most of the data comes from retrospective studies, observational cohort studies, case series, and case reports. In some studies, in vitro activity additive or synergy of various combinations of antimicrobials against CPOs has been demonstrated. The most studied combination was colistin in combination with a carbapenem.
In a systematic review of 22 observational studies, 7 studies analyzed treatment with polymyxins as monotherapy or combined with a carbapenem and identified higher all-cause mortality in patients treated with monotherapy compared with combination therapy (OR 1.58, 95% CI 1.03–2.42) [36]. It is noteworthy that there were important differences in the studies cited which may limit the overall interpretation of the results. For example, in four studies of blood stream infections caused by KPC-Klebsiella pneumoniae, two of them had polymicrobial infections at one or more sites of infection including one with Acinetobacter baumannii. In addition, the sample sizes were very small limiting the ability to draw meaningful conclusions.
In the INCREMENT cohort study, all-cause mortality was analyzed at 30 days in 437 patients with blood stream infections caused by carbapenemase-producing Enterobacteriaceae (CPE), of whom 86% were infected with Klebsiella pneumoniae and KPC was the most frequent type of carbapenemase encountered. In 78% of patients, treatment was considered appropriate (within the first 5 days of diagnosis, and with at least one active drug), with a difference in mortality of 22.1% (95% CI, 11.0–33.3) compared with patients with inappropriate treatment. In the analysis between monotherapy and combined therapy, there were no differences in total mortality (HR 0.76; 95% CI, 0.53–1.08; p = 0.12), except in the patients classified as having a “high mortality score” in which there was a protective effect with the combination therapy (HR 0.60; 95% CI, 0.39–0.93; p = 0.02). Colistin was used in 54% of monotherapy patients, followed by meropenem or imipenem and tigecycline [37].
In the randomized study by Paul et al. of 406 patients with carbapenem-resistant Gram-negative bacterial infections, no difference in either clinical failure at 14 days or mortality at 28 days was found between patients receiving monotherapy with colistin vs. combination therapy with colistin plus meropenem (RR 0.93; 95% CI, 0.83–1.03; p = 0.172). However, 77% of the patients had infection with A. baumannii and 5% by P. aeruginosa, so the results cannot be interpreted as equivalent to patients with CRE infections [38].
In a recent review on this topic, the combination of colistin plus fosfomycin appears to have good in vitro activity when the strains are susceptible to both drugs and this combination has been observed to increase bactericidal activity based on time-kill studies when both agents are used together [39]. The same effect has been described with aminoglycosides combined with tigecycline, fosfomycin, and colistin even with high MICs vs. the aminoglycosides alone. In addition, the combination of colistin plus a carbapenem has exhibited synergy in vitro [39].
Tigecycline is another antibiotic which has been studied for its activity against CRE. High doses of tigecycline in combination with carbapenems, β-lactamase inhibitors, or aminoglycosides were evaluated in 40 patients in a study with CR Klebsiella pneumoniae bloodstream infections. There were longer survival times in patients with high doses of tigecycline (100 mg every 12 h) [40]. This effect has also been observed in vitro (even when CRE have MICs for meropenem ≥ 16 mg/L) with the combination of tigecycline plus colistin plus a carbapenem [41].
A double carbapenem strategy has been explored as a salvage therapy in patients with no other treatment options. Experience with ertapenem plus meropenem was beneficial in two centers in Greece, with a reported clinical and microbiological cure of > 70% among the patients with KPC infections. There are no studies comparing this regimen with other combination therapies [42].
In a retrospective study in a center in Italy, 17 of 21 (76%) patients with KPC-Klebsiella pneumoniae infections treated with double carbapenems achieved clinical cure despite a MIC for meropenem ≥ 256 mg/L and ertapenem ≥ 128 mg/L. There were no differences compared with the use of other combinations (colistin, tigecycline, aminoglycosides) in terms of clinical cure, relapse, and death [43].
Clinical outcomes with the use of high-dose carbapenems alone or in combination with other agents in CRE infections have been evaluated in some studies. Giannella et al., in a retrospective analysis, reported on the mortality in patients with CR-K. pneumoniae bloodstream infections receiving combination therapy with high-dose meropenem (6 g/day) plus another agent vs. other combination therapy without a carbapenem where the majority (72%) of the isolates had a MIC ≥ 16 mg/L, and found that the use of this high-dose regimen was associated with a lower 14-day mortality (hazard ratio 0.69, 95% CI 0.47–1.00) on multivariate analysis but the confidence interval did reach one and was relatively wide [44]. There were no major differences in the 14-day mortality regardless of the MIC value. The authors recommended using meropenem in combination therapy in this setting with an extended infusion time [44]. A recent article focusing on antibiotic therapy of CRE infections and mortality, which included several large cohort studies, concluded that outcomes were found to be linked to the MIC of meropenem, with mortality > 35% in those with MIC ≥ 16 mg/L, while the best outcomes were found in patients with a MIC ≤ 8 mg/L [45].
“Newer” monotherapy and combination antibiotic options for CPO infections
In recent years, the development of new molecules that are effective against multidrug-resistant (MDR) microorganisms has been limited, but there are some new agents that have become available recently that hold promise for CPO infections. These new alternatives for CPO infections include the combination of known β-lactams with newer generation β-lactamase inhibitors and other drugs belonging to these groups. Additional therapeutic agents that hold promise for CPOs are in various research and development stages and include zidebactam and nacubactam (β-lactamase inhibitors) in combination with cefepime, ceftazidime-avibactam, and the new drugs murepavadin and arylomycin. We will discuss the evidence regarding drugs approved by the FDA and will also discuss cefiderocol which is not yet approved [46].
Ceftazidime-avibactam
This combination was approved by the FDA and EMA (European Medicines Agency) for the treatment of intraabdominal infections (in combination with metronidazole), urinary tract infections, and nosocomial pneumonia in 2015. Avibactam, an inhibitor of β-lactamases derived from diazabicyclooctane (DBO), has been shown to reduce the MIC of ceftazidime and has in vitro activity against class A β-lactamases (ESBL, KPC) and class C β-lactamases and partially against some class D β-lactamases [47].
The RECAPTURE Study, a phase III randomized clinical trial (RCT), demonstrated non-inferiority of ceftazidime/avibactam vs. doripenem in the clinical resolution of symptoms in hospitalized patients with urinary tract infections including pyelonephritis. Superiority was demonstrated in the microbiological response (difference 6.4%; 95% CI, 0.33 to 12.36). More than 95% of isolates in RECAPTURE were Enterobacteriaceae [48].
The REPROVE trial, another phase III RCT, compared ceftazidime/avibactam vs. meropenem in the treatment of nosocomial pneumonia including ventilator-associated pneumonia, demonstrating non-inferiority in clinical cure and microbiological response. Of the isolates in this study, 38% corresponded to Enterobacteriaceae [49]. In both trials, safety with the use of ceftazidime/avibactam was similar to ceftazidime alone.
Meropenem-vaborbactam
TANGO-II represents a multicenter RCT which evaluated the efficacy and safety of meropenem plus vaborbactam (a β-lactamase inhibitor derived from boronic acid) which when combined has an activity against class A and class C β-lactamase-producing organisms vs. other active best available therapy against CRE infections. This clinical trial found that meropenem-vaborbactam demonstrated a significantly higher clinical cure (65.6% vs. 33.3%, difference 32.3%, 95% CI 3.3–61.3%, p = 0.03) and higher microbiological cure. Safety and tolerability outcomes demonstrated a lower percentage of adverse events, mainly nephrotoxicity, which had an impact on all-cause mortality at 28 days. More than 70% of the strains isolated were KPC containing Klebsiella pneumoniae [50].
Imipenem-relebactam
Relebactam (a diazabicyclooctanone derivative) has been combined with imipenem/cilastatin and is active against class A and C β-lactamases. The combination of the two agents in different dosing regimens was compared with imipenem/cilastatin alone in the treatment of urinary tract infections demonstrating non-inferiority in the microbiological response including imipenem non-susceptible strains [51]. Data about the efficacy of this combination for severe infections compared with colistin combinations will be available soon from a phase III RCT.
Aztreonam-avibactam
One of the advantages of this combination is their activity against MBL and KPC carbapenemase containing organisms, ESBL, and AmpC-containing organisms [52]. The efficacy of the combination has been evaluated in vitro and in vivo in some clinical cases. Aztreonam plus ceftazidime/avibactam was effective in treating pyelonephritis in one patient with a NDM carbapenemase containing E. coli strain. Currently, a phase III RCT is scheduled to end in 2021.
Cefiderocol
Cefiderocol is a new drug, a siderophore of cephalosporins with in vitro activity against class A, B, C, and D β-lactamases, with activity in a broad range of Gram-negative bacteria, both fermentative and non-fermentative. It binds to PBP3 using iron transporters from Gram-negative bacteria. It was demonstrated to be non-inferior to imipenem/cilastatin in the treatment of complicated urinary tract infections in patients with high risk of MDR pathogens, but only 11% were carbapenem resistant in the cefiderocol group [47, 53]. Two phase III RCTs in serious infections are currently ongoing.
Plazomicin
Plazomicin is a new aminoglycoside derivative with activity against Gram-negative ESBL-producing organisms and CRE but with reduced activity in NDM producers. Its efficacy and safety were compared with meropenem therapy for 4 to 7 days in patients with urinary tract infections or acute pyelonephritis infections (EPIC RCT), and were found to be non-inferior in clinical cure and microbiological eradication. It was reported that a slightly higher percentage of patients achieved microbiologic eradication for aminoglycoside-resistant and ESBL-producing Enterobacteriaceae [54].
Eravacycline
Structurally eravacycline belongs to the tetracycline family and has an in vitro activity similar to tigecycline, with an activity against Acinetobacter baumannii. Its efficacy and safety were evaluated in the treatment of complicated intraabdominal infections in two phase 3 studies: IGNITE-1 comparing eravacycline with ertapenem and in IGNITE-4 comparing eravacycline with meropenem. In both studies, eravacycline demonstrated non-inferiority against standard doses of carbapenems in clinical cure, and in both studies, most of the isolates were ESBL-producing Enterobacteriaceae [55, 56].
Inhaled antibiotic options for CPO infections
Aerosolized antibiotics are a well-established therapy for respiratory infections primarily in cystic fibrosis (CF) populations and have been reviewed elsewhere [57]. Inhalation of antibiotics enables the delivery of high concentrations of the drug to the site of infection and largely mitigates issues of penetration, resistance, and systemic toxicity. Thus, inhaled antibiotics represent a potentially promising and innovative option for the treatment of resistant Gram-negative respiratory infections. However, the optimal delivery methods, drug combinations, and lack of safety and efficacy data have been limitations to widespread use of this modality [58].
A number of formulations and combinations have been examined both in in vitro and in clinical studies with highly varied results ranging from incompatibility of admixtures, issues of stability, lack of activity or synergy, and conflicting in vitro and in vivo results. As an example, a randomized controlled trial of inhaled amikacin and fosfomycin was undertaken (IASIS—NCT01969799) in patients with Gram-negative ventilator-associated pneumonia and was unable to demonstrate a difference in infection score or mortality between groups [59]. Results were similarly disappointing in the INHALE studies where aerosolized amikacin was unable to demonstrate superiority in a large controlled trial (NCT0179993 and NCT00805168) [60].
The lack of data for inhaled antibiotics for Gram-negative infections outside of CF should give one pause, but these challenges can be overcome. Given the rapidly rising prevalence of highly resistant organisms, a number of initiatives and societies are collaborating and this will lead to development of new agents and further assessment of currently existing agents. Robust studies ranging from mechanistic to clinical trials are necessary to evaluate the delivery, safety, and efficacy of inhaled therapies in the treatment of these serious infections.
Conclusions
New therapies require rapid and reliable identification of carbapenem resistance underscoring the necessity to have resources for phenotypic and/or genotypic detection of carbapenemases, thereby directing the optimal therapy according to the characteristics of the isolate (species, MIC), the site of infection, and severity of illness. In many low-income countries, new drugs are not available. Treatment regimens must be selected based on the best available evidence, which currently suggests double or triple therapy over monotherapy and with antimicrobial agents having an in vitro activity against the infecting CPO. New antimicrobials are on the horizon, but we need robust evidence about their efficacy as monotherapy in the context of multidrug resistance and in the setting of severe infections.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Resolution WHA 68-7 (Global Action Plan on Antimicrobial Resistance) of the sixty-eighth World Health Assembly, Geneva, Switzerland, 2015. http://apps.who.int/gb/ebwha/pdf_files/WHA68/A68_R7-en.pdf?ua=1. Accessed 5 May 2019.
Van Duin D, Doi Y. The global epidemiology pf carbapenemases-producing Enterobacteriaceae. Virulence. 2017;8(4):460–9.
Bonomo RA, Burd EM, Conly J, Limbago BM, Poirel L, Segre JA, et al. Carbapenemase-producing organisms: a global scourge. Clin Infect Dis. 2018;66(8):1290–7. https://doi.org/10.1093/cid/cix893.
World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. Geneva: World Health Organization; 2017. https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf?ua=1 Accessed 23 May 2019.
Ambler RP. The structure of b-lactamases. Philos Trans R Soc Lond Ser B Biol Sci. 1980;289:321–31.
Bush K, Jacoby GA, Medeiros AA. A functional classification scheme for beta-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–3.
Bush K, Jacoby GA. Updated functional classification of b-lactamases. Antimicrob Agents Chemother. 2010;54:969–76.
Cornaglia G, Giamarellou H, Rossolini GM. Metallo-β-lactamases: a last frontier for β-lactams. Lancet Infect Dis. 2011;11(5):381–93.
Walsh TR, Toleman MA, Poirel L, Nordmann P. Metallo-beta-lactamases: the quiet before the storm? Clin Microbiol Rev. 2005;18(2):306–25.
Kim JY, Jung HI, An YJ, et al. Structural basis for the extended substrate spectrum of CMY-10, a plasmid-encoded class C beta-lactamase. Mol Microbiol. 2006;60:907–16.
Watanabe M, Iyobe S, Inoue M, Mitsuhashi S. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1991;35:147–51.
Arakawa Y, Murakami M, Suzuki K, Ito H, Wacharotayankun R, Ohsuka S, et al. A novel integron-like element carrying the metallo-ß-lactamase gene blaIMP. Antimicrob Agents Chemother. 1995;39:1612–5.
Arakawa KY, Nakashima K, Ito H, Ichiyama S, Shimokata K, Kato N, et al. Multifocal outbreaks of metallo-ß-lactamase-producing Pseudomonas aeruginosa resistant to broad- spectrum ß-lactams, including carbapenems. Antimicrob Agents Chemother. 1996;40:349–53.
Cornaglia G, Riccio ML, Mazzariol A, Lauretti L, Fontana R, Rossolini GM. Appearance of IMP-1 metallo-ß-lactamase in Europe. Lancet. 1999;353:899–900.
Gibb AP, Tribuddharat C, Moore RA, Louie TJ, Krulicki W, Livermore DM, et al. Nosocomial outbreak of carbapenem-resistant Pseudomonas aeruginosa with a new blaIMP allele, blaIMP-7. Antimicrob Agents Chemother. 2002;46:255–8.
Lauretti L, Riccio ML, Mazzariol A, Cornaglia G, Amicosante G, Fontana R, et al. Cloning and characterization of blaVIM, a new integron-borne metallo-ß-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob Agents Chemother. 1999;43:1584–90.
Li H, Toleman MA, Bennett PM, Jones RN, Walsh TR. Complete Sequence of p07–406, a 24,179-base-pair plasmid harboring the blaVIM-7 metallo-beta-lactamase gene in a Pseudomonas aeruginosa isolate from the United States. Antimicrob Agents Chemother. 2008;52(9):3099–105.
Yong D, Toleman MA, Giske CG, et al. Characterization of a new metallo- β-lactamase gene, Bla (NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009;53:5046–54.
Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10(9):597–602.
Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, Steward CD, et al. Novel carbapenem-hydrolyzing β-lactamase KPC-1 from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2001;45:1151–61.
Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009;9(4):228–36.
European Centre for Disease Prevention and Control. Data from the ECDC Surveillance Atlas - antimicrobial resistance. https://ecdc.europa.eu/en/antimicrobial-resistance/surveillance-and-disease-data/data-ecdc. Accessed 31 May 2019.
Miller S, Humphries RM. Clinical laboratory detection of carbapenem- resistant and carbapenemase-producing Enterobacteriaceae. Expert Rev Anti-Infect Ther. 2016;14(8):705–17. This review article provides a detailed description of the methods that are used to detect the presence of carbapenem resistance.
CLSI, editor. Performance Standards for Antimicrobial Susceptibility Testing 29th ed. CLSI supplement M100. Clinical and Laboratory Standards Institute: Wayne; 2019.
Nielsen LE, Clifford RJ, Kwak Y, Preston L, Argyros C, Rabinowitz R, et al. An 11,000-isolate same plate/same day comparison of the 3 most widely used platforms for analyzing multi-drug resistant clinical pathogens. Diagn Microbiol Infect Dis. 83(2):93–8. https://doi.org/10.1016/j.diagmicrobio.2015.05.018.
Ong CH, Ratnayake L, Ang MLT, Lin RTP, Chan DSG. Diagnostic accuracy of BD Phoenix CPO Detect for carbapenemase production in 190 Enterobacteriaceae isolates. J Clin Microbiol. 2018;56:e01043–18.
The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 9.0, 2019. http://www.eucast.org.
Vading M, Samuelsen Ø, Haldorsen B, Sundsfjord AS, Giske CG. Comparison of disk diffusion, Etest and VITEK2 for detection of carbapenemase-producing Klebsiella pneumoniae with the EUCAST and CLSI breakpoint systems. Clin Microbiol Infect. 2011;17(5):668–74.
Tamma PD, Simner PJ. Phenotypic detection of carbapenemase-producing organisms from clinical isolates. J Clin Microbiol. 2018;56(11):e01140–18.
Tamma PD, Opene BNA, Gluck A, Chambers KK, Carroll KC, Simner PJ. Comparison of 11 phenotypic assays for accurate detection of carbapenemase producing Enterobacteriaceae. J Clin Microbiol. 2017;55:1046–55.
Choquet M, Guiheneuf R, Castelain S, Cattoir V, Auzou M, Pluquet E, et al. Comparison of MALDI-ToF MS with the Rapidec Carba NP test for the detection of carbapenemase-producing Enterobacteriacae. Eur J Clin Microbiol Infect Dis. 2018;37:149–55.
Rood IGH, Li Q. Review: Molecular detection of extended spectrum-β-lactamase- and carbapenemase-producing Enterobacteriaceae in a clinical setting. Diagn Microbiol Infect Dis. 2017;89:245–50.
Wang Q, Zhang Y, Yao X, Xian H, Liu Y, Li H, et al. Risk factors and clinical outcomes for carbapenem-resistant Enterobacteriaceae nosocomial infections. Eur J Clin Microbiol Dis. 2016;35:1679–89.
Martin A, Fahrbach K, Zhao Q, Lodise T. Association between carbapenem resistance mortality among adult, hospitalized patients with serious infections due to Enterobacteriaceae: results of a systematic literature review and meta-analysis. Open Forum Infect Dis. 2018;5(7):ofy150. This article is a recent systematic review and meta-analysis exploring 12 studies and reports the mortality in patients with severe CRE infections compared with those with severe non-CRE infections.
Tamma PD, Goodman KE, Harris AD, Tekle T, Roberts A, Taiwo A, et al. Comparing the outcomes of patients with carbapenemase-producing and non-carbapenemase-producing carbapenem-resistant Enterobacteriaceae bacteremia. Clin Infect Dis. 2017;64(3):257–64.
Zusman O, Altunin S, Koppel F, Benattar YD, Gedik H, Paul M. Polymyxin monotherapy or in combination against carbapenem-resistant bacteria: systematic review and meta-analysis. J Antimicrob Chemother. 2017;72:29–39.
Gutiérrez BG, Salamanca E, De Cueto M, Hsueh PR, Viale P, Paño-Pardo JR, et al. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis. 2017;17:726–34.
Paul M, Daikos GL, Durante-Magoni E, Yahav D, Carmeli Y, Dishon Y. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis. 2018;18(4):391–400.
Carrara E, Bragantini D, Tacconelli E. Combination versus monotherapy for the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Curr Opin Infect Dis. 2018;31(6):594–9.
Geng TT, Xu X, Huang M. High-dose tigecycline for the treatment of nosocomial carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Medicine. 2018;8(e9961):97.
Tsala M, Vourli S, Georgiou PG, Pournaras S, Daikos GL, Mouton JW, et al. Triple combination of meropenem, colistin and tigecycline was bactericidal in a dynamic model despite mere additive interactions in chequerboard assays against carbapenemase-producing Klebsiella pneumoniae isolates. J Antimicrob Chemother. 2019;74(2):387–94.
Souli M, Karaiskos I, Masgala A, Galani L, Barmpouti E, Giamarellou H. Double-carbapenem combination as salvage therapy for untreatable infections by KPC-2-producing Klebsiella pneumoniae. Eur J Clin Microbiol Infect Dis. 2017;36:1305–15.
Cancelli F, Oliva A, De Angelis M, Mascellino MT, Mastroianni CM, Vullo V. Role of double-carbapenem regimen in the treatment of infections due to carbapenemase producing carbapenem-resistant Enterobacteriaceae: a single-center observational study. Biomed Res Int. 2018;2018:2785696.
Giannella M, Trecarichi EM, Giacobbe DR, De Rosa FG, Basetti M, Bartoloni A, et al. Effect of combination therapy containing a high-dose carbapenem on mortality in patients with carbapenem-resistant Klebsiella pneumoniae bloodstream infection. Int J Antimicrob Agents. 2018;51(2):244–8.
Trecarichi EM, Tumbarello M. Therapeutic options for carbapenem-resistant Enterobacteriaceae infections. Virulence. 2017;8(4):470–84.
Mo Y, Lorenzo M, Farghaly S, Kaur K, Housman ST. What’s new in the treatment of multidrug-resistant gram-negative infections? Diagn Microbiol Infect Dis. 2019;93:171–81.
Jean SS, Gould IM, Hsueh PR. New drugs for multidrug-resistant Gram-negative organisms: time for stewardship. Drugs. 2019;79(7):705–14.
Wagenlenher FM, Sobel JD, Newell P, Armstrong J, Huang X, Stone GG, et al. Ceftazidime-avibactam versus doripenem for the treatment of complicated urinary tract infections, including acute pyelonephritis: RECAPTURE, a phase 3 randomized trial program. Clin Infect Dis. 2016;63(6):754–62.
Torres A, Zhong N, Pchl J, Timsit JF, Kollef M, Chen Z, et al. Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): a randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect Dis. 2018;18:285–95.
Wunderink RG, Giamarellos-Bourboulis EJ, Rahav G, Mathers AJ, Basetti M, Vazquez J, et al. Effect and safety of meropenem–vaborbactam versus best-available therapy in patients with carbapenem-resistant Enterobacteriaceae infections: the TANGO II Randomized Clinical Trial. Infect Dis Ther. 2018;7:439–55.
Sims M, Mariyanovski V, McLeroth P, Akers W, Lee YC, Brown ML. Prospective, randomized, double-blind, Phase 2 dose-ranging study comparing efficacy and safety of imipenem/cilastatin plus relebactam with imipenem/cilastatin alone in patients with complicated urinary tract infections. J Antimicrob Chemother. 2017;72:2616–26.
Emeraud C, Escaut L, Boucly A, Fortineau N, Bonnin RA, Naas T, et al. Aztreonam plus clavulanate, tazobactam or avibactam for the treatment of metallo-β-lactamase-producing-Gram negative related infections. Antimicrob Agents Chemother. 2019;63(5):e00010–19.
Portsmouth S, van Veenhuyzen D, Echols R, Machida M, Arjona-Ferreira JC, Ariyasu M, et al. Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: a phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2018;18:1319–28.
Wagenlehner FME, Cloutier DJ, Komirenko AS, Cebrik DS, Krause KM, Keepers TR, et al. Once-daily plazomicin for complicated urinary tract infections. N Engl J Med. 2019;380(8):729–40.
Solomkin J, Evans D, Slepavicius A, Lee P, Marsh A, Tsai L, et al. Assessing the efficacy and safety of eravacycline vs ertapenem in complicated intra-abdominal infections in the investigating Gram negative infections treated with eravacycline (IGNITE 1) Trial. JAMA Surg. 2017;152(3):224–32.
Solomkin J, Gardovskis J, Lawrence K, Montravers P, Sway A, Evans D, et al. IGNITE4: results of a phase 3, randomized, multicenter, prospective trial of eravacycline vs meropenem in the treatment of complicated intraabdominal infections. Clin Infect Dis. 2018. https://doi.org/10.1093/cid/ciy1029.
Dalhoff A. Pharmacokinetics and pharmacodynamics of aerosolized antibacterial agents in chronically infected cystic fibrosis patients. Clin Microbiol Rev. 2014;27:753–82.
Wenzler E, Fraidenburg DR, Scardina T, Danziger LH. Inhaled antibiotics for Gram-negative respiratory infections. Clin Microbiol Rev. 2016;29(3):581–632.
Kollef MH, Ricard JD, Roux D, et al. A randomized trial of the amikacin Fosfomycin inhalation system for the adjunctive therapy of Gram-negative ventilator associated pneumonia: IASIS trial. Chest. 2017;151(6):1239–46.
Bayer Ltd. http://press.bayer.com/baynews/baynews.nsf/id/Phase-III-study-program-Amikacin-Inhale-addition-standard-intubated-mechanically-ventilated-patients?OpenDocument&sessionID=1512472033. Accessed 25 May 2019.
Funding
E. Rennert-May was funded by an Alberta Innovates-Health Solutions Clinician Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
D. Martinez-Oliva declares that he has no conflict of interest. E. Rennert-May declares that she has no conflict of interest. R. Somayaji declares that she has no conflict of interest. J. Conly declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Treatment and Prevention of Hospital Infections
Rights and permissions
About this article
Cite this article
Martinez-Oliva, D., Rennert-May, E., Somayaji, R. et al. Diagnosis and Treatment of Carbapenemase-Producing Organisms—an Update. Curr Treat Options Infect Dis 11, 317–329 (2019). https://doi.org/10.1007/s40506-019-00202-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40506-019-00202-8