Abstract
Foliar fertilization for biofortification is a targeted, economic and environment friendly approach rather than soil fertilization. In this study, we identified the appropriate growth stage(s) and right foliar iron (Fe) formulation in enhancing growth and Fe biofortification in soybean. In addition, we studied the physiological mechanism adopted by plants to endure foliar supplied Fe. For this purpose, a field experiment was conducted to evaluate various organic and inorganic Fe formulations such as Fe-citrate, FePO4, humic acid (HA) + Fe, HA alone and nano-Fe along with control (deionized water) in soybean (Glycine max var. DS-2614). Plants were sprayed at flowering (Set I), pod filling (Set II) and at both stages (Set III). Biomass and leaf area were significantly enhanced with application of Fe-citrate and FePO4 followed by HA + Fe. The yield traits (pod number, seed yield, test weight) significantly increased with HA + Fe and nano-Fe application. Enhanced Fe accumulation in seed was observed with HA + Fe followed by Fe-citrate and nano-Fe treatment. Foliar application of Fe at pod filling stage improved growth whereas yield and Fe fortification improved in Set III. This response may be attributed to enhanced activities of antioxidant scavenging enzymes and reduced lipid peroxidation in leaves treated with HA + Fe followed by FePO4. Results suggest foliar application of HA + Fe (organic Fe) and nano-Fe to be promising for soybean in improving growth and seed Fe content.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soybean (Glycine max (L.) Merr.) is a leading oilseed as well as pulse crop with high nutritional and economic value. It is a major source of edible vegetable oil and high-protein feed supplement for humans and livestock. Iron (Fe) deficiency is a major yield-limiting factor for soybean production particularly in alkaline soil. Although Fe is abundant in earth’s crust but the uptake by roots is limited due to its low solubility and chemical instability. Increasing yield and Fe concentration in edible parts of crop plants is a necessity to meet the demands of rising population and to improve Fe deficiency-induced anaemia in people. One of the approaches for fortifying Fe in crop plants is ‘Foliar feeding’ which is targeted, cost-effective and environment friendly and thus, a sustainable agronomic approach to increase grain Fe concentration. Efficient foliar fertilization takes into account not only the successful penetration of Fe through the leaf, but also its translocation to the edible part. Various inorganic and chelated forms of Fe fertilizers viz. ferrous sulphate (FeSO4), Fe-EDTA (ethylene diamine tetra acetic acid), Fe-DTPA (diethylene triamine penta acetic acid), Fe-EDDHA (ethylenediamine-N, N′-bis (2-hydroxyphenyl acetic acid) and Fe-citrate have been used for foliar application (Fernandez et al. 2009). The concentration and physico-chemical properties of sprayed ion as well as physiological factors of the plant including growth stage, leaf age and metabolic processes determine the efficacy of foliar fertilization (Fernandez and Ebert 2005).
The range of applied foliar Fe in different crops varies from 1.0 to 29.0 mM (Fernandez and Ebert 2005). Plants at anthesis or grain filling stages are more tolerant to higher concentration of foliar fertilizers compared to early growth stage (tillering) (Fageria et al. 2009). The most critical time to apply foliar fertilizer is when a plant is in transition phase, that is, from vegetative to reproductive phase. Efficiency of foliar application also depends upon the mobility of nutrients as relatively immobile nutrients show positive results only in the targeted tissue sprayed with nutrient (Fageria et al. 2009). Fe is relatively immobile in tomato (Lycopersicon esculentum), cucumber (Cucumis sativus) and navy bean (Phaseolus vulgaris) but shows high mobility in wheat (Triticum aestivum) and muskmelon (Cucumis melo) during reproductive stage (Guzman et al. 1990; Garnett and Graham 2005). The growth stage of crop and number of sprays may differentially influence the accumulation of Fe in seeds. Rice (Oryza sativa) showed improved growth with single Fe spray at anthesis (Zhang et al. 2009), wheat responded well after two sprays, that is, at tillering and stem elongation (Armin et al. 2014) while strawberry (Frageria vesca) performed best when foliar application was repeated at different growth stages (Erdal et al. 2004). With regard to Fe concentration, a lower dose (1%) of FeSO4 was found to be more effective in improving plant Fe content than the higher dose (2%) (Moosavi and Ronaghi 2011). However, a few studies reported no increase in yield and biomass with Fe application but it increased grain Fe concentration (Zhang et al. 2010).
In recent years, humic acid (HA) has gained importance as an effective organic substance for improving plant growth. HA influences growth by improving processes like nutrient uptake, maintenance of membrane stability, enhancement of photosynthesis by improved photosystem II activity and hormonal activity (Chen and Aviad 1990; Katkat et al. 2009; Pizzeghello et al. 2013). With the advancement of nano-technology, the use of nano fertilizers has also been proposed for improving plant nutrition (Dimkpa and Bindraban 2016). Due to large surface area of nano-materials, they can be efficiently absorbed by the plants. Nano-Fe fertilizers are considered as a potential foliar spray to enhance growth and yield of crops by improving photosynthesis and activating the antioxidant defense system of the plant (Wang et al. 2016; Shokri-Gharelo and Ghader 2017).
Fe is essential for many metabolic processes in plants while its deficiency as well as toxicity affects plant growth by influencing plant’s antioxidative system. Fe plays triple role during oxidative stress; first, it facilitates the decomposition of lipid peroxidation; second, it is involved in generation of reactive oxygen species (ROS) and third, it acts as a constituent of various antioxidative enzymes (Halliwell and Gutteridge 1986; Becana et al. 1998). The excess concentration of Fe generates oxidative stress by an increase in the steady state concentration of ROS within the plant cell (Halliwell and Gutteridge 1986). These ROS include superoxide anion radical (O −.2 ), hydrogen peroxide (H2O2), hydroxyl radical (\( {\text{OH}}^{ \cdot } \)) and singlet oxygen (1O2) which are produced during Fe toxicity (Choudhury et al. 2017). The increased oxidative stress due to Fe toxicity reduced photosynthesis and yield in soybean, canola (Brassica napus) and tobacco (Nicotiana tabacum) (Sinha et al. 1997). To overcome the damaging effect of these ROS, plant cells are equipped with enzymatic and non-enzymatic mechanisms. The detoxifying enzymes include superoxide dismutase (SOD), peroxidases like ascorbate (APOX) and guaiacol peroxidase (GPOX), glutathione reductase (GR) and catalase (CAT) while ascorbate is most important non-enzymatic substance that helps in detoxification of ROS. Excess Fe application to foliage at improper growth stage might cause Fe toxicity leading to oxidative stress in plants. Thus, the balance between free radical generation and free radical defense determines the survival of the system.
From literature survey, it was found that only a few studies have investigated whether foliar penetrated Fe could translocate to edible parts under Fe non-deficient conditions, and a very few have studied the physiological basis for such responses. Therefore, we hypothesised that foliar Fe application under non-deficient condition will improve translocation to grains. In the present study, we investigated the effect of various foliar Fe formulation in soybean at flowering (R2) and pod filling (R5) and at both R2 and R5 stages. The main objectives of the study were (1) to identify the best combination of growth stage and foliar formulation which might result in maximum translocation of Fe to seed along with improved yield under non-deficient Fe conditions, and (2) to study the plants’ response in terms of detoxification mechanism when supplied with external Fe.
Materials and methods
Plant materials and growth conditions
Soybean plants (var. DS 2614) were raised in soil under natural conditions at ICAR-Indian Agricultural Research Institute, New Delhi. Seeds were soaked overnight in deionized water and were inoculated with Bradyrhizobium japonicum before sowing. Recommended dose of 20 kg N, 60 kg P2O5 and 40 kg K2O ha−1 was added to soil as urea, single super phosphate and muriate of potash, respectively. Plants were irrigated with normal tap water as and when required. The soil properties were as follows: pH (soil:water::1:5) 8.44, electrical conductivity 0.29 mS m−1, soil texture sandy loam with clay 12.3%, silt 22.5% and sand 63.2%. The available P (Olsen 1954) in soil was measured to be 26.8 kg P2O5 ha−1 (high) and Fe was 4.8 mg kg−1 soil (medium).
Foliar Fe treatment
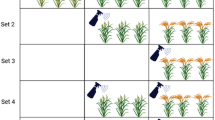
The experiment was divided into three sets having equal number of plots (net area 2.0 m2 per plot) and each Set differed in timing of foliar application. The Set I and Set II plants received single foliar spray at flowering (R2, plants beginning to full bloom and flowers at most nodes, 50 days after sowing) and pod filling (R5, beginning of seed setting, 75 days after sowing) stages, respectively while Set III plants were sprayed twice at R2 and R5. Fe compounds used for foliar application included Fe-citrate (Sigma F3388) (4.0 mM), Fe-phosphate (Sigma 436011) (2.0 mM), humic acid (Sigma 53680) (HA, 50 mg), humic acid (25 mg) with FeCl3 (Sigma 7705-08-0) (2.0 mM) (HA + Fe), nano-Fe (Sigma 544884) (4.0 mM) and deionized water as control. In a preliminary experiment, low (2.0 mM) and high (4.0 mM) concentration of each compound was used for foliar application and on the basis of physiological response and yield (data not presented), the above-specified concentrations were selected for this experiment. The spray formulations were prepared by dissolving required amount of chemical and adding 100 μL of surfactant (Triton × 100) in 1.0 L of solution. The pH of spray formulation was set at 6.0 using HCl or KOH. Care was taken to avoid any dripping of excess solution into the soil during spraying on plants by covering the soil surface with polythene which was removed the next day.
Physiological traits
In all three Sets, the observation on biomass, leaf area and chlorophyll were recorded on 6th day after foliar spray. Plants were harvested to measure total green leaf area using leaf area meter (Model LICOR-3000). Chlorophyll concentration was measured by non-maceration method (Hiscox and Israelstam 1979) and expressed as mg per g leaf fresh weight. Total shoot biomass was recorded by drying the samples in a hot-air oven at 65 °C until a constant weight was obtained and expressed as g per plant.
Tissue Fe concentration
The Fe concentration was estimated in leaf, stem and seed. The leaf and stem were thoroughly rinsed with distilled water to remove any adhered Fe applied as foliar spray. Estimation of Fe was done by wet digestion with diacid (HNO3:HClO4) mixture. The digested sample was used for Fe analysis by atomic absorption spectrometer (ECIL, India). Fe uptake or accumulation was calculated by multiplying the respective tissue Fe concentration with their dry weight and expressed as mg or µg per plant.
Oxidative stress markers
To estimate the oxidative stress, fully expanded young leaves were collected after 3rd day of foliar application from each Set. For estimation of O −.2 production, its capacity to reduce nitroblue tetrazolium (NBT) and forming blue colour formazones was measured (Chaitanya and Naithani 1994). Estimation of H2O2 was done by measuring the intensity of light yellow coloured titanium-hydro-peroxide complex with titanium reagent at 415 nm (Rao et al. 1997). Ascorbic acid estimation was carried out by measuring absorbance of pink coloured complex at 530 nm formed due to the reduction of dinitrophenyl hydrazine by ascorbic acid to phenyl hydrazone in acidic medium (Mukherjee and Choudhari 1983). Lipid peroxidation was estimated by measuring the concentration of thiobarbituric acid reactive substances (TBARS) and expressed as equivalents of malondialdehyde (MDA) (Heath and Packer 1968). MDA content was calculated according to extinction coefficient € = 155 mM−1 cm−1 by subtracting from the non-specific values (absorption at 600 nm) from the specific values (absorption at 532 nm).
Antioxidative enzyme assay
For enzyme assay, fully expanded leaves collected after 3rd day of foliar application from each Set was used. Extraction of antioxidant enzymes (SOD, CAT, APOX, GPOX and GR) was carried out with 0.1 M phosphate buffer, pH 7.5, containing 0.5 mM EDTA and 1 mM ascorbic acid was added in case of APOX. SOD activity was estimated by recording the decrease in absorbance of formazone at 560 nm produced by O −.2 and nitroblue tetrazolium dye (Dhindsa et al. 1981). For CAT activity, the reduction of H2O2 to water and molecular oxygen catalyzed by CAT was measured as a decrease in absorbance at 240 nm at an interval of 30 s for 1 min (Aebi 1984). The GPOX activity was measured as an increase in absorbance due to the oxidation of guaiacol to tetra-guaiacol (Castillo et al. 1984). GR was assayed by addition of NADPH and oxidized glutathione and measuring the decreased in absorbance at 340 nm (Anderson et al. 1990). APOX activity assay was based on the decrease in absorbance of ascorbic acid at 290 nm due to oxidation of ascorbic to mono-dehydroascorbic acid and dehydroascorbic acid (Nakano and Asada 1981).
Yield traits
At maturity (R8), the yield traits viz. number of pods per plant, seeds per pod, test weight (100-seed weight) and total seed yield per plant were recorded.
Statistical analysis
Experiments were laid out as randomized block design with two factors, growth stages and foliar treatments, and six replications of each treatment. Experiments were repeated twice over time and data were pooled for calculation of mean. Data were subjected to one-way analysis of variance (ANOVA). Differences between treatments were detected by Tukey’s test at P ≤ 0.05 level of significance using SAS. Graphs were plotted in Graph Pad Prism version 6.00 (GraphPad Software, La Jolla, CA) and MS Excel.
Results and discussion
Effect of foliar Fe application at different stages on growth and yield traits
Plants receiving foliar spray at flowering stage (Set I) showed significant (P < 0.05) increase in leaf area and chlorophyll concentration as compared to control (Fig. 1b, c), but no significant effect on shoot biomass was obtained (Fig. 1a). Maximum increase in leaf area was recorded with foliar application of Fe-citrate and FePO4 (2.1 fold). Foliar application of FePO4 also resulted in increased total chlorophyll concentration. The yield traits, pod weight and pod number per plant, total seed weight per plant and test weight, improved significantly due to foliar Fe application at flowering stage as compared to the control, except for nano-Fe (Fig. 3). Among treatments, the maximum increase in pod weight (95%), total seed weight (78%) and test weight (31%) were recorded with HA (Fig. 2b–d). However, maximum pod number obtained with Fe-citrate was statistically at par with HA (Fig. 2a). Except for test weight, all other yield traits exhibited significant reduction due to application of nano-Fe at flowering stage as compared to control suggesting that nano-Fe at 4.0 mM in soybean may be too high concentration for plants to detoxify Fe at cellular level. This result corroborates with Jalali et al. (2017) who applied 100 ppm of Fe-nano particles on maize and the seeds of second generation were again given the same treatment. The second progeny plants showed reduced biomass, lower contents of chlorophyll and protein as well as lower H2O2 scavenging capacity with higher amount of total Fe content.
Influence of foliar application of Fe compounds in soybean at flowering (Set I), pod filling (Set II) and both stages (Set III) on growth and Fe uptake. a Total shoot biomass, b total leaf area, c total chlorophyll concentration. Data correspond to mean ± SEm (n = 5). Data analysis was carried out using one-way ANOVA separately for each stage and calculated least significant difference. Mean with same letter are not significantly different at P ≤ 0.05
Influence of foliar application of Fe compounds in soybean at flowering (Set I), pod filling (Set II) and both stages (Set III) on growth and Fe uptake. a Leaf Fe content, b stem Fe content, c seed Fe concentration and d seed Fe content. Data correspond to mean ± SEm (n = 5). Data analysis was carried out using one-way ANOVA separately for each stage and calculated least significant difference. Mean with same letter are not significantly different at P ≤ 0.05
Foliar application of various Fe formulations at pod filling stage (Set II) in soybean had no significant effect on biomass (Fig. 1a). However, a significant increase in leaf area was observed with foliar treatments of FePO4 (82%), Fe-citrate (81%) and HA + Fe (52%) as compared to control (Fig. 1b). A small increase was noted in leaf chlorophyll concentration with all foliar treatments, except HA which showed a reduction over control (Fig. 1c). The yield traits were also significantly affected by foliar applied Fe formulations at pod filling stage (Fig. 3). Compared to control, higher pod number and pod weight per plant were obtained in FePO4 and HA + Fe sprayed plants. The total seed weight was negatively affected with application of Fe-citrate, HA and nano-Fe while the test weight increased with for these treatments, by 43% with FePO4, and remained unchanged with nano-Fe. Plants receiving foliar spray twice (at R2 and R5; Set III) showed significant effect of treatments on shoot biomass and leaf area (Fig. 1a, b). Maximum biomass and leaf area were recorded with FePO4 (63%, 82%), followed by HA + Fe (40% for shoot biomass) and Fe-citrate (81% for leaf area). Similarly, Fe formulation significantly affected leaf chlorophyll concentration (Fig. 1c). Spraying twice significantly improved yield traits (Fig. 2a–d). Application of HA + Fe recorded maximum pod number, pod weight and total seed weight per plant which was > twofold but the test weight increased only by 14%. However, the test weight increased with HA (47%) and nano-Fe (44%) as compared to control.
Influence of various Fe formulations applied as foliar spray at different stages on yield attributes in soybean grown in soil. a Pod number, b pod weight, c total seed weight, d 100 seed weight. Data correspond to means ± SEm (n = 5). Data analysis was carried out using one-way ANOVA separately for each stage and calculated least significant difference. Mean with same letter are not significantly different at P ≤ 0.05. F flowering, PF pod filling, F + PF flowering + pod filling
Comparing the effect of Fe formulation at various stages, it was observed that Fe could improve growth and yield in soybean. The increase in leaf area and chlorophyll concentration was highest in Set I while increases in shoot biomass and yield traits were highest in Set III. This suggests that foliar Fe-induced improvement in physiological traits by spraying only at one stage may not be sufficient to improve growth and yield. Seed yield rather than growth may be considered more important for selecting the timing of Fe application. As lowest yield traits were recorded in Set II, this may not be the appropriate stage for foliar Fe supplement. Foliar Fe spray at flowering stage might have enhanced the number of flowers, thus resulting in higher number of pods per plant. Repeating the spray at pod filling stage might have a positive effect on the seed development thus, increasing the test weight leading to increased seed weight per plant.
Effect of foliar Fe application at different stages on Fe partitioning
Fe content or uptake in leaf and stem was significantly (P < 0.05) increased by foliar application at flowering stage (Fig. 2a, b). Highest Fe content in leaf (2.2 fold) and stem (1.6 fold) was obtained with HA + Fe treatment as compared to control. Foliar application of various Fe compounds at flowering stage showed significant effect on Fe concentration and uptake in seeds (Fig. 2c, d). Seed Fe concentration was maximum with nano-Fe and Fe-citrate treatment. The total Fe content in seeds per plant was significantly higher with application of HA (94%) and Fe-citrate (67%) which might be due to increased seed yield obtained with these treatments. Thus, Set I treatment suggests that Fe was absorbed by leaves and was mobilized through stem to seeds. At pod filling stage, Fe treatments significantly enhanced leaf Fe content, maximum being observed with FePO4, HA and Fe-citrate treatments (Fig. 2a). However, increased Fe was accumulated in stem with HA + Fe (2.1 fold) and nano-Fe (2.4 fold) treatments. Moreover, the Fe application at pod filling stage also showed significant influence on Fe concentration in seed with 5–20% increase. The total Fe uptake in seed increased significantly by 42% and 40% with HA + Fe and FePO4, respectively in Set II. Thus, Set II showed comparable results with Set I though less apparent evidence for high impact on concentration and content of Fe in seed.
Foliar Fe applied at two growth stages significantly influenced Fe accumulation in different plant organs. Application of FePO4 increased Fe accumulation in leaf by 2.6-fold while in stem it was increased by 2.2-fold. The Set III resulted in significant increase in concentration and total Fe content in seed for all treatments except HA. Seed Fe concentration was maximum (36%) with Fe-citrate while HA + Fe treatment also resulted in 16% increase in comparison to control. Conversely, total Fe uptake in seed increased by > twofolds by Fe-citrate, nano-Fe and HA + Fe, with the latter showing maximal increase (2.8 fold). Thus, Set III suggests an additive effect of sprays in both stages but it was not apparent in all the treatments. This additive effect was most prominent in nano-Fe, less so with HA-Fe and FePO4 in that order, while Fe-citrate and HA revealed an aberrant trend.
In the present study, the Fe concentration in seed and total Fe accumulation was maximal when foliar application was done twice on the same plant (Set III). Similar increase in seed yield as well as seed Fe content was reported after foliar Fe application in common bean but the stage of application was not mentioned (Sida-Arreola et al. 2015). In another study on wheat, foliar application of Fe at three stages (early heading, 10 days after flowering and milky ripe stage) had positive effect on grain Fe concentration though the yield remained unaffected (Zhang et al. 2010). However, these studies partially confirm our findings as we observed that foliar application at both flowering and pod filling stages improved yield and Fe fortification in soybean seeds. Further, we found increased accumulation of Fe in leaf and stem in all treatments as compared to control which was maximum with HA + Fe application. Increased Fe accumulation in foliage have been reported in wheat (Katkat et al. 2009), perennial ryegrass (Maibodi et al. 2015), tomato (Adani et al. 1998) and gerbera (Gerbera jamesonii) (Nikbakht et al. 2008). The higher Fe accumulation in leaf with HA might be due to the fact HA improves membrane permeability leading to ion uptake (Katkat et al. 2009). Among treatments, Fe-citrate and nano-Fe resulted in increased Fe concentration in seeds. About 38% increase in wheat grain Fe concentration was reported with foliar application of 2.0 g L−1 nano-Fe oxide (Razmjoo and Ghafari 2015). This might be attributed to smaller size of nano-Fe particles that help in efficient absorption of Fe on plant surface (Shokri-Gharelo and Ghader 2017). However, the total Fe uptake in seed was maximum with HA + Fe treatment but not significantly different from nano-Fe, which was due to higher seed yield obtained in both. Interestingly, foliar application of HA resulted in better yield in Set I, but twice spray might have caused toxicity due to higher concentration at 50 mg as against 25 mg of HA along with Fe. This is also evident from increased MDA levels (Supplementary Fig. 3a) as discussed below resulting in membrane damage by higher concentration of HA sprayed twice in Set III. Response of plants to foliar application of HA was found to be dose as well as species dependent with lower dose having beneficial effects over higher doses on growth and yield traits (Adani et al. 1998; Nikbakht et al. 2008; Maibodi et al. 2015).
Effect of foliar Fe application at different stages on antioxidant scavenging system
Foliar application of various Fe compounds at flowering stage significantly (P < 0.05 and 0.01) affected the oxidative stress markers, including MDA, O −.2 and H2O2 (Supplementary Fig. 1a–d). Among Fe treatments, FePO4 resulted in marked reduction in MDA while nano-Fe showed highest MDA levels in comparison to control. The production of O −.2 decreased significantly with all foliar Fe treatment except nano-Fe. In contrast, H2O2 content increased in all Fe treatments except nano-Fe. The ascorbate content significantly increased with nano-Fe application as compared to control suggesting that the plants were exposed to oxidative stress. Ascorbate in reduced form is involved in non-enzymatic scavenging of ROS generated during stress. Synthesis and recycling of ascorbate is crucial via dehydroascorbate and mono-dehydroascorbate reductases for ROS scavenging (Conklin and Barth 2004). The results of detoxification system revealed that foliar application of Fe at flowering stage significantly affected the activities of enzyme involved in ROS scavenging in soybean leaves (Supplementary Fig. 1e–i). Activity of SOD and CAT increased significantly with Fe-citrate and HA while SOD was reduced with nano-Fe treatment. The CAT activity increased by more than 45% for Fe-citrate and HA sprayed plants. Activity of GPOX and APOX increased with all Fe treatments, except nano-Fe. The GPOX activity increased more than twofold with all foliar treatments and the highest was obtained with HA while APOX activity was maximum with FePO4 treatment. The GR activity also increased by 3.2 folds with HA while nano-Fe resulted in significant reduction as compared to control.
Foliar application of Fe at pod filling stage significantly affected the oxidative stress markers in soybean leaves (Supplementary Fig. 2a–d). In HA and nano-Fe treatments, MDA production was almost doubled while it was significantly lower in HA + Fe as compared to control. There was twofold reduction in accumulation of O −.2 in FePO4 treated plants while with nano-Fe treatment it was similar to control. However, the amount of H2O2 increased significantly with all foliar treatments with maximum noted in FePO4 and HA + Fe treated plants. Ascorbate content also increased with HA while FePO4 and Fe-citrate treated plants showed lower ascorbate values than control. Significant variation in the activities of antioxidative enzymes was obtained due to foliar treatments (Supplementary Fig. 2e, i). Highest SOD and CAT activities were observed with FePO4 as compared to control while HA + Fe also resulted in maximum CAT activity. The activity of GPOX increased significantly by 2.1–3.8 folds with all formulations being highest in HA + Fe while the APOX activity increased with HA + Fe and Fe-citrate in comparison to control. Plants sprayed with HA and nano-Fe failed to show any increase in CAT, SOD and APOX activities. In addition to this, foliar application of HA at pod filling stage reduced GR activity but other treatments showed a significant increase in GR activity.
Foliar-Fe fertilization at both flowering and pod filling stages significantly influenced the production of oxidative stress markers (Supplementary Fig. 3a–d). MDA content in leaves was reduced by all treatments except nano-Fe and HA in comparison to control. Similar to Set I and Set II, nano-Fe application resulted in increased MDA content. Production of O −.2 content in Fe-citrate and HA + Fe treated plants was significantly reduced while other foliar treatments had no significant difference. This was supported by 90% increased production of H2O2 in plants sprayed with HA + Fe indicating efficient scavenging of O −.2 . Also, plants sprayed with FePO4 and Fe-citrate showed increased production of H2O2. Significant difference was observed in the activity of enzymes involved in scavenging ROS due to repeated foliar application on soybean plants (Supplementary Fig. 3e–i). A significant increase in activity of SOD, CAT, GPOX, APOX and GR was recorded in foliar treatments FePO4, HA + Fe and Fe-citrate as compared to control. Application of HA + Fe resulted in increased activity of SOD, CAT, GPOX, APOX and GR as compared to control. Similarly, application of FePO4 and Fe-citrate also exhibited markedly higher activity of these enzymes. It was observed that high concentration (4 mM) of nano-Fe exposed the plants to oxidative stress.
MDA is considered as a marker for evaluating membrane damage and loss of membrane permeability under toxic or deficient conditions (Agarwal et al. 2010). In this study, MDA production was consistently and significantly reduced in all Sets sprayed with HA + Fe whereas nano-Fe showed maximal MDA concentration as compared to other treatments as well as control. There may be possibility that nano-particles could be physically damaging the lipid membranes (Chen and Bothun 2014). In Brassica napus exposed to drought stress, application of HA resulted in reduced production of MDA indicating less stress which was attributed to increased activity of APOX and POD, thereby maintaining higher chlorophyll a and total chlorophyll concentration (Lotfi et al. 2015). Likewise, Fe-citrate resulted in significantly lower values of MDA in Set I and Set III. This suggested that external Fe supplied via foliar route either did not induce lipid peroxidation or the detoxification system was successfully induced in plants to prevent the damage caused by ROS. Further, humic substances are known to increase cell membrane permeability and enhance metallic ion (Fe and Zn) uptake (Chen and Aviad 1990). Activities of the antioxidant scavenging enzymes, SOD, APOX, GPOX, GR and CAT, increased in leaves sprayed with HA + Fe, FePO4 and Fe-citrate. Plants were able to efficiently scavenge O −.2 by SOD to H2O2 and O2 which was evident from corresponding reduction in amounts of O −.2 and increased concentration of H2O2. In Set II and Set III, CAT was involved in catalysis of H2O2 while GPOX and APOX were active in all three Sets and were several folds higher than the control. This suggests that latter two enzymes were most efficiently involved in detoxification of ROS in case of HA + Fe, Fe-citrate and FePO4 treatments. Increase in GR activity was prominent in Set III with all three Fe treatments whereas in Set I and Set II, only HA + Fe resulted in maximum GR activity. Increased GR activity resulted in production of reduced ascorbate which in turn was oxidized after it reacts with ROS, thus resulting in reduced ascorbate levels as evident from corresponding decreased levels in all Sets with HA + Fe treatment (Supplementary Figs. 1d, 2d and 3d). Similar increase in activity of antioxidant enzymes with a corresponding decrease in lipid peroxidation was reported in B. napus with foliar application of 6.0 mg L−1 HA (Lotfi et al. 2015). Since free Fe2+ in cells is toxic and catalyze the decomposition of H2O2 to hydroxy radical (Becana et al. 1998), so the chelated form of Fe proved to be better than FeSO4 in inducing antioxidant enzymes (CAT, SOD and GPOX) with reduced level of H2O2 in common bean and wheat (Agarwal et al. 2010, Sida-Arreola et al. 2015). This supports our result with respect to Fe chelated with citrate and humic acid however, performance of FePO4 as foliar spray needs further attention. Inorganic FePO4 may be recommended for foliar application as the plants are benefitted with dual nutrient that are relatively unavailable in soil.
Application of nano-Fe increased yield and seed Fe fortification in Set III and the results were at par with HA + Fe. Many studies have linked this to the ability of nano-Fe to reduce ROS production by the activation of antioxidant system (Babaei et al. 2017; Shokri-Gharelo and Ghader 2017). However, in our study the ROS production and lipid peroxidation was noted to be quite high in case of nano-Fe. In a similar study, it was found that MDA levels were high in the first 2 weeks of exposure of watermelon (Citrullus lanatus) to nano-Fe. However, it decreased after 3 weeks of nano-Fe exposure which was accompanied by an increase in antioxidative enzymes, i.e., SOD, POD and CAT (Wang et al. 2016). Similarly, cucumber plants treated with nano-Cu exposed them to oxidative stress and generation of ROS (Zhao et al. 2016). Thus, our results indicate that the plants’ defense response may not have been fully activated by nano-Fe at the time of sampling and later activation of antioxidants might have improved the seed yield and Fe.
Conclusions
In conclusion, the experiment revealed that growth and yield along with Fe fortification in soybean seeds can be enhanced when foliar Fe is applied at both flowering and pod filling stage, most likely due to their additive effects. Application at flowering stage increases pod number while at pod filling, it increases test weight. In general, Fe applied in any form (organic or inorganic) increased Fe concentration in vegetative organs and even in seeds. The highest impact on yield and seed Fe concentration in soybean was found with HA (25 mg) + Fe (2 mM). Foliar application of nano-Fe was also found to be promising in increasing the seed Fe concentration, irrespective of the time of application. Moreover, the yield obtained with nano-Fe application was next to HA + Fe when sprayed at both flowering and pod filling stages. Thus, the present study explains the physiological basis of plants’ response to agronomic biofortification through foliar feeding of Fe.
References
Adani, F., Genevini, P., Zaccheo, P., & Zocchi, G. (1998). The effect of commercial humic acid on tomato plant growth and mineral nutrition. Journal of Plant Nutrition, 21(3), 561–575. https://doi.org/10.1080/01904169809365424.
Aebi, H. (1984). Catalase in vitro. Methods in Enzymology, 105, 121–126.
Agarwal, S., Sairam, R. K., Meena, R., Tyagi, A., & Srivastava, G. C. (2010). Effect of excess and deficient levels of iron and copper on oxidative stress and antioxidant enzymes activity in wheat. Journal of Plant Sciences, 5(1), 105–116.
Anderson, J. V., Hess, J. L., & Chevone, B. I. (1990). Purification, characterization, and immunological properties of two isoforms of glutathione reductase from eastern white pine needles. Plant Physiology, 94, 1402–1409. https://doi.org/10.1104/pp.94.3.1402.
Armin, M., Akbari, S., & Mashhadi, S. (2014). Effect of time and concentration of nano-Fe foliar application on yield and yield components of wheat. International Journal of Biosciences, 4, 69–75. https://doi.org/10.12692/ijb/4.9.69-75.
Babaei, K., Sharifi, R. S., Pirzad, A., & Khalilzadeh, R. (2017). Effects of bio fertilizer and nano Zn–Fe oxide on physiological traits, antioxidant enzymes activity and yield of wheat (Triticum aestivum L.) under salinity stress. Journal of Plant Interactions, 12, 381–389. https://doi.org/10.1080/17429145.2017.1371798.
Becana, M., Moran, J. F., & Iturbe-Ormaetxe, I. (1998). Iron-dependent oxygen free radical generation in plants subjected to environmental stress: Toxicity and antioxidant protection. Plant and Soil, 201, 137–147.
Castillo, F. I., Penel, I., & Greppin, H. (1984). Peroxidase release induced by ozone in Sedum album leaves. Plant Physiology, 74, 846–851. https://doi.org/10.1104/pp.74.4.846.
Chaitanya, K. K., & Naithani, S. C. (1994). Role of superoxide, lipid peroxidation and superoxide dismutase in membrane perturbation during loss of viability in seeds of Shorea robusta Gaertn. F. New Phytologist, 126, 623–627. https://doi.org/10.1111/j.1469-8137.1994.tb0295.
Chen, Y., & Aviad, T. (1990). Effects of humic substances on plant growth. Humic substances in soil and crop sciences: Selected readings, (humic substances). https://doi.org/10.2136/1990.humicsubstances.c7.
Chen, K. L., & Bothun, G. D. (2014). Nanoparticles meet cell membranes: probing nonspecific interactions using model membranes. Environmental Science and Technology, 48, 873–880. https://doi.org/10.1021/es403864v.
Choudhury, F. K., Rivero, R. M., Blumwald, E., & Mittler, R. (2017). Reactive oxygen species, abiotic stress and stress combination. The Plant Journal, 90, 856–867. https://doi.org/10.1111/tpj.13299.
Conklin, P. L., & Barth, C. (2004). Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant, Cell and Environment, 27, 959–970.
Dhindsa, R. A., Plumb-Dhindsa, P., & Thorpe, T. A. (1981). Leaf senescence: Correlated with increased permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. Journal of Experimental Botany, 126, 93–101. https://doi.org/10.1093/jxb/32.1.93.
Dimkpa, C., & Bindraban, P. S. (2016). Micronutrients fortification for efficient agronomic production. Agronomy for Sustainable Development, 36, 1–26.
Erdal, I., & Kepenek, K. (2004). Kizilgoz İ (2004) Effect of foliar iron applications at different growth stages on iron and some nutrient concentrations in strawberry cultivars. Turkish Journal of Agriculture and Forestry, 28(6), 421–427.
Fageria, N. K., Filho, M. B., Moreira, A., & Guimarães, C. M. (2009). Foliar fertilization of crop plants. Journal of Plant Nutrition, 32(6), 1044–1064. https://doi.org/10.1080/01904160902872826.
Fernandez, V., & Ebert, G. (2005). Foliar iron fertilization: A critical review. Journal of Plant Nutrition, 28, 2113–2124. https://doi.org/10.1080/01904160500320954.
Fernandez, V., Orera, I., Abadía, J., & Abadía, A. (2009). Foliar iron-fertilisation of fruit trees: Present knowledge and future perspectives—A review. The Journal of Horticultural Science & Biotechnology, 84, 1–6. https://doi.org/10.1080/14620316.2009.11512470.
Garnett, T. P., & Graham, R. D. (2005). Distribution and remobilization of iron and copper in wheat. Annals of Botany, 95, 817–826.
Guzman, M., Valenzuela, J. L., Sanchez, A., & Romero, L. (1990). A method for diagnosis the status of horticulture crops. II. Micronutrients. Phyton International Journal of Experimental Botany, 51, 43–56.
Halliwell, B., & Gutteridge, J. M. (1986). Iron and free radical reactions: two aspects of antioxidant protection. Trends in Biochemical Sciences, 11, 372–375.
Heath, R. L., & Packer, L. (1968). Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics, 125, 189–198. https://doi.org/10.1016/0003-9861(68)90654-1.
Hiscox, J. T., & Israelstam, G. F. (1979). A method for the extraction of chlorophyll from leaf tissue without maceration. Canadian Journal of Botany, 57, 1332–1334. https://doi.org/10.1139/b79-163.
Jalali, M., Ghanati, F., Modarres-Sanavi, A. M., & Khoshgoftarmanesh, A. H. (2017). Physiological effects of repeated foliar application of magnetite nanoparticles on maize plants. Journal of Agronomy and Crop Science, 203, 593–602. https://doi.org/10.1111/jac.12208.
Katkat, A. V., Çelik, H., Turan, M. A., & Asik, B. B. (2009). Effects of soil and foliar applications of humic substances on dry weight and mineral nutrients uptake of wheat under calcareous soil conditions. Australian Journal of Basic and Applied Sciences, 3, 1266–1273.
Lotfi, R., Gharavi-Kouchebagh, P., & Khoshvaghti, H. (2015). Biochemical and physiological responses of Brassica napus plants to humic acid under water stress. Russian Journal of Plant Physiology, 62, 480–486. https://doi.org/10.1134/S1021443715040123.
Maibodi, D. M., Kafi, N. M., Nikbakht, A., & Rejali, F. (2015). Effect of foliar applications of humic acid on growth, visual quality, nutrients content and root parameters of perennial ryegrass (Lolium perenne L.). Journal of Plant Nutrition, 38, 224–236. https://doi.org/10.1080/01904167.2014.939759.
Moosavi, A. A., & Ronaghi, A. (2011). Influence of foliar and soil applications of iron and manganese on soybean dry matter yield and iron–manganese relationship in a Calcareous soil. Australian Journal of Crop Science, 5, 1550–1556.
Mukherjee, S. P., & Choudhari, M. A. (1983). Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Plant Physiology, 58, 166–170. https://doi.org/10.1111/j.1399-3054.1983.tb04162.x.
Nakano, Y., & Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology, 22, 867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232.
Nikbakht, A., Kafi, M., Babalar, M., Xia, Y. P., Luo, A., & Etemadi, N. A. (2008). Effect of humic acid on plant growth, nutrient uptake, and postharvest life of gerbera. Journal of Plant Nutrition, 31, 2155–2167. https://doi.org/10.1080/01904160802462819.
Olsen, S. R. (1954). Estimation of available phosphorus in soils by extraction with sodium bicarbonate. Washington: United States Department of Agriculture.
Pizzeghello, D., Francioso, O., Ertani, A., Muscolo, A., & Nardi, S. (2013). Isopentenyladenosine and cytokinin-like activity of different humic substances. Journal of Geochemical Exploration, 129, 70–75. https://doi.org/10.1016/j.gexplo.2012.10.007.
Rao, M. V., Paliyath, G., Ormrod, D. P., Murr, D. P., & Watkins, C. B. (1997). Influence of salicylic acid on H2O2 production, oxidative stress and H2O2 metabolizing enzymes. Plant Physiology, 115, 137–149. https://doi.org/10.1104/pp.115.1.137.
Razmjoo, J., & Ghafari, H. (2015). Response of durum wheat to foliar application of varied sources and rates of iron fertilizers. Journal of Agricultural Science and Technology, 17, 321–331.
Shokri-Gharelo, Derakhti-Dizaji M., & Ghader, R. (2017). Effects of nano-iron spraying on the antioxidant activities of canola leaf under drought stress. Journal of Biodiversity and Environmental Sciences, 11, 304–311.
Sida-Arreola, J. P., Sánchez-Chávez, E., Ávila-Quezada, G. D., Zamudio-Flores, P. B., & Muñiz, C. A. (2015). Iron biofortification and its impact on antioxidant system, yield and biomass in common bean. Plant, Soil and Environment, 61, 573–576. https://doi.org/10.17221/643/2015-PSE.
Sinha, S., Gupta, M., & Chandra, P. (1997). Oxidative stress induced by iron in Hydrilla verticillata (l.f.) Royle: Response of antioxidants. Ecotoxicology and Environmental Safety, 38, 286–291. https://doi.org/10.1006/eesa.1997.1598.
Wang, Y., Hu, J., Dai, Z., Li, J., & Huang, J. (2016). In vitro assessment of physiological changes of watermelon (Citrullus lanatus) upon iron oxide nanoparticles exposure. Plant Physiology and Biochemistry, 108, 353–360. https://doi.org/10.1016/j.plaphy.2016.08.003.
Zhang, Y., Shi, R., Rezaul, K. M., Zhang, F., & Zou, C. (2010). Iron and zinc concentrations in grain and flour of winter wheat as affected by foliar application. Journal of Agriculture and Food Chemistry, 58, 12268–12274. https://doi.org/10.1021/jf103039k.
Zhang, J., Wang, M. Y., & Wu, L. H. (2009). Can foliar iron-containing solutions be a potential strategy to enrich iron concentration of rice grains (Oryza sativa L.)? Acta Agriculturae Scandinavica B, 59, 389–394. https://doi.org/10.1080/09064710802203545.
Zhao, L., Huang, Y., Hu, J., Zhou, H., Adeleye, A. S., & Keller, A. A. (2016). 1H NMR and GC-MS based metabolomics reveal defense and detoxification mechanism of cucumber plant under nano-Cu stress. Environmental Science and Technology, 50, 2000–2010. https://doi.org/10.1021/acs.est.5b05011.
Acknowledgements
Financial assistance from Virtual Fertilizer Research Center, USA [Grant no. 02838/14] to conduct this research is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharma, S., Malhotra, H., Borah, P. et al. Foliar application of organic and inorganic iron formulation induces differential detoxification response to improve growth and biofortification in soybean. Plant Physiol. Rep. 24, 119–128 (2019). https://doi.org/10.1007/s40502-018-0412-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-018-0412-6