Abstract
Purpose
The purpose of this systematic review is to assess the accuracy of contrast-enhanced ultrasound (CEUS) to computed tomography angiography (CTA) for the detection of endoleaks within EVAR surveillance program.
Material and methods
A systematic review in Pubmed, Embase and Cochrane database was performed. Articles assessing diagnostic accuracy and comparative modality (CTA vs. CEUS) for endoleaks in adult patients within surveillance programs were retrieved. Methodological assessment was performed, using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) tools. The sensitivity and specificity of data were extracted and statistical analysis was performed using MetaDiSc version 1.4.
Results
Eight articles were found eligible (n = 454 patients). The pooled sensitivity of CEUS at detecting endoleak is 0.914 (CI 0.866–0.949) and pooled specificity is 0.782 (CI 0.741–0.820).
Conclusion
The CEUS with its dynamic nature and longer scanning window demonstrated to be a highly sensitive modality for endoleak detection in comparison to CTA in delayed endoleaks type II.
Riassunto
Scopo
Scopo di questa revisione è stato valutare l'accuratezza dell'ecografia con mezzo di contrasto (CEUS) rispetto all'Agio Tomografia Computerizzata (CTA) per il rilevamento di endoleak, nell'ambito del programma di sorveglianza EVAR.
Materiali e Metodi
E' stata eseguita una revisione sistematica nei database Pubmed, Embase e Cochrane. Sono stati valutati gli articoli che prendevano in considerazione l'accuratezza diagnostica e il confronto (CTA Vs CEUS) per endoleak, in pazienti adulti nell'ambito di programmi di sorveglianza. La valutazione metodologica è stata effettuata utilizzando come strumento il Quality Assessment of Diagnostic Accuracy Studiesla (QUADAS). Sono state estratte sensibilità e specificità ed è stata effettuata l'analisi statistica utilizzando MetaDiSc version 1.4.
Risultati
Sono stati trovati otto articoli adatti allo studio (n = 454 pazienti). La sensibilità della CEUS nell'individuare endoleak è 0,914 (CI 0,866-0,949) e la specificità 0,782 (CI 0,741-0,820).
Conclusione
La CEUS, per la sua natura dinamica e la possibilità di scansione più lunga, ha dimostrato di essere una modalità altamente sensibile per la rilevazione di endoleak rispetto alla CTA negli endoleak di tipo II.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endovascular (EVAR) approach in AAA repair, described in 1991, is an accepted alternative to open surgery for selected group of patients [1]. If therapy for AAA is indicated, then treatment via open surgery or EVAR depends on variables and patient risk factors [2]. Despite significant reduction in peri-operative morbidity and mortality in EVAR patients [3–5], endoleak post-EVAR remains an important complication. The current data exhibit endoleak in 15–45 % of EVAR cohort [6] and their correct understanding demands special attention as different treatment strategies depend on their initial classification.
The Society of Interventional Radiology states that there are five goals of post-EVAR imaging, two of which involve aneurysm sac measurement and endoleak detection. The imaging modalities deployed to achieve these goals including plain radiography, computed tomography angiography (CTA), magnetic resonance angiography (MRA), conventional angiography and colour duplex ultrasound. The diagnostic accuracy of each imaging modality in detecting endoleaks varies but the ideal modality should be inexpensive, widely available, reproducible, accurate and with minimum radiation exposure [7].
Currently, CTA remains the main surveillance modality as it enables accurate evaluation of aneurysm morphological changes, sac diameter, graft anchorage and integrity [8]. Colour duplex ultrasound (US) is an adjunct modality commonly used with CTA for in post-EVAR surveillance. US is non-invasive, inexpensive, portable, and involves no exposure to radiation and nephrotoxic contrast. Doppler waveform analysis can also provide information on flow direction. However, rapid advances in imaging techniques combined with the development of new generation of contrast agents have improved the application of the ultrasound. Contrast-enhanced ultrasound (CEUS) is a relatively new, cost effective and minimally invasive modality in detection of endoleaks.
Therefore, the purpose of this systematic review and meta-analysis is to examine the accuracy of CEUS in comparison to CTA in detection of endoleaks in post-EVAR patients with emphasis on delayed type II.
Materials and methods
Literature search
An electronic search in Pubmed, Embase and Cochrane database for original articles from 1997 to January 2013 was performed. The language was restricted to English only. Key search terms were ultrasound, abdominal aortic aneurysm, surveillance, computed tomography, contrast agents, levovist, optison, sonovue, endoleak, sensitivity and specificity (Fig. 1).
Inclusion criteria
The inclusion criteria were: (1) retrospective and prospective cohort studies comparing CEUS and CTA for the detection of endoleaks (2) CTA defined as the reference standard or as a comparative modality (3) Concurrent CEUS and CTA within EVAR surveillance programs (4) Unselected patients after EVAR (5) Sufficient data for determination of both true- and false-positive results.
Exclusion criteria
The exclusion criteria included: (1) review articles, (2) individual studies with less than ten patients, (3) operators not being blinded to other diagnostic results, (4) non-concurrent scans within 1 month along with delay CEUS and CTA, (5) non-fully paired study design with subjects receiving only a subset of tests, (6) inadequate CTA or CEUS protocol for reproducibility, (7) selected patients based on previous test outcomes, and (8) non-consecutive enrolment of subjects.
Quality assessment
Quality assessment was carried out using QUADAS, a generic tool specifically designed for the use in diagnostic test accuracy reviews [9]. QUADAS was utilised to examine key attributes of each included study to minimising bias and assure methodological integrity. QUADAS contains 14 recommended quality items, 3 of which (items 2, 8, 9) are omitted by the Cochrane Collaboration as they are clarity items rather than items that assess methodological validity. Studies included for this review were all evaluated against all 14 QUADAS criteria. Heterogeneity and differences in clinical or methodological characteristics of studies were noted and considered when analysing study results for both excluded and included studies (Table 1).
Data analysis
Execution of meta-analysis depended on the number, methodological quality of the primary studies and the degree of their heterogeneity in estimation of the diagnostic accuracy. The result demonstrated the sensitivity and specificity of the studies to be fairly homogeneous. No implicit cutoff effect was applied in all studies and a fixed effect model was, therefore, utilised for statistical pooling. A random effect model was avoided as this weights smaller studies proportionally higher than a fixed effect model when estimating a summary effect. According to the Cochrane collaboration guideline, the individual studies were analysed and reported by paired forest plots of sensitivity and specificity, confidence intervals and summary receiver operator curve (sROC) (Fig. 2). The statistical analysis of the data was performed using Meta-DiSc version 1.4. Individual data (true positive, false positive, false negative, true negative) for each study were entered into the program. This allowed pooled sensitivity and specificity values to be computed along with diagrammatical forest plots and a plot for sROC. The 95 % confidence intervals were computed with outcomes.
Results
Search results
The electronic search yielded a total of 45 publications and abstracts. Six papers were excluded due to language. Furthermore, 12 papers were excluded due to the lack of design or abstract formatting. Twenty-seven papers were retrieved and after application of inclusion/exclusion criteria only eight papers were found eligible (Fig. 1). All included studies were evaluated against all 14 QUADAS (Table 1) criteria and their detailed characteristics are also tabulated (Table 2).
Discussion
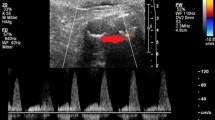
Contrast-enhanced ultrasound introduced for the first time in 1997 is a technique aimed to reduce radiation dose and obviate the need for nephrotoxic contrast reported in EVAR surveillance cohort [10]. The ultrasound contrast agents are characterised by inert gas micro-bubbles encapsulated within a lipid shell that under the acoustic pressure wave of an ultrasound transducer can increase signal strengths 100–1,000 times [11]. These micro-bubbles oscillate, resonate, or burst, giving continuous contrast enhancement on grey-scale images for approximately 3–4 min. Intravenously administered, ultrasound contrast agents are not nephrotoxic and have excellent safety profiles [12]. The reported, life-threatening anaphylactic reaction is limited to only 0.001 % [13].
The only limitation of CEUS is that it can only perform analysis on one defined area of the aneurysm with continuous imaging. If the site of endoleak is not known at the time of scanning, a second bolus of microbubble-based agent may be required to scan the aneurysm at a different level [14]. However, CEUS cannot accurately show surveillance goals, such as stent position and/or kinking.
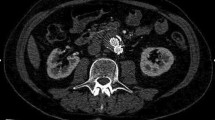
Endoleak, first described and classified by White et al. [15], is the persistent peri-graft blood flow outside the stent graft lumen but within the aneurysm sac. The aneurysm sac is, therefore, no longer excluded, increasing the risk of rupture. There is also a fifth type termed Endo tension [16] defined as a state of elevated pressure within the aneurysm sac. This essentially represents continuous sac expansion but with no visible sac perfusion.
Type I and Type III endoleaks are uncommon, imparting systemic or near systemic pressure within the excluded sac. These are significantly related to high risk of aneurysm rupture [17]. The general consensus is that type I and III endoleaks should be treated as soon as possible. Conversely, urgent treatment is not indicated with type II endoleaks, but there is frequent association with continued aneurysm enlargement [18]. There is continued debate about the significance and treatment of type II endoleaks, but there are no clinical guidelines on how to manage such leaks [19, 20]. Type IV endoleaks are now rare since the availability of new and improved endovascular stent grafts.
An analysis of AAA ruptures after EVAR by Schlosser et al. [21] demonstrated that most ruptures occur within the first 3 years with a mortality rate of 60 %. The majority of these ruptures were preceded by endoleaks but in only 35 % of patients, the endoleaks were detected during follow-up. Only three of the studies examined subjects to this timeframe (3 years). Two papers showed the most discordance between CTA and CEUS. In the McWilliams study [22], all discordant endoleaks were type II endoleaks. Similarly, out of the 40 endoleaks that showed discrepancy in Ten Bosch’s study [23], 38 were characterised as type II. This shows that the majority of additional endoleaks detected or missed by CEUS were type II endoleaks, the importance of which is still debated. As discussed earlier, type II endoleaks are classified as low-pressure leaks and in most cases, intervention is not indicated unless significant increase in sac diameter is demonstrable. In areas where CEUS did not reveal an endoleak, no aneurysm growth was observed which also raises the question of when, or, if intervention is necessary.
It is important that detected endoleaks are correctly classified. This important factor coupled with measured sac dimensions primarily determines patient management. The performance of CTA and CEUS in classification of endoleaks is not within the remit of this review. Although, the current data suggest that CEUS can be safely and accurately used as a surveillance tool in detection of high-pressure endoleaks (type I and type III), which ordinarily expedite immediate clinical intervention.
A meta-analysis deemed feasible given the methodological homogeneity of the studies (Table 3). However, the pooled sensitivity (Fig. 3) showed some degree of heterogeneity. Each study utilised either first generation (Levovist) or 2nd generation (SonoVue & Optison) US contrast agents. But the first generation agent is known to be associated with a blooming artefact during initial stages of enhancement [24, 25], which can give rise to false-positive outcomes. Six studies that used 2nd generation contrast agent showed excellent sensitivity. In addition, two studies that deployed both generation contrast agents also demonstrated 100 % sensitivity in detection of endoleaks type II. Therefore, omitting studies that utilise 1st generation contrast in sub–group analysis shows CEUS to be as accurate as CTA in detecting endoleaks.
Heterogeneity was noted in the pooled specificity of the results. The specificity values (Fig. 4) for each study deemed poor to excellent (55–95 %), with lower than expected values due to the high number of false positives. The authors of each study, however, question whether the false positives were in fact true endoleaks with CT making incorrect diagnoses. This difference in specificity can be attributed to improved CT protocol or by the use of Tissue Harmonic Imaging (THI). THI is a ultrasound contrast-specific software that reduces the blooming artefact effect of the contrast [25, 26] and is a common feature among the more recent studies. In addition, the method of contrast injection in CEUS (Bolus Vs Continuous) has also shown to alter the specificity. The continuous method allows longer examination time, which in turn permits detection of the slowest of endoleaks (continuous 81 Vs bolus 55 %) [27, 28]. Moreover, the lower than expected specificity of CEUS can also be attributed to the longer examination time available in CTA image analysis than CEUS. But the dynamic nature of CEUS permits detection of those slow endoleaks that the static nature of CTA might miss.
For dual or triple-phase CTA, the delay time for image acquisition varied significantly between studies (60 s–3 min). In the study by Napoli’s et al. [29], contrast enhancement was depicted in CEUS images 150 s after contrast injection. In CTA, the image acquisition in the venous phase was up to 80-s post-contrast injection, which resulted in inadequate and missing of the endoleaks (delay arterial phase). This was echoed in the study by Iezzi et al. where four false positive results were found 150 s after US contrast injection. These slow leaks can only be seen with adequate delay times in CTA protocols. It is likely that these leaks were in fact true leaks missed by CTA.
Currently, there is no general consensus on the CTA protocol as shown by the various CT protocols advocated in the included studies. The study by Iezzi et al. [30] showed that the combination of arterial phase and unenhanced imaging performed at 1-month follow-up offers improved specificity and positive predictive values compared with arterial phase alone. The delayed phase, however, did not significantly (P > 0.5) increase sensitivity for detection of the endoleaks, but does depict low-flow endoleaks not seen at the arterial phase. Macari et al. [31] even question the necessity of the arterial phase as in 110 tri-phasic post-EVAR scans (in 85 patients), in no more than 3.1 % of all examinations; there was 95 % confidence that arterial phase imaging would depict an endoleak missed at venous phase imaging.
The discordance between the 2 modalities (CEUS Vs CTA) could also be explained by the differences in contrast resolution. Minutes after contrast injection in CTA (the venous phase), the contrast density, within the aorta and an endoleak, is relatively low compared with the arterial phase. With CEUS, however, contrast resolution remains high even when the agent becomes diluted through time. With extended examination time and a consistent contrast resolution, CEUS is able to detect the slower leaks that CTA can easily miss. Discordance is stated to be highly influenced by differences in contrast resolution and course of its appearance [32].
One important source of heterogeneity is varying operator experience. Only Iezzi et al. explicitly gave the experience of their operators rather than stating ‘experienced’. The systematic review by whiting et al. showed that only 8 out of 55 publications that focused on bias or variation in diagnostic accuracy considered observer variation. In seven of the included studies, this source of heterogeneity affected estimates of accuracy.
Binary test outcomes are defined on the basis of a threshold for test positivity and change if the threshold is altered [33]. For endoleaks, test positivity threshold is subjective and not met by simply satisfying a set number. Variation in this threshold is, therefore, likely as there is no explicit numerical cut-off point, and a definition of a test positive outcome (presence of endoleak) is based on judgment rather than measurement. Operator and equipment variability also introduces heterogeneity in observed test results. The summarised sensitivity and specificity are, therefore, a reflection of the average observed accuracy and heterogeneity in positivity threshold. Consequently, results are obtained from the inherent operator dependency of the ultrasound. This inherent operator dependency is not considered a weakness in CTA imaging [34, 35].
Conclusion
The result of this systematic review and meta-analysis suggests that CEUS can be as sensitive as CTA in detecting the type of endoleaks that would normally warrant an immediate intervention (type I and III) and slow endoleaks (type II) that CTA might miss. However, the outcome does not provide sufficient evidence for the replacement of CTA with CEUS in surveillance programme. Furthermore, if CEUS is to replace CTA due to patient co-morbidities, it must be utilised in addition to other modalities or within an imaging algorithm.
References
Parodi JC, Palmaz JC, Barone HD (1991) Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg 5:491–499
Bush RL, Mureebe L, Bohannon WT, Rutherford RB (2008) The impact of recent european trials on abdominal aortic aneurysm repair: is a paradigm shift warranted? J Surg Res 48:264–271
Blankensteijn JD, de Jong SECA, Prinssen M, van der Ham AC, Buth J, van Sterkenburg SMM, Verhagen HJM, Buskens E, Grobbee DE, DREAM Trial Group (2005) Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. N Engl J Med 352:2398–2405
Greenhalgh RM, Brown LC, Kwong GPS, Powel JT, Thompson SG, EVAR trial participants (2004) Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet 364:843–848
Lederle FA, Freischlag JA, Kyriakides TC, Padberg FT Jr, Matsumura JS, Kohler TR, Lin PH, Jean-Claude JM, Cikrit DF, Swanson KM, Peduzzi PN, OVER Veterans Affairs Cooperative Study Group (2009) Outcomes following endovascular vs open repair of abdominal aortic aneurysm. A randomized trial. JAMA 302:535–1542
Stavropoulos SW, Charagundla SR (2007) Imaging techniques for detection and management of endoleaks after endovascular aortic aneurysm repair. Radiology 243:641–655
Tsoumakidou G, Brountzos E (2010) Detection of complications after aortic stent grafting. Eur Cardiol 6:83–87
Carrafiello G, Recaldini C, Lagana D, Piffaretti G, Fugazzola C (2008) Endoleak detection and classification after endovascular treatment of abdominal aortic aneurysm: value of CEUS over CTA. Abdom Imaging 33:357–362
Whiting P, Rutjes AWS, Reitsma JB, Glas AS, Bossuyt PM, Kleijnen J (2004) Sources of variation and bias in studies of diagnostic accuracy: a systematic review. Ann Intern Med 140:189–202
Heilberger P, Schunn C, Ritter W, Weber S, Raithel D (1997) Postoperative color flow duplex scanning in aortic endografting. J Endovasc Surg 4:262–271
Bendick PJ, Bove PG, Long GW, Zelenock GB, Brown OW, Shanley CJ (2003) Efficacy of ultrasound scan contrast agents in the noninvasive follow-up of aortic stent grafts. J Vasc Surg 37:381–385
EFSUMB Study Group (2008) Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS)—update 2008. Ultraschall Med 29:28–44
Piscaglia F, Bolondi L (2006) The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol 33:180–186
Quaia E (2005) Detection of endoleak after endovascular abdominal aortic aneurysm repair. Med Radiol Contrast Media Ultrason Part 2:11–115
White GH, Yu W, May J, Chaufour X, Stephen MS (1997) Endoleak as a complication of endoluminal grafting of abdominal aortic aneurysms: classification, incidence, diagnosis, and management. J Endovasc Surg 4:152–168
Gilling-Smith G, Brennan J, Harris P, Bakran A, Gould D, McWilliams R (1999) Endotension after endovascular aneurysm repair: definition, classification, and strategies for surveillance and intervention. J Endovasc Surg 6:305–307
van Marrewijk C, Buth J, Harris PL, Norgren L, Nevelsteen A, Wyatt MG (2002) Significance of endoleaks after endovascular repair of abdominal aortic aneurysms: the Eurostar experience. J Vasc Surg 35:461–473
Buth J, Harris PL, van Marrewijk C, Fransen G (2003) The significance and management of different types of endoleaks. Semin Vasc Surg 16(2):95–102
Veith FJ, Baum RA, Ohki T, Amor T, Adiseshiah M, Blankensteijn JD, Buth J, Chuter TAM, Fairman RM, Gilling-Smith G, Harris PL, Hodgson KJ, Hopkinson BR, Ivancev K, Katzen BT, Lawrence-Brown M, Meier GH, Malina M, Makaroun MS, Parodi JC, Richter GM, Rubin GD, Stelter WJ, White GH, White RA, Wisselink W, Zarins CK (2002) Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg 35:1029–1035
Sternbergh WC III, Greenberg RK, Chuter TAM, Tonnessen BH (2008) Redefining postoperative surveillance after endovascular aneurysm repair: recommendations based on 5 year follow up in the US Zenith multicentre trial. J Vasc Surg 48:278–285
Schlosser FJV, Gusberg RJ, Dardik A, Lin PH, Verhagen HJM, Moll FL, Muhs BE (2009) Aneurysm rupture after EVAR: can the ultimate failure be predicted? Eur J Vasc Endovasc Surg 37:15–22
McWilliams RG, Martin J, White D, Gould DA, Rowlands PC, Haycox A, Brennan J, Gilling-Smith GL, Harris PL (2002) Detection of endoleak with enhanced ultrasound imaging: comparison with biphasic computed tomography. J Endovasc Ther 9:170–179
Ten Bosch JA, Rouwet EV, Peters CTH, Jansen L, Verhagen HJM, Prins MH, Teijink JAW (2010) Contrast-enhanced ultrasound versus computed tomographic angiography for surveillance of endovascular abdominal aortic aneurysm repair. J Vasc Interv Radiol 21:638–643
McWilliams RG, Martin J, White D, Gould DA, Harris PL, Fear SC, Brennan J, Gilling-Smith GL, Bakran A, Rowlands PC (1999) Use of contrast-enhanced ultrasound in follow-up after endovascular aortic aneurysm repair. J Vasc Interv Radiol 10:1107–1114
Giannoni MF, Fanelli F, Citone M, Acconcia MC, Speziale F, Gossetti B (2007) Contrast ultrasound imaging: the best method to detect type II endoleak during endovascular aneurysm repair follow-up. Interact CardioVasc Thorac Surg 6:359–362
Sun Z (2006) Diagnostic value of color duplex ultrasonography in the follow-up of endovascular repair of abdominal aortic aneurysm. J Vasc Interv Radiol 17(5):759–764
Henao EA, Hodge MD, Felkai DD, McCollum CH, Noon GP, Lin PH, Lumsden AB, Bush RL (2006) Contrast-enhanced Duplex surveillance after endovascular abdominal aortic aneurysm repair: improved efficacy using a continuous infusion technique. J Vasc Surg 43:259–264
Iezzi R, Basilico R, Giancristofaro D, Pascali D, Cotroneo AR, Storto ML (2009) Contrast-enhanced ultrasound versus color duplex ultrasound imaging in the follow-up of patients after endovascular abdominal aortic aneurysm repair. J Vasc Surg 49:552–560
Napoli V, Bargellini I, Sardella SG, Petruzzi P, Cioni R, Vignalli C, Ferrari M, Bartolozzi C (2004) Abdominal aortic aneurysm: contrast-enhanced US for missed endoleaks after endoluminal repair. Radiology 233:217–225
Iezzi R, Cotroneo AR, Filippone A, Di Fabio F, Quinto F, Colosimo C, Bonomo L (2006) Multidetector CT in abdominal aortic aneurysm treated with endovascular repair: are unenhanced and delayed phase enhanced images effective for endoleak detection? Radiology 241:915–921
Macari M, Chandarana H, Schmidt B, Lee J, Lamparello P, Babb J (2006) Abdominal aortic aneurysm: can the arterial phase at CT evaluation after endovascular repair be eliminated to reduce radiation dose? Radiology 241:908–914
Dill-Macky MJ, Wilson SR, Sternbach Y, Kachura J, Lindsay T (2007) Detecting endoleaks in aortic endografts using contrast-enhanced sonography. AJR 188:W262–W268
Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y (2010) Analysing and presenting results [Internet], Version 1.0. In: Deeks JJ, Bossuyt PM, Gatsonis C (eds) Cochrane handbook for systematic reviews of diagnostic test accuracy, chap 10. http://srdta.cochrane.org/handbook-dta-reviews
Clevert DA, Minaifar N, Weckbach S, Kopp R, Meimarakis G, Clevert DA, Reiser M (2008) Color duplex ultrasound and contrast-enhanced ultrasound in comparison to MS-CT in the detection of endoleak following endovascular aneurysm repair. Clin Hemorheol Microcirc 39:121–132
Mauro R, Maioli F, Freyrie A, Testi G, Palumbo N, Serra C, Stella A (2010) Is CEUS a valid alternative to CTA in endoleak’s detection? Ital J Vasc Endovasc Surg 17(4):253–258
Conflict of interest
The authors (J. Chung, A. Kordzadeh, I. Prionidis, Y. Panayiotopoulos, T. Browne) have no conflict of interest.
Human and Animal Studies
The study described in this article does not contain studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chung, J., Kordzadeh, A., Prionidis, I. et al. Contrast-enhanced ultrasound (CEUS) versus computed tomography angiography (CTA) in detection of endoleaks in post-EVAR patients. Are delayed type II endoleaks being missed? A systematic review and meta-analysis. J Ultrasound 18, 91–99 (2015). https://doi.org/10.1007/s40477-014-0154-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40477-014-0154-x