Abstract
Purpose
To evaluate diagnostic accuracy and to perform an activity-based cost analysis of contrast-enhanced ultrasonography (CEUS) compared to computed tomography (CT) during annual surveillance after abdominal aortic aneurysm repair with endovascular procedure (EVAR).
Materials and methods
This retrospective study included 137 patients in post-EVAR follow-up over a 6-year period (average post-operatory follow-up without aneurysm sac volumetric reduction). Sensitivity, specificity, positive predictive values, negative predictive values and accuracy were considered for CEUS using CT angiography (CTA) as reference standard. An activity-based cost analysis was performed to evaluate potential savings due to the introduction of CEUS as an alternative to CT, after the first year of postoperative negative controls.
Results
CEUS reported accuracy, sensitivity, specificity, positive predictive values, negative predictive values of 97.4, 96, 100, 100 and 93.1% in the detection and characterization of endoleaks. CEUS cost was € 84.7, and CTA cost was € 157.77, with a differential cost of € 73.07; using CEUS as an alternative to CT allowed a potential saving of 50.052,95 € during follow-up.
Conclusions
CEUS is an accurate and cheap imaging method in post-EVAR follow-up patients, and it could be considered as a valid alternative to CTA, after the first year of negative controls, reducing the number of unnecessary CT examinations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endovascular treatment of abdominal aortic aneurysms (endovascular aneurysm repair, EVAR) has been introduced since the 1990s; compared to laparotomic surgery, it has several advantages, including less perioperative morbidity and mortality. However, endovascular procedure is more expensive than traditional surgery, with higher frequency of re-interventions to treat complications, such as endoleak, endograft occlusion, migration and post-implantation rupture [1].
Endoleak is defined as incomplete exclusion of aneurysm sac due to persistent blood flow outside a graft and within the sac itself. It represents the most frequent complication following EVAR, with an incidence of 10–35%. Early diagnosis is crucial for preventing the risk of aneurysm sac enlargement and rupture [2].
According to EUROSTAR guidelines (European Collaborators on Stent/Graft Techniques for Aortic Aneurysm Repair), computed tomography angiography (CTA) is an established “gold standard” and noninvasive method to evaluate these patients during follow-up. During the first year, follow-up observations should be performed at 3, 6 and 12 months after procedure, with successive annual checks for an average period of 6 years [3].
Several studies have already shown that alternative imaging techniques (MR, eco-color-Doppler) and contrast-enhanced ultrasonography (CEUS) permit increased recognition and characterization of endoleak [4,5,6,7,8,9,10,11,12,13,14,15].
After 1 year of negative CT exams, use of CEUS as an alternative investigation method could lead to substantial cost savings, related to its lower costs. Also, CEUS could avoid radiation exposure and the administration of iodine contrast material in patients with impaired renal function.
The aim of this study is therefore to evaluate diagnostic accuracy and the theoretical economic benefits provided by CEUS in comparison with CTA in patients treated with EVAR, after the first year of negative controls.
Materials and methods
Patients, inclusion and exclusion criteria
This single-center retrospective study received IRB approval, and informed consent was obtained from all the patients enrolled. The study population included 157 patients treated with EVAR in clinical–radiological follow-up over a period of 6 years between 2011 and 2016.
Exclusion criteria consisted of the presence of insufficient image quality or the lack of any imaging study.
Twenty patients were excluded because of the lack of one of the imaging methods. Therefore, we included 137 patients treated with EVAR (122 males and 15 females, mean age 70.3 years, range 54–90 years), 69 with Zenith endograft, Cook Medical and 68 with Excluder, Gore Medical: all patients underwent endoprosthesis evaluation with CEUS and CTA, both performed within 1 week (average 4.3 days, range 2–7 days). At the time of the study, average time after positioning vascular endoprosthesis was 4 years (range 1–7 years). None of the patients had a reduction in the maximum diameter of the aneurysm sac. None of the patients had allergic diathesis after iodinated contrast injection.
Twenty-three of 137 patients had a history of ischemic heart disease (with only one case of previous myocardial infarction): this was not an exclusion criterion, because it occurred 6 months before CEUS examination. In 9 out of 137 patients was performed an angiographic study with femoral artery approach, based on previous ultrasound or CT findings, in order to characterize and possibly treat endoleaks.
Imaging technique
In CEUS, all ultrasound examinations were performed by the same operator on a Sequoia 512 6.0 ultrasound system (Acuson, Mountain View CA, USA), with harmonic microbubble-specific imaging, convex probes and low acoustic ultrasound pressure (2–4 MHz cadence contrast pulse sequencing; 0.2 mechanical index; 12–13 frames a second). Longitudinal and axial scans were obtained, oriented in relation to aorta and iliac arteries major axis. Each investigation started with standard B-mode evaluation. The color-Doppler technique was adopted according to the radiologist choice. The exam was completed after intravenous administration of 2.4 ml bolus of second-generation contrast agent (SonoVue®, Bracco, Milan, Italy).
Aorta insonation was continuous with dynamic observation from the unenhanced phase to the contrast-enhanced phase. All CEUS examinations were recorded on DVD to review the dynamic study.
CT angiography
Abdominal aorta CT study was performed with a 64-row scanner (Brilliance CT-64; Philips, Eindhoven, The Netherlands) before and after intravenous administration of 1.5 ml/kg of water-soluble iodine contrast agent (Ultravist 370, Bayer Schering Pharma, Germany) at a flow rate of 4.5 ml/s by automatic power injectors, followed by a bolus 50 ml of saline solution. Patients underwent a cranio-caudal scan from the diaphragm to the symphysis pubis, with triphasic protocols including unenhanced acquisitions, arterial contrast-enhanced acquisitions (timed with a bolus-tracking technique, performed with a 4-s delay from 150-HU threshold of enhancement) and venous contrast-enhanced acquisitions (60 s later). Scan parameters of unenhanced and venous contrast-enhanced phases included: 2 mm slice thickness; 1 mm pitch; 120 kV; and 250 mAs. For arterial contrast-enhanced phases, parameters were: 0.8 mm slice thickness; 0.4 mm pitch; 120 kV; and 310 mAs.
Image analysis
Both CTA and CEUS images were double-blinded analyzed by two radiologists; in case of doubt (n = 7), discrepancies were subsequently resolved by consensus. Endoleak was defined as a persistent contrast flow into the aneurysm sac during contrast-enhanced phase [2].
CEUS
During basal ultrasound study, aneurysm sac diameter was evaluated to document any dilatation and stent position, and then, most adequate scan for contrast-enhanced phase was chosen. During contrast-enhanced phase, the presence of endoleaks was evaluated, which showed persistent flow of contrast microbubbles outside the endoprosthesis lumen. CEUS examination lasted 4 min after contrast administration, in order to detect also late type endoleaks (in particular type II) [13].
CTA
CT images were analyzed on a dedicated workstation in order to generate multi-planar two-dimensional reconstructions (MPR), MIP (maximum intensity projections) and 3D reconstructions (Volume Rendering). During the study, we evaluated correct stent position and aneurysm sac diameter. Endoleaks were diagnosed in the case of persistent contrast flow inside the excluded sac.
Statistical analysis
Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy of CEUS were calculated as compared to CTA, representing the reference standard. Student’s t test and McNemar’s test were used for result comparison. p value < 0.05 was considered as statistically significant.
Economic analysis
Potential savings were evaluated by relating the differential cost of CTA and CEUS, according to a 6-year simulation model, applied to our 137 patients in post-procedural follow-up. After the first year with negative CTA, starting from the second year, cost savings due to the introduction of CEUS as an alternative to CTA in the annual routine examinations were assessed. For the entire surveillance period, we assumed that no patient should be re-operated.
For economic analysis, differential costs of CEUS and CTA were considered (defined as the sum of equipment, materials and staff costs) according to the modalities of examinations in our institute based on the activity-based costing (ABC) method and similarly as other papers in the literature [17,18,19]. Five components of differential costs including 20% VAT were considered:
-
1.
Equipment cost (purchase and maintenance) with 8 years of amortization.
-
2.
Variable costs, depending on materials and devices (contrast media, saline solution, needles and DVDs).
-
3.
Medical staff cost estimated at € 1.02 per minute, depending on the time required for each examination (medical history, informed consent, examination and report), and obtained from the SIRM document determining activity volumes of radiologists [16]. Time for CEUS execution was 25 and 27 min for CTA.
-
4.
Technician costs, equal to € 0.37 per minute.
-
5.
Nursing staff costs, equal to € 0.37 per minute.
Results
No adverse events related to administration of ultrasound and iodinated contrast agents were recorded. In one case, CEUS examination was partially limited by a median abdominal wall laparocele. The mean BMI of patients enrolled was 22.2 (range 18.8–37.9, with 4.3 SD).
No stenoses or occlusions of endoprosthesis were observed during both CEUS and CTA. A non-relevant difference between CTA and CEUS has been demonstrated for an average diameter of excluded aneurysm sacs (at CT, 50 mm, range 30–79 mm; at CEUS, 48 mm, range 30–74 mm; p > 0.05) and an average diameter of aneurysm sacs with endoleak (at CTA, 53 mm, range 42–81 mm; at CEUS, 50 mm, range 40–78 mm, p > 0.05).
A total of 102 cases of endoleak were diagnosed using CTA, 3 of type I and 99 of type II; 99 cases were diagnosed using CEUS, all of type II.
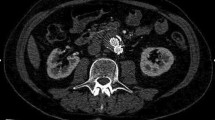
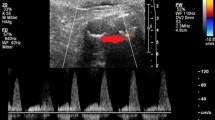
CEUS showed, compared to CTA, 97.1% sensitivity, 100% specificity, 100% PPV, 92.1% NPV and 98.0% accuracy to detect endoleak. In 99 cases, there was a concordance between CEUS and CTA on the presence of endoleak (Figs. 1, 2), whereas the absence of endoleak was confirmed by CEUS in 35 cases (true negatives).
At CEUS, in the axial (a) and sagittal scans (b), an endoleak of type II (arrow) is depicted posteriorly to the right branch of the endoprosthesis. In the same site (arrow), a leak of iodinated contrast material is demonstrated by means of CT angiography in the MIP images on the axial (c) and coronal planes (d)
The leak of contrast material (arrow) is better depicted at CEUS in the axial scans acquired during the arterial phase (a, b) if compared to the CT angiography, pointing out a tiny endoleak (arrow) located near the posterior aspect of the excluded aneurysm lumen, demonstrated with axial (c, d) and sagittal MIP reconstructions (e) (arrows)
Economic analysis: savings of CEUS vs CTA
According to equipment, materials and staff costs, a CEUS exam has an average cost of € 84.7, compared to € 157.77 of a single CT exam (p < 0.001). According to the model we proposed and considering the differential cost (CT–CEUS) for a single exam of € 73.07 (Table 1), introducing CEUS as an alternative to CT allowed a total saving of € 50.052,95 (73.07 × 137 × 5) within the 6 years of follow-up planned for the 137 patients enrolled, after the first year.
Significant differences between CT and CEUS regarded amortization and maintenance costs (p < 0.001), equipment costs for single exam (p < 0.0001), examination average cost (p < 0.001) and technician costs (required for CT, 9.99 € per exam and not required for CEUS; p < 0.001). No significant differences for materials cost, mainly consisting of contrast media used (p > 0.05) and staff cost (p > 0.05).
Discussion
Since endovascular prostheses were introduced in clinical practice, they are increasingly considered as an alternative to traditional surgical techniques in abdominal aortic aneurysms treatment. Although results are excellent in the immediate postoperative period, the medium- and long-term duration and their effectiveness in preventing aneurysm rupture are still under discussion. After the first year, main causes of failure of EVAR procedure are endoleaks, a reperfusion of the excluded aneurysm sac, and endotension, defined as high pressure within the aneurysm sac [1, 3].
Although mechanisms underlying these complications are unclear and there is no agreement on the therapeutic approach, radiologists play a crucial role in endoleak diagnosis and treatment. While CTA represents the gold standard in endoleaks identification and characterization, many studies emphasize the potential role of CEUS [5,6,7,8,9,10,11,12,13,14,15]. Our results confirm the accuracy in post-EVAR follow-up; the failure to identify three endoleaks was correlated with intrinsic limitations, such as patient’s constitution or intestinal interposition. It must be underlined that the average BMI of patients enrolled was not far from reference values, allowing the radiologist to easily perform a reliable CEUS exam in the majority of cases.
However, CTA can not correctly diagnose all endoleaks, as the hypodynamic ones, due to slow flow of exile lumbar arteries. Correct evaluation of the diameters of excluded aneurysm sac is decisive because an increase in pressure inside the excluded bag, and consequently an increase in its caliber, may confirm the suspicion of endoleak. In this regard, despite the sample is relative small, we found a nonsignificant difference between CEUS and CTA in diameters determination, both for correctly excluded sacs and for those with endoleak.
Our activity-based cost analysis showed that CEUS could provide significant savings compared to CTA for the management of these patients and could make the basis for an alternative follow-up protocol in the future.
As previously suggested [17,18,19], it is clear that activity-based cost analysis is merely the first step in a cost-effectiveness analysis that encompasses diagnostic benefits, including effects on treatment and biological costs.
The economic factor along with advantages of CEUS (low invasiveness, high tolerance, absence of ionizing radiation and safety in subjects with renal insufficiency) makes it an alternative to CTA for post-procedural follow-up [15].
However, CTA offers some advantages: relatively non-operator dependent, not conditioned by physical constitution, provides information on endograft implantation, integrity, migration, distortion, length and diameter of the neck; it remains the gold standard examination for first year of post-EVAR follow-up. Consequently, in a reasoned follow-up, CTA should be performed only in patients with the increase in sac diameter or for the detection of endoleak before surgery [14]. On the opposite, CTA has some drawbacks including radiation exposure, that may represent a problem in long-term follow-up, and iodine contrast material administration that could represent a contraindication in patients with renal impairment.
This study suffers from several limitations. First the patient population is relatively limited so far and additional prospective comparative study would be necessary to corroborate our hypothesis. Second, activity-based costing (ABC) analysis is only a part of the health tecnology assessment (HTA), that also includes budget impact and cost-efficacy analysis.
Furthermore, CEUS has several intrinsic limitations. For example, it may be difficult to observe deeply situated lesions, in particular in obese patients; moreover, bowel gas interposition and the presence of diffuse mural calcification could obscure the penetration of ultrasound beam resulting in a poor acoustic window. Second, compared with CT or MRI, the performance of CEUS is more strongly influenced by the experience of the investigator and by patient-related factors (cooperativeness).
It should be noted that in our study, we recorded a higher incidence of endoleak than the literature (15–40%) [7,8,9,10,11,12,13,14,15], explained by the small study population and by selection bias.
Conclusions
In conclusion, CEUS is an accurate and a cheap imaging method in post-EVAR follow-up patients, and it could be considered as a valid alternative to CTA, after the first year of negative controls, reducing the number of unnecessary CT examinations.
References
Adriaensen M, Bosch JL, Halpern EF et al (2002) elective endovascular versus open surgical repair of abdominal aortic aneurysms: systematic review of short-term results. Radiology 224(3):739–747. https://doi.org/10.1148/radiol.2243011675
Carrafiello G, Recaldini C, Laganà D et al (2007) Endoleak detection and classification after endovascular treatment of abdominal aortic aneurysm: value of CEUS over CTA. Abdom Imaging 33(3):357–362. https://doi.org/10.1007/s00261-007-9268-3
Harris P, Vallabhaneni S, Desgranges P et al (2000) Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: the EUROSTAR experience. J Vasc Surg 32(4):739–749. https://doi.org/10.1067/mva.2000.109990
Piscaglia F, Nolsøe C, Dietrich C et al (2011) The EFSUMB guidelines and recommendations on the clinical practice of contrast enhanced ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall in der Medizin - European Journal of Ultrasound 33(01):33–59. https://doi.org/10.1055/s-0031-1281676
Carrafiello G, Laganà D, Recaldini C et al (2006) Comparison of contrast-enhanced ultrasound and computed tomography in classifying endoleaks after endovascular treatment of abdominal aorta aneurysms: preliminary experience. Cardiovasc Intervent Radiol 29(6):969–974. https://doi.org/10.1007/s00270-005-0267-x
Dill-Macky MJ, Wilson SR, Sternbach Y et al (2007) Detecting endoleaks in aortic endografts using contrast-enhanced sonography. Am J Roentgenol. https://doi.org/10.2214/ajr.05.0532
Manning BJ, Kristmundsson T, Sonesson B et al (2009) Abdominal aortic aneurysm diameter: a comparison of ultrasound measurements with those from standard and three-dimensional computed tomography reconstruction. J Vasc Surg 50(2):263–268. https://doi.org/10.1016/j.jvs.2009.02.243
Iezzi R, Basilico R, Giancristofaro D et al (2009) Contrast-enhanced ultrasound versus color duplex ultrasound imaging in the follow-up of patients after endovascular abdominal aortic aneurysm repair. J Vasc Surg 49(3):552–560. https://doi.org/10.1016/j.jvs.2008.10.008
Cantisani V, Ricci P, Grazhdani H et al (2011) Prospective comparative analysis of colour-doppler ultrasound, contrast-enhanced ultrasound, computed tomography and magnetic resonance in detecting endoleak after endovascular abdominal aortic aneurysm repair. J Vasc Surg 53(2):551. https://doi.org/10.1016/j.jvs.2010.12.025
Giannoni M, Citone M, Rossini M et al (2012) Role of contrast-enhanced ultrasound in the follow-up of endo-vascular aortic aneurysm repair: an effective and safe surveillance method. Curr Pharm Des 18(15):2214–2222. https://doi.org/10.2174/138161212800099928
Perini P, Sediri I, Midulla M et al (2012) Contrast-enhanced ultrasound versus CT angiography in fenestrated EVAR surveillance: a single-center comparison. J Endovasc Ther 19(5):648–655. https://doi.org/10.1583/jevt-12-3909r.1
Cantisani V, Grazhdani H, Clevert DA et al (2015) EVAR: benefits of CEUS for monitoring stent-graft status. Eur J Radiol 84(9):1658–1665. https://doi.org/10.1016/j.ejrad.2015.07.001
Chung J, Kordzadeh A, Prionidis I et al (2015) Contrast-enhanced ultrasound (CEUS) versus computed tomography angiography (CTA) in detection of endoleaks in post-EVAR patients. Are delayed type II endoleaks being missed? A systematic review and meta-analysis. J Ultrasound 18(2):91–99. https://doi.org/10.1007/s40477-014-0154-x
Cantisani V, Grazhdani H, Di Marzo L et al (2016) What is the role of contrast-enhanced ultrasound in the evaluation of the endoleak of aortic endoprostheses? A comparison between CEUS and CT on a widespread scale. J Ultrasound 19(4):281–287. https://doi.org/10.1007/s40477-016-0222-5
Abraha I, Luchetta ML, De Florio R et al (2017) Ultrasonography for endoleak detection after endoluminal abdominal aortic aneurysm repair. Cochrane Database of Syst Rev. https://doi.org/10.1002/14651858.cd010296.pub2
Gruppo di lavoro misto SIRM-SNR IMS (2006) Sago S.p.A. Metologia di determinazione dei volumi di attività e della produttività dei medici radiologi. Nomenclatore SIRM-SNR delle prestazioni radiologiche; p 15, Tav III
Grisi G, Stacul F, Cuttin R et al (2000) Cost analysis of different protocols for imaging a patient with acute flank pain. Eur Radiol 10(10):1620–1627. https://doi.org/10.1007/s003300000549
Faccioli N, D’Onofrio M, Comai A et al (2007) Contrast-enhanced ultrasonography in the characterization of benign focal liver lesions: activity-based cost analysis. Radiol Med (Torino) 112(6):810–820. https://doi.org/10.1007/s11547-007-0185-x
Jonk YC, Kane RL, Lederle FA et al (2007) Cost-effectiveness of abdominal aortic aneurysm repair: a systematic review. Int J Technol Assess Health Care 23(02):205–215. https://doi.org/10.1017/s0266462307070316
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All the authors declare that he/she has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent for CEUS, CT and MR was obtained from all individual participants included in the study. This single-center retrospective study received IRB approval.
Rights and permissions
About this article
Cite this article
Faccioli, N., Foti, G., Casagranda, G. et al. CEUS versus CT Angiography in the follow-up of abdominal aortic endoprostheses: diagnostic accuracy and activity-based cost analysis. Radiol med 123, 904–909 (2018). https://doi.org/10.1007/s11547-018-0926-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-018-0926-z