Abstract
Purpose of Review
We reviewed and summarized the clinical, experimental, and epidemiological evidence examining the link between the microbiome and adiposity in the pathogenesis and progression of breast, endometrial, and colorectal cancer. Investigation of this intersection offers a novel approach for both the prevention and treatment of these cancers.
Recent Findings
The complexity of the gut microbiome and its association with the risk and progression of multiple cancers has gained increasing attention in recent years. Evidence suggests that gut dysbiosis may contribute to carcinogenesis through lowered microbial diversity, production of harmful metabolites, and increased inflammation. Additional risk factors for cancer, such as excess adiposity, may also affect the microbiome to alter metabolic and immune pathways, suggesting an obesity-associated gut microbiome may play a significant role in the development of cancer.
Summary
We found an abundance of evidence for bidirectional communication between the microbiome and adiposity and its significance in the development of obesity-related cancers. Current therapeutic approaches for restoring microbiome homeostasis as well as targeting adiposity are also discussed herein and offer potential to reduce the cancer burden in populations with a higher risk and prevalence of obesity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is the second leading cause of death in the United States and is projected to be responsible for over 610,000 deaths in the United States in 2024 [1]. Well-established risk factors for cancer include aging, excessive alcohol consumption, chronic inflammation, dietary pattern, obesity, tobacco use, radiation and sunlight exposure, exposure to certain chemicals, and family history [1]. Over the past decade, the gut microbiome—the trillions of microbes that inhabit the human gastrointestinal tract—has been associated with the risk and progression of multiple cancers. However, ongoing research seeks to investigate whether associations of the gut microbiome with cancer are causative or consequential.
Importantly, the gut microbiome can be modified, such as through diet and lifestyle interventions. Multiple lines of evidence demonstrate that obesity may be bidirectionally associated with the gut microbiome [2,3,4]. Obesity is one of many lifestyle factors associated with an increased risk for incidence and poor outcomes of multiple cancers, especially colorectal, post-menopausal breast, and endometrial cancers [5]. Mechanisms driving this association are suspected to be due to increased levels insulin and other hormones that can promote cancer growth and the pro-inflammatory environment that can occur as a result of excess adiposity [6]. Obesity can also impact patient response to cancer treatment and increase several treatment-related adverse effects, including immune dysfunction, resistance to radiation, lymphedema, and chemotherapy-induced toxicities (i.e., cardiotoxicity, peripheral neuropathy, etc.) [7, 8].
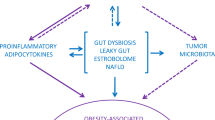
The bidirectional influence between the microbiome and adiposity offers a novel approach to investigate cancer prevention and treatment. Taken together, an obesity-associated gut microbiome may contribute to carcinogenesis, for example, through lowered diversity of the gut microbes and increased production of pro-inflammatory molecules like lipopolysaccharide and bile acids (Fig. 1) [9]. However, the impact of the obesity-associated microbiome on the response to and efficacy of chemotherapeutic drugs remains poorly understood. This targeted review provides a summary of the most recent epidemiologic evidence regarding interrelationships between obesity, the gut microbiome, and three potentially obesity- and gut microbiome-associated cancers (colorectal, breast, and endometrial cancers) (Table 1).
Methods
During the summer of 2023, the literature search strategy for this narrative review was developed by the Medical Librarian (MH), Biomedical Library at Moffitt Cancer Center, in collaboration with the research team. The resulting strategy is composed of 7 sections: Sentinel Articles, Gastrointestinal Microbiome, Adiposity, Breast Neoplasms, Colorectal Neoplasms, Ovarian Neoplasms, and Uterine Neoplasms. Medical Subject Headings [MeSH], synonyms, acronyms, and phrase searching were incorporated within the topical sections. Each section’s results were evaluated based upon the number of Sentinel Articles retrieved.
Utilizing Ovid MEDLINE(R) ALL < 1946 to August 23, 2023 > the search strategy was run on August 24, 2023. As specified by the team, the date limits applied were January 1, 2018, through December 31, 2023. No published search filters were utilized within this strategy. Search details and results are listed in Appendix 1.
Microbiome and Adiposity in Colorectal Cancer Risk and Outcomes
Colorectal cancer (CRC) is the third most common cancer type and the second leading cause of cancer death among men and women in the United States [1]. Several studies have shown that specific bacterial taxa linked to obesity could play a role in the etiology of CRC [10,11,12,13,14,15,16]. Specifically, Fusobacterium nucleatum, Helicobacter pylori, Streptococcus bovis, Clostridium septicum, Bacteroides fragilis, and others that often originate in the oral cavity may be involved in the carcinogenesis of CRC [14, 17, 18]. Additionally, lower abundance of butyrate-producing bacteria Eubacterium rectale, Faecali bacterium prausnitzii, and Enterococcus faecalis have been found repeatedly in CRC patients [19,20,21,22]. Environmental exposures largely contribute to the composition of the gut microbiome, potentially leading to inflammation, dysplasia, and the onset of CRC [16, 23].
There are several mechanisms by which obesity may increase the risk of CRC via the gut microbiome. For example, obesity may increase the risk of CRC by reducing the abundance of short chain fatty acid (SCFA)-producing bacteria. Obesity may also contribute to systemic inflammation, which is mechanistically linked to CRC, by altering intestinal barrier function which can lead to leakage of microbial products [24]. In a study comparing the gut microbiome of CRC patients (N = 45) with and without obesity to non-obese healthy controls (N = 20), significantly higher levels of the microbial-related metabolites zonulin, trimethylamine N-oxide (TMAO), and IL-Iβ and lower levels of IL-10 were reported among CRC patients with obesity compared to patients without obesity and healthy control. These findings were hypothesized to result from an obesity-related microbial profile linked to CRC [25]. A few years later, a multi-center study also characterized the gut microbiota of obese and lean patients with CRC and found further distinct differences in microbial composition between the two groups [26]. Interestingly, patients with comorbid CRC and obesity had an increased abundance of Clostidrium difficile, which has been previously associated with obesity [27] and could drive tumorigenesis of CRC by secreting toxin TcdB [28]. When analyzing functional alterations, increased metabolism of microbially-regulated D-Arginine and D-ornithine were found for obese CRC patients, while pathways of inositol phosphate metabolism, synthesis, and degradation of ketone bodies were specifically enriched in lean CRC patients [26].
Dietary intake is well known to contribute to both obesity and the composition of the gut microbiome, and, in turn, to CRC risk. Red and processed meats are hypothesized to increase CRC risk by increasing production of gut bacteria-related secondary bile acids and TMAO [29]. Ultra-processed foods, which are associated with risk of weight gain and obesity and lead to unfavorable alterations of the gut microbiome, may also contribute to CRC. A study analyzing data from three large prospective US cohorts [(Nurses’ Health Study (1986–2014; n = 64,425 women), Nurses’ Health Study II (1991–2015; n = 92,782 women), and the Health Professionals Follow Up Study (1986 − 204; n = 46,341 men)] reported that, among men, high consumption of ultra-processed foods was associated with a 29% increased risk of CRC. Subgroups of ultra-processed foods (e.g., meat-based ready to eat meals, sugar sweetened beverages) were also associated with increased CRC risk for men and women [30]. In addition, a high fat diet was shown to alter gut metabolite production of, for example, lysophosphatidic acid, which impairs cell junctions and promotes CRC cell proliferation [31]. Finally, growing evidence supports a role of the gut microbiome and body composition in the prognosis of CRC patients, potentially modulating sensitivity to radiation and chemotherapy [32].
Microbiome and Adiposity in Breast Cancer Risk and Outcomes
Breast cancer is the leading cause of cancer among women in the United States, and the second leading cause of cancer death [1]. It is likely that some established breast cancer risk factors (e.g., obesity, diet) [33] and other unknown risk factors, influence the composition and function of the gut microbiome. The gut microbiome influences multiple pathways that are mechanistically linked to the initiation and growth of breast neoplasms [34,35,36]. Accumulating evidence supports the role of the gut microbiome in regulating endogenous estrogens [34, 37] and systemic inflammation and immunity [38, 39]. The fecal microbiome was measured via the 16 S rRNA gene among Ghanaian women with and without breast cancer in a large population-based breast cancer case-control study (N = 379 cases and N = 414 controls) [40]. Fecal microbiome alpha diversity was strongly inversely associated with breast cancer and the overall composition of the gut microbiome (beta diversity) differed substantially between breast cancer cases and healthy controls [40]. In another study using shotgun metagenomics to characterize the fecal microbiota of 62 breast cancer cases and 71 controls, the pathway for the short chain fatty acid, butyrate, was suggested to be inversely associated with postmenopausal breast cancer [41]. In other smaller studies, the gut microbiome was also associated with breast cancer risk factors, tumor characteristics, prognostic factors, and adverse treatment side effects [42, 43]. Further, higher cumulative days of antibiotics were associated with higher risk of incident and fatal breast cancer [44]. Taken together, emerging data support the hypothesis that the gut microbiome is an important factor mediating breast carcinogenesis.
Obesity may be inversely associated with risk of premenopausal breast cancer, and positively associated with risk of postmenopausal breast cancer, especially with postmenopausal hormone receptor-positive breast cancer risk [45]. Further, obesity is associated with a shorter time to disease recurrence and higher mortality for both premenopausal and postmenopausal breast cancers. It is plausible that there may be strong interrelationships between the gut microbiome, obesity, and breast cancer risk and progression. For example, mammographic breast density is an established risk factor for breast cancer, with higher breast densities associated with higher breast cancer risk. In a study that investigated 16 S rRNA sequenced fecal microbiome profiles in relation to mammographic breast density and body mass index (BMI) among N = 69 healthy postmenopausal women, alpha diversity and Firmicutes to Bacteroides ratio was found to be associated with breast density, and alpha diversity was highest among women with both a high breast density and low BMI [46]. Further, energy dense diets are risk factors for both breast cancer and obesity. The gut microbiome may play a key role in weight gain and loss, such as through harvesting energy from food [3, 47]. Finally, some forms of breast cancer are driven by estrogens that may be enhanced by adipose tissue. Some bacteria contain genes that are capable of metabolizing estrogens (hence the coined term, the ‘estrobolome’) [34]. Bile acids have bidirectional associations with the gut microbiome, and bile acid levels in circulation may also be influenced by obesity. Lithocholic acid is a secondary bile acid generated by bacteria from primary bile acids that escape enterohepatic circulation. Lithocholic acid biosynthesis was lower in patients with breast cancer compared to breast cancer free controls [48]. Taken together, there is strong plausibility that obesity is linked with both the gut microbiome and breast cancer risk.
The gut microbiome is likely influenced by breast cancer therapy [49] and may modify therapy efficacy, toxicity, and longer term outcomes [42, 50]. For example, among 24 primary HER2-positive breast cancer patients treated with neoadjuvant therapy containing trastuzumab, compared to non-responders, responders had higher alpha diversity and higher abundance of Clostridiales, Bifidobacteriaceae, Turicibacteraceae, and Bacteroidales [51]. In a prospective study among 40 early-stage breast cancer patients, the women both gained weight on average and had concomitant changes in the gut microbiome [52]. Further research should explore the significance of body composition before cancer treatment, during cancer treatment, and interactions with the gut microbiome in breast cancer outcomes.
Microbiome and Adiposity in Endometrial Cancer Risk and Outcomes
Endometrial cancer is the most common gynecological malignancy, with endocrine and metabolic pathways yet to be fully elucidated [1, 53]. Excess abdominal fat, metabolic syndrome, and higher circulating estrogen associated with obesity are well recognized risk factors for endometrial cancer [54]. Chronic inflammation may also independently, and in interaction with other factors like insulin resistance and estrogen production, mediate obesity-related risk of endometrial cancer [55]. For example, Friedenreich et al. 2013 found that circulating levels of CRP, TNF-alpha and IL-6 were statistically significantly associated with endometrial cancer in a large case-control study [56]. Obesity is associated with the diagnosis of endometrial cancer at an earlier age, particularly the endometroid subtype [57]. Of the two types of endometrial cancer, the most prevalent (80–90%) Type 1 endometroid adenocarcinomas are estrogen-dependent. Most endometrial cancers have sporadic mutations and arise from endometrial hyperplasia. Type 1 endometroid carcinomas may have gene mutations in phosphatase and tensin homolog gene (PTEN), the Kirsten ras gene (KRAS), the beta-catenin gene (CTNNB1), and DNA characterized by microsatellite instability (MSI) [58].
Much like in colorectal and breast cancer, the mechanisms by which obesity influences the gut microbiome also play a role in endometrial cancer development. Female sex hormones are bidirectionally associated with the gut microbiota [59]. Gut dysbiosis involves decreased microbial diversity, decreased gram negative and increased gram-positive bacteria, estrobolome dysfunction, and disruption of estrogen metabolism Ruminococcaceae, Proteobacteria, Gammaproteobacteria, Enterobacteriales, Enterobacteriaceae, and Shigella are prevalent [60, 61]. Endometrial dysbiosis is characterized by increased Atopobium vaginae and Porphyromonas somerae which set the stage for inflammation [60, 62]. Porphyromonas somerae combined with high pH in the vagina could be a promising risk biomarker for endometrial cancer [58, 60].
Longitudinal cohort studies and clinical trials, including Iowa Women’s Health Study, European Prospective Investigation into Cancer and Nutrition (EPIC)-Oxford trial, and Re-Energize with Nutrition, Exercise and Weight Loss (RENEW) investigating the associations of diet, weight loss and associated changes in biomarkers with cancer, collectively support a role for modifiable risk factors such as metabolic syndrome and obesity in endometrial cancer risk [55, 63]. American Cancer Society estimates 70% of endometrial cancer is attributable to lifestyle factors of maintaining excess body weight through high fat, calorie dense diets and insufficient physical activity [1]. Diet has been shown to modulate estrogen and inflammation levels [64]. Physical activity improves insulin sensitivity and reduces glucose levels. Reducing adiposity reduces pro-inflammatory cytokines and increases anti-inflammatory mediators (IL-10 and adiponectin). Exercise decreases risk by reducing serum estradiol and increasing sex hormone binding globulin and reduces circulating insulin and insulin resistance [65]. Methods to manipulate hormone and immune function through biobehavioral lifestyle changes offer hope for endometrial cancer risk reduction and treatment.
Obesity, the Gut Microbiome, and Cancer Disparities
There are extensive disparities in the cancers described above. The CRC mortality rate among non-Hispanic Black individuals is approximately 37% higher than in non-Hispanic White individuals [66]. In the United States, Black women suffer higher breast cancer mortality than other racial and ethnic groups. Indeed, Black women are 42% more likely to die from breast cancer than white women [67]. Furthermore, Black women with endometrial cancer have an 80% higher mortality rate compared to white women, due to more advanced tumor stages at diagnosis and more aggressive histologic subtypes even though occurrence is overall less common [68]. Explanations for disparities in cancer incidence and survival are likely multifactorial, including various social and structural drivers of health (SSDOH) and differences in molecular and clinical characteristics. Beyond race and ethnicity, individuals who live in highly deprived or rural areas may also fare poorer than other individuals. Further, there are differences in obesity rates by racial and ethnic group. For example, obesity disproportionately affects African American communities, with an estimated prevalence of 49.6% among non-Hispanic Black adults [69]. The gut microbiome is likely influenced by known and unknown factors related to race and ethnicity including obesity. Differences in obesity rates and the gut microbiome may reflect various SSDOH. Racial and ethnic microbiome differences may be evident as early as a few months after birth [70, 71] and can potentially result from exposures that occur as early as in utero or at birth. For example, caesarean sections are known to alter the microbiome among offspring and are performed at higher rates among racial minorities [72]. Other microbiome and adiposity influencing exposures can also accumulate over the life course such as food insecurity, access to spaces for physical activity, chronic stress due to discrimination, and other exposures [72]. Taken together, future research should aim to elucidate the interactions among obesity, the gut microbiome, and SSDOH in racial and ethnic disparities in cancer.
Interventions Targeted to the Gut Microbiome and Obesity
Microbiota dysbiosis contributes to weight gain by inducing inflammation, reducing fat and cholesterol metabolism, and decreasing insulin sensitivity [73]. Importantly, reduced biodiversity of the gut microbiome is linked to different conditions such as obesity-associated inflammatory characteristics, gastrointestinal diseases, and risk of several cancers [47, 74, 75]. The increase of microbial biodiversity related to various interventions could have beneficial effects on the pathogenesis of these conditions.
Diet
Prebiotics stimulate the growth and activity of gut microbes, in order to improve overall health [76]. Studies have revealed that the gut microbiota can be rapidly modified by dietary changes and increasing fiber leads to better mucosal health [33, 77, 78]. Previous seminal research has demonstrated that consumption of a very high fiber diet can lead to improvements in the gut environment in a very rapid manner [33, 77, 78]. Experimental studies have shown that the consumption of food rich in prebiotics (i.e. dietary fiber) is strongly related to beneficial effects against obesity and alteration of the obese microbiome [79, 80]. Prebiotics are evidenced to stimulate the growth of Lactobacillus and Bifidobacterium in obese animals [81], as well as reduce the abundance of pathogenic microorganisms, such as Firmicutes and Bacteroidetes [82]. Furthermore, targeted prebiotic diets increase genes inversely associated with obesity (i.e., TYK2, CXCL1, NXPH1) and suppress adipokines (FGF2) to improve metabolic function [80]. Some studies have shown that these changes in gene expression were related to improved entero-endocrine cell activity, glucose homeostasis, and leptin sensitivity in both obese and diabetic mice treated with oligofructose [83, 84]. In addition, prebiotics can also improve lipid and glucose metabolism, resulting in lower triglycerides levels, adipose tissue mass, and muscle lipid infiltration [83]. Interestingly, prebiotics can be used in combination with probiotic bacteria, termed “synbiotics”, in order to improve their beneficial effects against obesity and related metabolic disorders and diseases [85, 86].
Probiotics
Probiotics are live strains of microorganisms that, when integrated into the intestinal ecosystem, can effectively reorganize the intestinal flora and provide health benefits [87]. Their integration allows for interaction with immune cells and alteration of cytokine production to inhibit inflammatory responses, while also strengthening the gut mucosal barrier to maintain intestinal barrier stability and mucosal permeability [73]. However, probiotics are limited to specific bacterial strains for regulatory reasons (mostly within the genera Lactobacillus, Bifidobacterium, and Saccharomyces for yeasts), and their properties should be defined at the species and strain level [88]. A recent review of the literature summarizes that current clinical and preclinical studies have demonstrated that probiotics can potentially reduce body weight, lower blood lipids, and improve metabolism [73]. Furthermore, many clinical studies indicate the effectiveness of probiotics in preventing, treating, and reducing the progression of several types of cancer including colorectal, liver, breast, bladder, colon, and cervical [89,90,91,92,93,94]. The results of many in vitro studies indicate anti-cancer properties of probiotics in modulating the proliferation/apoptosis of cancer cells (e.g., gastric, colonic, and myeloid leukemia cells) suggesting their beneficial use in cancer prevention and as an adjuvant treatment [95]. Despite potential benefits, probiotics have a number of limitations, including variations in formulation, differences across microbial strains included, passage through the gastrointestinal tract, and potential safety issues (e.g., especially among vulnerable cancer patients).
Exercise
Exercise counteracts obesity progression and has been demonstrated to modulate the gut microbiota. Indeed, the composition of the intestinal microflora was evidenced to be different in obese and nonobese rats and exercise improved both the composition and diversity of gut bacteria [96]. Beneficial effects of exercise on the microbiome might be mediated by decreased intestinal permeability, which prevents pathogens from crossing the intestinal barrier and reduces inflammation [88]. Furthermore, bacterial diversity is increased, including SCFA-producing species, while potential disease-causing species such as E. coli or E. faecalis are decreased [88]. The anti-inflammatory metabolite, SCFA butyrate, has been linked to exercise-related outcomes [97], which allows this to be a highly effective intervention to target both obesity and the microbiome, as obesity may increase the risk of cancers through reduced SFCA production.
Future Directions
In addition to the cancer disparities explained in this review, further gaps in knowledge regarding the link between the microbiome, adiposity, and cancer, warrant further investigation on their interrelationships. Current interventional studies include confounding factors such as diet, body composition, study design, and analytical methods, which limit the conclusions of the existing studies and add difficulty applying findings to all populations. With exercise interventions specifically, it is unclear whether other aspects of exercise prescription (such as frequency, duration, and type of exercise) may also impact the degree of changes in the microbiota. Interestingly exercise-induced changes in the microbiota were also largely reversed after cessation of activity [98]. Further experimental studies are also needed to improve knowledge about possible combinations of diet, pre- and probiotics, the timing of intervention, and their potential anti-obesogenic effects. Interesting preclinical data on fecal microbiota transplant (FMT) may prove beneficial to the microbiota. However, most FMT research is conducted on mouse models, as fecal transplants in humans are associated with safety and ethical issues. A combination of treatments to target the microbiome and adiposity may be most beneficial for overall improved health and prevention of cancer and cancer-related outcomes.
Conclusions
Taken together, there appears to be substantial evidence for a role of the gut microbiome and adiposity, potentially interactively, in obesity-related risk and outcomes of colorectal, breast, and endometrial cancer. As accumulating evidence highlights the potential for specific microbes to be involved in carcinogenesis, interventions to change the gut microbiome and/or improve weight management offer enormous opportunity to reduce the cancer burden in all populations, and especially those disproportionately impacted by obesity and cancer.
Data Availability
No datasets were generated or analysed during the current study.
References
American Cancer Society. Cancer Facts and Fig. 2023. Atlanta: American Cancer Society; 2023.
Greathouse KL, et al. Gut microbiome meta-analysis reveals dysbiosis is independent of body mass index in predicting risk of obesity-associated CRC. BMJ Open Gastroenterol. 2019;6(1):e000247.
Turnbaugh PJ, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31.
Zhang C, et al. Dietary modulation of Gut Microbiota contributes to alleviation of both genetic and simple obesity in children. EBioMedicine. 2015;2(8):968–84.
Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–91.
Song M, Chan AT. Environmental factors, gut microbiota, and Colorectal Cancer Prevention. Clin Gastroenterol Hepatol. 2019;17(2):275–89.
Pati S, et al. Obesity and Cancer: a current overview of Epidemiology, Pathogenesis, outcomes, and management. Volume 15. Cancers (Basel); 2023. 2.
Slawinski CGV, et al. Obesity and Cancer Treatment outcomes: interpreting the Complex evidence. Clin Oncol (R Coll Radiol). 2020;32(9):591–608.
Turnbaugh PJ, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–4.
Gagniere J, et al. Gut microbiota imbalance and colorectal cancer. World J Gastroenterol. 2016;22(2):501–18.
Bullman S, et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science. 2017;358(6369):1443–8.
Zackular JP et al. Manipulation of the gut microbiota reveals role in Colon tumorigenesis. mSphere, 2016. 1(1).
Zackular JP, et al. The gut microbiome modulates colon tumorigenesis. mBio. 2013;4(6):e00692–13.
Thomas AM, et al. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat Med. 2019;25(4):667–78.
Coker OO, et al. Altered gut metabolites and microbiota interactions are implicated in colorectal carcinogenesis and can be non-invasive diagnostic biomarkers. Microbiome. 2022;10(1):35.
Feng Q, et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat Commun. 2015;6:6528.
Wirbel J, et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat Med. 2019;25(4):679–89.
Dalal N, et al. Gut microbiota-derived metabolites in CRC progression and causation. J Cancer Res Clin Oncol. 2021;147(11):3141–55.
Shoji M, et al. Characteristics of the gut microbiome profile in obese patients with colorectal cancer. JGH Open. 2021;5(4):498–507.
Balamurugan R, et al. Real-time polymerase chain reaction quantification of specific butyrate-producing bacteria, Desulfovibrio and Enterococcus faecalis in the feces of patients with colorectal cancer. J Gastroenterol Hepatol. 2008;23(8 Pt 1):1298–303.
Wu N, et al. Dysbiosis signature of fecal microbiota in colorectal cancer patients. Microb Ecol. 2013;66(2):462–70.
Wang T, et al. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2012;6(2):320–9.
Tu P et al. Gut Microbiome Toxicity: connecting the Environment and Gut Microbiome-Associated diseases. Toxics, 2020. 8(1).
Song M, Chan AT, Sun J. Influence of the gut Microbiome, Diet, and Environment on Risk of Colorectal Cancer. Gastroenterology. 2020;158(2):322–40.
Sanchez-Alcoholado L et al. Gut microbiota-mediated inflammation and gut permeability in patients with obesity and colorectal Cancer. Int J Mol Sci, 2020. 21(18).
•• Zhu X et al. Multi-kingdom microbial signatures in excess body weight colorectal cancer based on global metagenomic analysis Commun Biol, 2024. 7(1): p. 24. Global metagenomic analysis reveals specific multi-kingdom microbial signatures for obese-CRC and lean CRC, which may contribute to precision diagnosis and treatment of CRC.
Bishara J, et al. Obesity as a risk factor for Clostridium difficile infection. Clin Infect Dis. 2013;57(4):489–93.
Drewes JL, et al. Human Colon cancer-derived Clostridioides difficile strains drive Colonic Tumorigenesis in mice. Cancer Discov. 2022;12(8):1873–85.
Diakite MT et al. Relationships between gut microbiota, red meat consumption and colorectal cancer. J Carcinog Mutagen, 2022. 13(3).
Hang D, et al. Ultra-processed food consumption and risk of colorectal cancer precursors: results from 3 prospective cohorts. J Natl Cancer Inst. 2023;115(2):155–64.
Yang J, et al. High-Fat Diet promotes colorectal tumorigenesis through modulating gut microbiota and metabolites. Gastroenterology. 2022;162(1):135–49. e2.
Ding C, et al. Intestinal microbiota: a novel perspective in colorectal cancer biotherapeutics. Onco Targets Ther. 2018;11:4797–810.
David LA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63.
Kwa M et al. The intestinal microbiome and estrogen receptor-positive female breast Cancer. J Natl Cancer Inst, 2016. 108(8).
Tobias DK, et al. Markers of inflammation and incident breast Cancer risk in the women’s Health Study. Am J Epidemiol. 2018;187(4):705–16.
Garcia-Estevez L, Moreno-Bueno G. Updating the role of obesity and cholesterol in breast cancer. Breast Cancer Res. 2019;21(1):35.
Flores R, et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J Transl Med. 2012;10:253.
Maynard CL, et al. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489(7415):231–41.
Belkaid Y, Timothy W, Hand. Role of the Microbiota in immunity and inflammation. Cell. 2014;157(1):121–41.
Byrd DA, et al. Associations of fecal microbial profiles with breast cancer and nonmalignant breast disease in the Ghana breast Health Study. Int J Cancer. 2021;148(11):2712–23. Study analyzed fecal bacteria associated with breast cancer and nonmalignant breast disease.
Zhu J, et al. Breast cancer in postmenopausal women is associated with an altered gut metagenome. Microbiome. 2018;6(1):136.
Terrisse S, et al. Intestinal microbiota influences clinical outcome and side effects of early breast cancer treatment. Cell Death Differ. 2021;28(9):2778–96.
Wu AH, et al. Gut microbiome associations with breast cancer risk factors and tumor characteristics: a pilot study. Breast Cancer Res Treat. 2020;182(2):451–63.
Velicer CM, et al. Antibiotic use in relation to the risk of breast cancer. JAMA. 2004;291(7):827–35.
Picon-Ruiz M, et al. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67(5):378–97.
Yaghjyan L, et al. Gut microbiome, body weight, and mammographic breast density in healthy postmenopausal women. Cancer Causes Control. 2021;32(7):681–92.
Ley RE, et al. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–5.
Miko E, et al. Lithocholic acid, a bacterial metabolite reduces breast cancer cell proliferation and aggressiveness. Biochim Biophys Acta Bioenerg. 2018;1859(9):958–74.
Guan X, et al. Gut microbiota profiling in patients with HER2-Negative metastatic breast Cancer receiving metronomic chemotherapy of Capecitabine compared to those under conventional dosage. Front Oncol. 2020;10:902.
Alexander JL, et al. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol. 2017;14(6):356–65.
Di Modica M, et al. Gut Microbiota Condition the therapeutic efficacy of Trastuzumab in HER2-Positive breast Cancer. Cancer Res. 2021;81(8):2195–206.
Walker J, et al. Chemotherapy-induced weight gain in early-stage breast cancer: a prospective matched cohort study reveals associations with inflammation and gut dysbiosis. BMC Med. 2023;21(1):178.
Borghi C, et al. Biomolecular basis related to inflammation in the pathogenesis of endometrial cancer. Eur Rev Med Pharmacol Sci. 2018;22(19):6294–9.
Yasin HK, Taylor AH, Ayakannu T. A narrative review of the role of Diet and Lifestyle factors in the Development and Prevention of Endometrial Cancer. Cancers (Basel), 2021. 13(9).
Dossus L, et al. Obesity, inflammatory markers, and endometrial cancer risk: a prospective case-control study. Endocr Relat Cancer. 2010;17(4):1007–19.
Friedenreich CM, et al. Case-control study of inflammatory markers and the risk of endometrial cancer. Eur J Cancer Prev. 2013;22(4):374–9.
Perez-Martin AR, et al. Impact of metabolic syndrome on the risk of endometrial cancer and the role of lifestyle in prevention. Bosn J Basic Med Sci. 2022;22(4):499–510.
Boutriq S et al. Gut and Endometrial Microbiome Dysbiosis: A New Emergent Risk Factor for Endometrial Cancer J Pers Med, 2021. 11(7).
Alizadehmohajer N, et al. Association between the microbiota and women’s cancers - cause or consequences? Biomed Pharmacother. 2020;127:110203.
Zhao SS, et al. Altered gut Microbial Profile accompanied by abnormal fatty acid metabolism activity exacerbates endometrial Cancer Progression. Microbiol Spectr. 2022;10(6):e0261222.
Li Y, et al. Gut Microbiome Dysbiosis in patients with Endometrial Cancer vs. healthy controls based on 16S rRNA gene sequencing. Curr Microbiol. 2023;80(8):239. Study analyzed gut microbiota composition and endometrial cancer development.
Lu W, et al. Dysbiosis of the endometrial microbiota and its association with inflammatory cytokines in endometrial cancer. Int J Cancer. 2021;148(7):1708–16.
Linkov F, et al. Longitudinal evaluation of cancer-associated biomarkers before and after weight loss in RENEW study participants: implications for cancer risk reduction. Gynecol Oncol. 2012;125(1):114–9.
Ricceri F, et al. Diet and endometrial cancer: a focus on the role of fruit and vegetable intake, Mediterranean diet and dietary inflammatory index in the endometrial cancer risk. BMC Cancer. 2017;17(1):757.
Friedenreich CM, et al. Case-control study of the metabolic syndrome and metabolic risk factors for endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2011;20(11):2384–95.
American Cancer Society. Cancer facts & figures for African American/Black people 2022–2024. Atlanta: American Cancer Society; 2022.
Yedjou CG, et al. Health and racial disparity in breast Cancer. Adv Exp Med Biol. 2019;1152:31–49.
Farley J, et al. Racial disparities in blacks with gynecologic cancers. Cancer. 2007;110(2):234–43.
Hales CM et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief, 2020(360): p. 1–8.
Mallott EK, et al. Human microbiome variation associated with race and ethnicity emerges as early as 3 months of age. PLoS Biol. 2023;21(8):e3002230.
Li J, et al. Individuality and ethnicity eclipse a short-term dietary intervention in shaping microbiomes and viromes. PLoS Biol. 2022;20(8):e3001758.
Amato KR et al. The human gut microbiome and health inequities. Proc Natl Acad Sci U S A, 2021. 118(25).
Cai Y, et al. Probiotics therapy show significant improvement in obesity and neurobehavioral disorders symptoms. Front Cell Infect Microbiol. 2023;13:1178399.
Monda V, et al. Exercise modifies the gut microbiota with positive Health effects. Oxid Med Cell Longev. 2017;2017:p3831972.
Claesson MJ, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–84.
Pineiro M, et al. FAO Technical meeting on prebiotics. J Clin Gastroenterol. 2008;42(Suppl 3 Pt 2):S156–9.
O’Keefe SJ, et al. Fat, fibre and cancer risk in African americans and rural africans. Nat Commun. 2015;6:6342.
Wu GD, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–8.
Delannoy-Bruno O, et al. Evaluating microbiome-directed fibre snacks in gnotobiotic mice and humans. Nature. 2021;595(7865):91–5.
She J, Wong CC, Yu J. Targeted prebiotics alter the obese gut microbiome in humans. Signal Transduct Target Ther. 2021;6(1):363.
Connolly ML, Lovegrove JA, Tuohy KM. In vitro fermentation characteristics of whole grain wheat flakes and the effect of toasting on prebiotic potential. J Med Food. 2012;15(1):33–43.
Parnell JA, Reimer RA. Effect of prebiotic fibre supplementation on hepatic gene expression and serum lipids: a dose-response study in JCR:LA-cp rats. Br J Nutr. 2010;103(11):1577–84.
Everard A, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60(11):2775–86.
Posovszky C, Wabitsch M. Regulation of appetite, satiation, and body weight by enteroendocrine cells. Part 2: therapeutic potential of enteroendocrine cells in the treatment of obesity. Horm Res Paediatr. 2015;83(1):11–8.
Liong MT, Dunshea FR, Shah NP. Effects of a synbiotic containing Lactobacillus acidophilus ATCC 4962 on plasma lipid profiles and morphology of erythrocytes in hypercholesterolaemic pigs on high- and low-fat diets. Br J Nutr. 2007;98(4):736–44.
Cerdo T et al. The role of Probiotics and Prebiotics in the Prevention and treatment of obesity. Nutrients, 2019. 11(3).
Food F. a.A.O.F.G.f.t.E.o.P.i. Report of a Joint FAO/WHO Working Group on Drafting guidelines for the evaluation of Probiotics in Food. FAO; 2002.
Clauss M, et al. Interplay between Exercise and Gut Microbiome in the Context of Human Health and Performance. Front Nutr. 2021;8:637010.
Toi M, et al. Probiotic Beverage with soy isoflavone consumption for breast Cancer Prevention: a case-control study. Curr Nutr Food Sci. 2013;9(3):194–200.
Pala V, et al. Yogurt consumption and risk of colorectal cancer in the Italian European prospective investigation into cancer and nutrition cohort. Int J Cancer. 2011;129(11):2712–9.
Goldin BR, Gorbach SL. Effect of Lactobacillus acidophilus dietary supplements on 1,2-dimethylhydrazine dihydrochloride-induced intestinal cancer in rats. J Natl Cancer Inst. 1980;64(2):263–5.
Ohara T, Yoshino K, Kitajima M. Possibility of preventing colorectal carcinogenesis with probiotics. Hepatogastroenterology. 2010;57(104):1411–5.
Liu ZH, et al. The effects of perioperative probiotic treatment on serum zonulin concentration and subsequent postoperative infectious complications after colorectal cancer surgery: a double-center and double-blind randomized clinical trial. Am J Clin Nutr. 2013;97(1):117–26.
Ohashi Y, et al. Habitual intake of lactic acid bacteria and risk reduction of bladder cancer. Urol Int. 2002;68(4):273–80.
Slizewska K, Markowiak-Kopec P, Slizewska W. Role Probiotics Cancer Prev Cancers (Basel), 2020. 13(1).
Petriz BA, et al. Exercise induction of gut microbiota modifications in obese, non-obese and hypertensive rats. BMC Genomics. 2014;15(1):511.
Erlandson KM, et al. An exercise intervention alters stool microbiota and metabolites among older, sedentary adults. Ther Adv Infect Dis. 2021;8:20499361211027067.
Allen JM, et al. Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc. 2018;50(4):747–57.
Author information
Authors and Affiliations
Contributions
TLC conceptualized the article. MKH conducted literature searches and article retrieval. TLC, DR, DAB completed screening for article inclusion. DR, VD, and DAB prepared tables. VD created Fig. 1. TLC, DR, VD, MKH, and DAB wrote the main text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Disclosures
The authors have nothing to disclose. This article does not contain any studies with human or animal subjects performed by any of the authors.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Carson, T.L., Rivers, D., Doerr, V. et al. The Intersection of the Microbiome and Adiposity in Cancer Risk and Outcomes: Breast, Endometrial, and Colorectal Cancers. Curr Epidemiol Rep 11, 140–152 (2024). https://doi.org/10.1007/s40471-024-00351-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40471-024-00351-5