Abstract
The domatium presents a diversity of forms, and the understanding of the structural aspect of the domatia is very important because it can provide diagnostic characters for families and genera, and favors the establishment of mutualistic relations. The objectives of the present study were to characterize the developmental stages of the domatia of Schinus terebinthifolius Raddi. (Anacardiaceae) located at the base of the leaflet, and based on these data to determine if the structural aspects provide good shelter for mites and ants. Domatia were observed under a stereomicroscope and then processed following standard techniques for light and scanning electron microscopy. The domatia of S. terebinthifolius develop early during the development of the leaves and go through four stages of development. The domatia have a spiraling architecture with internal chambers that contain trichomes and stomata, suggesting good conditions of shelters for arthropods. Deposits of phenolic compounds and calcium crystals were identified in the domatia, suggesting an investment in alternative forms of protection from herbivory. Structural analysis showed the developmental stages of domatia and how the architecture of these structures can provide efficient shelter for arthropods, thereby contributing to the protection of these plants from herbivory and facilitating ecological studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Domatia are small structures that often harbor predaceous arthropods which are potentially beneficial to the plant (Agrawal et al. 2000). These structures are very important for taxonomy because they provide diagnostic characters for botanical families and genera (Nickol 1998; Michelangeli 2000; Kim and Ngondya 2010; Leroy et al. 2010). In this regard, these structures have been reported in approximately 277 families and more than 2000 species of Angiospermae (Agrawal and Karban 1997).
In addition to their contribution to taxonomy, domatia also play an important ecological role. Numerous studies have reported the establishment of a mutualistic relationship between domatia-bearing plants and mites (Agrawal 1997; Norton et al. 2001; Romero and Benson 2005). Domatia favor mites by providing them favorable environments for oviposition and the effectiveness survival of mite offspring, in addition to providing protection against desiccation and attack by predators (Pemberton and Turner 1989; Grostal and O’Dowd 1994; Norton et al. 2001; Romero and Benson 2005). Mites in turn remove spores and hyphae of fungal pathogens, in addition to protecting the plant from attack by phytophagous insects (Roda et al. 2001; Kreiter et al. 2003; Romero and Benson 2005). This mutualistic relationship has been increasingly drawing the attention of ecologists, who seek to understand more completely the interactions between domatia and mites (Nishida et al. 2006).
The diversity of forms of domatia may favor these interactions. The principle types are in the form of pits or pouches and dense tufts of hair between the main and secondary veins (Romero and Benson 2005; Moraes et al. 2011), or a combination of tufts of hairs with pits and pouches (O’Dowd and Willson 1991; Romero and Benson 2005). Studies on Cinnamomum camphora (L.) J. Presl (Lauraceae) revealed that the different forms of domatia could be related to specific taxa of mites (Nishida et al. 2006). Domatia that are in the form of pouches are preferentially inhabited by herbivorous or fungivorous mites, and the domatia that are in the form of pits are commonly used by carnivorous mites (Nishida et al. 2006). The coevolution of domatia and mites was proposed by Pemberton and Turner (1989); however, there is little concrete evidence to support this hypothesis.
Studies aim at characterizing the morphology and anatomy of the different forms of domatia are rare, and most of them have been mainly dedicated to investigating the ecological relationships involving mites (O’Dowd 1989; Agrawal 1997; Norton et al. 2001; Romero and Benson 2004). However, some authors have dedicated themselves to investigating morphoanatomical aspects and the development of the different forms of domatia (Tillberg 2004; Nishida et al. 2006; Leroy et al. 2008; Leroy et al. 2010; Richards and Coley 2012). Solís and Ferrucci (2006) described morphoanatomical characteristics of the domatia of two species of Cardiospermum L. (Sapindaceae) in order to collect data that contribute to the systematic of the genus. In addition, Solís-Montero et al. (2009) characterized the domatia of nine species in Mortoniodendron Standl. & Steyerm. (Malvaceae), with the aim of evaluating if there is any relation between the domatia type and the species. Val and Dirzo (2003) investigated how the ontogeny of domatia of Cecropia peltata L. (Cecropiaceae) can influence the interaction with mites, causing changes in plant defense strategies. Leroy et al. (2008) found changes in leaf blade tissue during the formation of domatia of Hirtella physophora Mart. & Zucc. (Chrysobalanaceae).
The species in question, Schinus terebinthifolius Raddi (Anacardiaceae), is dioecious, and can be found in the form of medium-sized trees (3–4 m) or small bushes. The leaves are compound, imparipinnate, and with the petiole and rachis narrowly winged. The leaflets are opposite or subopposite, usually sessile and may be oval or elliptical in shape (Cronquist 1981). Two types of domatia have been described for this species, one located in the region of the junction of the secondary veins with the midrib, referred to as “tufts of hair,” and the other located at the base of the leaflet, referred to as the “cavity form” (Barros 1961). Wiggers et al. (2005) reported a high diversity of mites inhabiting the domatia of S. terebinthifolius located at the base of the leaflet, and the great majority of fungivorous and predatory mites.
Following this reasoning, some anatomical characteristics of leaf may be modified by pressures exerted by the mutualistic relationship. Thus, this study aims to characterize the stages of development of the of S. terebinthifolius located at the base of the leaflet and determine if there are developmental strategies of structure.
2 Materials and methods
Individual leaflets of S. terebinthifolius Idem were collected during the month of January 2014, in the Grussaí/Iquipari lagoon complex (Complexo Lagunar de Grussaí/Iquipari; 21°72654′S and 41°03645′W), in the municipality of São João da Barra in the state of Rio de Janeiro, Brazil.
For structural and development of domatia analyses we select 10 individuals from which five buds and five fully expanded leaves were collected. Later, domatia at different stages of development were removed from the base of buds and leaves. For morphological characterization of the domatia, samples were observed and images captured with a camera (Power Shot A640, CANON, New York, USA) attached to a stereomicroscope (Zeizz Stemi SV 11).
Histochemical tests were performed using freehand sections in all development stages of domatia. The sections were then deposited on slides and submitted to different reagents to determine the chemical groups present in the cells of the domatia, such as lugol to determine the presence of starch (Berlyn and Miksche 1976); ruthenium red for detection of mucilage/pectic (Chamberlain 1932); ferric chloride for identification of phenolic compounds (Johansen 1940); sudan IV to verify the presence of lipids (Gerlach 1984), and for identification of calcium oxalate crystals was utilized polarized light microscopy. Standard control procedures were performed simultaneously. Observation of cuts and obtaining the images were made using a light microscope Axioplan ZEISS (Oberkohen, Germany), using camera Cannon Power Shot A640 and software Analysis®-LINK/ISIS/ZEISS (Oxford, UK). The chemical composition of the crystals, which was calcium oxalate, was confirmed because they were insoluble in acetic acid and soluble in chloridric acid (Mclean and Cook 1958).
For anatomical analysis, the material was fixed in a solution of 2.5% glutaraldehyde, 4% formaldehyde and 0.05 M sodium cacodylate buffer at pH 7.2, then washed in the same buffer and postfixed in 1% osmium tetroxide and 0.05 M sodium cacodylate buffer for 2 h at ambient temperature. Next, the samples were dehydrated in an ascending series of acetone. After dehydration, the material was infiltrated and embedded in epoxy resin (Epon 812 Polybed®, Warrington, USA). Semi-thin sections of approximately 2–5 µm were obtained and stained with 1% toluidine blue and 1% borax buffer (Johansen 1940). The slides were then observed and documented with a camera (PowerShot A640, CANON, New York, USA) attached to a light microscope (Axioplan, ZEISS, Oberkochen, Germany).
The thickness of the domatia, mesophyll, and number of mesophyll cells were calculated from cross sections of the domains. We examined 25 fields for each domatium developmental stage. The images obtained were processed and analyzed using image processing digital system Image Pro-Plus.
Domatia were also subjected to scanning electron microscopy. For this, the domatia were fixed and dehydrated following the same methods used for optical microscopy. Then, the samples were subjected to critical-point drying with CO2 (CPD 030, Baltec, Heerbrugg, Switzerland) and adhered to stubs using carbon adhesive tape and covered with a layer of approximately 20 nm of gold (SCD 050, Baltec, Heerbrugg, Switzerland). Images were obtained using a ZEISS-DSEM 962 scanning electron microscope (Oberkochen, Germany) at a voltage of 25 kV.
3 Results
The rachis of S. terebinthifolius is characterized by being cylindrical in shape in the distal region and pseudo-winged in the basal region of the leaflets. This wing-like rachis is most developed in the region of the third leaflet, as can be seen in Fig. 1. This structure is very close to the base of the leaflets, with folds forming an enclosure called the domatium (Fig. 1–13). Domatia were observed only at the base of the second and third leaflets, while none of the first leaflets analyzed had domatia (Fig. 1). Only this form of domatium was found in individuals investigated.
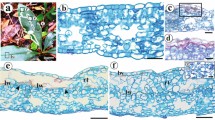
Characterization of the stages of development of domatia of Schinus terebinthifolius. 1 Overview of a leaf showing domatia at the base of the leaflets. 2, 6, 10 Initial stage of development. 3, 7, 11 Initial intermediate stage of development. 4, 8, 12 Late intermediate stage of development. 9, 13 Complete development stage. 1–5 Images taken using a stereomicroscope. 6–9 Images taken using a scanning electron microscope. 10–13 Images taken using a optical microscope. Identification of leaflets (1°, 2°, 3°); Domatia (arrows); Wing-like rachis (WR); Trichomes (T). Bars: 2, 3 1 mm; 4, 5 2 mm; 6 1 mm; 7 100 µm; 8 200 µm; 9 500 µm; 10–13 200 µm
Four stages of development of domatia were characterized. The initial stage (IS) is characterized by small projections that emerge from the winged structures located at the base of the leaflets (Fig. 2, 6, 10). At this stage, a great quantity of trichomes are distributed over the entire surface of this projection (Fig. 6). Transverse sections at this stage revealed that the winged projections show undifferentiated mesophyll, and a subepidermal layer consisting of 2–5 layers of cells underlying the two surfaces of epidermis (Fig. 14). The adaxial epidermal cells are higher than those of the abaxial epidermis, and a thickening of the cuticular layer on both sides was found (Fig. 15, 16).
Anatomical characterization of the domatia of Schinus terebinthifolius. 14–16 Transverse section of a domatium in initial stage. 17–19 Transverse section of a domatium in initial intermediate stage. 20–22 Transverse section of a domatium in late intermediate stage. 23–25 Transverse section of a domatium in complete development stage. Asterisks indicate cell compaction. Adaxial epidermis (Ad); Subepidermal layer (UE); Mesophyll (ME); Secretory canal (SC); Abaxial epidermis (Ab). Bars: 14, 17, 20, 21 100 µm; 15, 16, 18, 19, 22, 24, 25 50 µm
The second stage, called the initial intermediate stage (IIS), exhibits further development of these projections and a slight curvature of the structure, in which is formed a concave region facing the abaxial surface of the leaves (Fig. 3, 7, 11). This event resulted in an increase in the mesophyll between the adaxial and abaxial surfaces (Fig. 17). In IIS, the subepidermal layer is comprised of three layers of cells below the adaxial surface and is absent from the abaxial surface (Fig. 18, 19).
The third stage, called the late intermediate stage (LIS), has differentiated mesophyll, consisting of palisade and spongy parenchyma, differently of earlier stages (Fig. 20, 22). Exhibits more accentuated curvature of the projection compared to the second stage (IIS). The curvature is the result of the continuous process of developing and folding of the projection upon itself resulting in a domatium, and culminating in the initial formation of a structure similar to a capsule/enclosure (Fig. 4, 8, 12). In this stage, the domatia are at different points of folding, causing cell compaction in different regions of the structure (Fig. 20, 22). In the basal region of the insertion of the rachis where there is less compaction of the tissues, intercellular spaces are present between the cells of the mesophyll (Fig. 20). On the other hand, in the median region, mesophyll and the epidermis presented more cellular compaction (Fig. 21). In the region distal to the rachis, cellular compaction was more intense in the region of mesophyll. A larger number of cells were also observed on the abaxial surface of the epidermis that were smaller than the cells of the epidermis of the adaxial surface (Fig. 22).
The last stage of development, called the complete development stage (CDS) of the domatia, a capsule/enclosure is already fully formed due to process of continuous folding of the projections (Fig. 5, 9, 13). The anatomical characteristics encountered at this stage were similar to those encountered in the LIS, differing only with respect to the lesser degree of cellular compression in the middle region of this structure (Fig. 23–25).
Through the morphometric analysis of the domatium, it was possible to verify a reduction of the domatia thickness, the mesophyll thickness, and the number of mesophyll cells in the LIS and CDS stages when compared to the IS and IIS stages (Table 1). These results help to understand the processes of folding of the domatium.
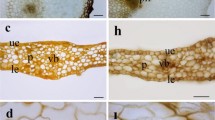
In the transverse section of the rachis, more details of formation of the domatium can be observed, leaving their enclosure inclined toward facing the abaxial surface of the leaf (Fig. 26, 29). The internal structure of the domatium is organized by spiral chamber that were produced by the folding (Fig. 27, 30). It is in this chamber that mites can be found (Fig. 28) among the tectory trichomes (Fig. 27) and the glandular trichomes (Fig. 30). Stomata are restricted to just inside of the domatium (Fig. 32). The microchemical test with ferric chloride detected the presence of phenolic compounds (Fig. 33), and polarized light microscopy revealed the presence of calcium oxalate crystals of druse type in mesophyll cells (Fig. 34). There were no starches, lignin or lipid droplets detected.
Morphological aspects of domatia of Schinus terebinthifolius in complete development stage. 26 Transverse section of rachis detailing the spiral organization of a portion of a domatium. 27 Transverse section showing the presence of trichomes inside of a domatium. 28 Presence of mites in the interior of a domatium. 29 Front view of a domatium. 30 Transverse section of a domatium. 31 Glandular trichomes inside of a domatium. 32 Stomata inside of a domatium. Detail highlighting the presence of stomata inside of a domatium. 33 Detection of phenolic compounds in a domatium. 34 Calcium crystals in a domatium. 26–28 Stereomicroscopy. 29–32 Scanning electron microscopy. 33 Light microscopy and i polarized light microscopy. Petiole (PT); Pore (PO); Trichomes (T); Internal chambers (IC); Glandular trichomes (GT); Mite (star); Stomata (arrow). Bars: 26 2 mm; 27, 28 1 mm; 29 200 µm; 30, 31, 32 500 µm; 33 100 µm; 34 50 µm
4 Discussion
The best-known types of domatia are structures found on the abaxial surfaces of leaves, usually in the region of the contact between the midrib and secondary veins on the edges of the leaves (Agrawal 1997; Moraes et al. 2009, 2011). According to Barros (1961), S. terebinthifolius present two types of domatia: (1) tuft of hair; (2) cavity shaped. However, only the second type was found and analyzed in this work. In this study, we utilized the terminology capsule/enclosure type of domatia to differentiate them from the cavity-shaped domatia that have already be unclassified between veins of leaves of other species of plants.
The ontogeny of the leaves domatia of S. terebinthifolius occurs along with the development of the leaves, suggesting that they contribute to the early establishment of the mutualistic relationships with ants or mites, thus providing protection throughout the life cycle of the leaf (Brouat and McKey 2000).
The development of domatia on only the second and third leaflets is a constant characteristic of all leaves of S. terebinthifolius, suggesting that the distribution of domatia is an intrinsic genetic characteristic of the species and not a response to environmental variables. Similar patterns of distribution of domatia were observed in Miconia tristis Spring. and M. doriana Cogn. (Melastomataceae) (Souza and Marquete 2000), and in Miconia sellowiana Naudin (Melastomataceae) (Larcher de Carvalho et al. 2012). However, the hypothesis that the distribution of domatia is part of a strategy for the mutualistic relationship cannot be ruled out.
The formation of domatia in S. terebinthifolius can be explained by the processes of development and folding of the rachis winged structures located at the base of the leaflets. This pattern of formation of domatia was described by Leroy et al. (2008), for pouch-shaped domatia of Hirtella physophora (Chrysobalanaceae). Structural alterations of tissue also contribute to the formation of domatia. In general, the compaction of parenchyma cells is one of the main causes of the folding of leaves during the domatia development (Santos and Almeida 1995; Nishida et al. 2006). Similar processes were encountered in the present study for the formation of capsule-/enclosure-type domatia of S. terebinthifolius. Another factor that can contribute to the folding of the winged structure is the existence of smaller and more numerous cells in the abaxial epidermis in comparison with the adaxial epidermis. Studies of development of leaves reported with the primary characteristic for the winding of the leaves (Paiva et al. 1993).
The internal organization of chambers in domatia can provide efficient shelter for species of mites, as confirmed by the presence of these arthropods within these chambers. Wiggers et al. (2005) showed that Lorryia formosa Cooreman (Tydeidae) is the most abundant species of mites in domatia of the S. terebinthifolius, representing 95.9% of the total mites observed. In addition, the same authors reported that mites were found in different stages of development, in the form of eggs, juveniles and adults, proving that domatia of the S. terebinthifolius are good environment for the shelter and for spawn mites. This characteristic may be part of a strategy for the mutualistic relationship in that provides a more comfortable and camouflaged shelter for the arthropods. This reinforces the hypothesis defended by several authors that the establishment of this relationship between plant and arthropods can be beneficial to both (Walter and O’Dowd 1992; Agrawal 1997; Agrawal et al. 2000; Norton et al. 2001; Romero and Benson 2004).
The occurrence of trichomes inside of domatia has already been described for other species of plants and is considered to serve as a physical defense for mites, as well as contribute to the diet of these organisms, since these trichomes can trap pollen and mold spores that the mites will eat (Romero and Benson 2005; Moraes et al. 2011). The presence of stomata inside of domatia indicates that they can act jointly with trichomes to establish a more humid environment, thus providing mites with protection from desiccation (O’Dowd and Willson 1991; Romero and Benson 2005).
Another strategy against herbivory of this type of domatia of S. terebinthifolius include the presence of phenolic compounds and calcium crystals. Herbivores mites were rarely found in domatia of the S. terebinthifolius (Wiggers et al. 2005) that can is related to large amounts of phenolic compounds and crystals present in the leaves. Phenolic compounds may inhibit herbivores since they are astringent substances that promote unpalatability to herbivores including phytophages (Roshchina and Roshchina 1993; Carvalho et al. 2000). But this species also have low levels of herbivory. In Cecropia peltata L. (Cecropiaceae), Val and Dirzo (2003) reported the presence of phenolic compounds in domatia, suggesting an inefficient interaction between the arthropods and plants in the protection against herbivory, since the plant is developing additional resources for protection from herbivory.
The presence of druse crystals can also be related to protection against herbivores. Some studies have linked increased amounts of calcium crystals on plants that suffer attacks from hervbivores (Molano-Flores 2001; Nakata 2003; Franceschi and Nakata 2005). The presence of calcium crystals in the domatia of S. terebinthifolius may be another indication of the investment these plants make in alternative forms of protection against herbivory.
Structural analysis showed that the stages of development and the architecture of domatia can provide a more efficient shelter for mites that in turn can to contribute to plant protection. Various plant strategies are related to the distribution of domatia, the development and folding of the lateral projections for the formation of domatia to shelter mites, and phenolic compounds and druse crystals for efficient protection against herbivory, thereby corroborating ecological studies. Furthermore, this study provides information that than can also contribute to the study of the systematic of this plant family and genus.
Change history
27 October 2017
The panels in Figs. 1–34 were incorrectly labelled in the initial online publication. The original article has been corrected.
References
Agrawal AA (1997) Do leaf domatia mediate a plant–mite mutualism? An experimental test of the effects on predators and herbivores. Ecol Entomol 22:371–376
Agrawal AA, Karban R (1997) Domatia mediates plant-arthropod mutualism. Nature 387:562–563
Agrawal AA, Karban R, Colfer G (2000) How leaf domatia and induced plant resistance affect herbivores, natural enemies and plant performance. Oikos 89:70–80
Barros MAA (1961) Domácias nas angiospermas—variações na forma e na localização. An E S A Luiz de Queiroz 18:132–146
Berlyn GP, Miksche JP (1976) Botanical microtechnique and cytochemistry. The Iowa State University Press, Iowa
Brouat C, McKey D (2000) Origin of caulinary ant domatia and timing of their onset in plant ontogeny: evolution of a key trait in horizontally transmitted ant—plant symbioses. Biol J Linn Soc 7:801–819
Carvalho JCT, Gosmann G, Schenkel EP (2000) Compostos fenólicos simples e heterosídicos. In: Simões CMO et al. (eds) Farmacognosia: da planta ao medicamento, 5th edn. Universidade Federal do Rio Grande do Sul, Florianópolis, pp 451–469
Chamberlain CJ (1932) Methods in plant histology. The University of Chicago Press, Chicago
Cronquist A (1981) An integrated system of classification of flowering plants. Columbia University Press, New York
Franceschi VR, Nakata PA (2005) Calcium oxalate in plants: formation and function. Annu Rev Plant Biol 56:41–71
Gerlach D (1984) Botanishe mikrotechnik. Georg Thieme Verlag, Stuttgart
Grostal P, O’Dowd DJ (1994) Plants, mites and mutualism: leaf domatia and the abundance and reproduction of mites on Viburnum tinus (Caprifoliaceae). Oecologia 97:308–315
Johansen DA (1940) Plant microtechnique. McGraw-Hill Book Co. Inc, New York
Kim BG, Ngondya IB (2010) Taxonomic importance of leaf domatia of the five species of the genus Cornus in Korea. J Agri Life Sci 44:79–82
Kreiter S, Tixier MS, Bourgeois T (2003) Do generalist phytoseiid mites (Gamasida: Phytoseiidae) have interactions with their host plants? Insect Sci Appl 23:35–50
Larcher de Carvalho L, Boeger MR, Brito AF, Goldenberg R (2012) Morfologia das domácias foliares de Miconia sellowiana Naudin (Melastomataceae). Biotemas 25:1–9
Leroy C, Jauneau A, Quilichini A, Dejean A, Orivel J (2008) Comparison between the anatomical and morphological structure of leaf blades and foliar domatia in the ant-plant Hirtella physophora (Chrysobalanaceae). Ann Bot 101:501–507
Leroy C, Jauneau A, Quilichini A, Dejean A, Orivel J (2010) Comparative structure and ontogeny of the foliar domatia in three neotropical myrmecophytes. Am J Bot 97:557–565
McLean RC, Cook WRL (1958) Plant science formulae. Macmillan & Company Ltd, London
Michelangeli FA (2000) A cladistic analysis of the genus Tococa (Melastomataceae) based on morphological data. Syst Bot 25:211–234
Molano-Flores B (2001) Herbivory and calcium concentration affect calcium oxalate crystal formation in leaves of Sida (Malvaceae). Ann Bot 88:387–391
Moraes TMS, Barros CFS, Silva Neto SJ, Gomes VM, Da Cunha M (2009) Leaf blade anatomy and ultrastructure of six Simira species (Rubiaceae) from the Atlantic Rain Forest, Brazil. Biocell 33:155–165
Moraes TMS, Rabelo GR, Alexandrino CR, Silva Neto SJ, Da Cunha M (2011) Comparative leaf anatomy and micromorphology of Psychotria species (Rubiaceae) from the Atlantic Rain Forest. Acta Bot Bras 25:178–190
Nakata PA (2003) Advances in our understanding of calcium oxalate crystal formation and function in plants. Plant Sci 164:901–909
Nickol MG (1998) Characterization of the domatia of Apollonias (Lauraceae) on the Atlantic Islands. Bol Mu Munic Funchal (História Natural) 5:273–281
Nishida S, Tsukaya H, Nagamasu H, Nozaki M (2006) A comparative study on the anatomy and development of different shapes of domatia in Cinnamomum camphora (Lauraceae). Ann Bot 97:601–610
Norton AP, English-Loeb G, Belden E (2001) Host plant manipulation of natural enemies: leaf domatia protect beneficial mites from insect predators. Oecologia 126:535–542
O’Dowd DJ (1989) Leaf domatia and mites on Australasian plants: ecological and evolutionary implications. Biol J Linn Soc 37:191–236
O’Dowd DJ, Willson MF (1991) Associations between mites and leaf domatia. Trends Ecol Evol 6:179–182
Paiva LV, Carvalho SA, Souza M (1993) Limpeza clonal da laranjeira ‘Seleta Folha Murcha’ através da microenxertia in vitro. Pesq Agropec Bras 28:1341–1344
Pemberton RW, Turner CE (1989) Occurrence of predatory and fungivorous mites in leaf domatia. Am J Bot 76:105–112
Richards LA, Coley PD (2012) Domatia morphology and mite occupancy of Psychotria horizontalis (Rubiaceae) across the Isthmus of Panama. Arthropod Plant Interact 6:129–136
Roda A, Nyrop J, English-Loeb G (2001) Leaf pubescence and two-spotter pider mite webbing influence phytoseiid behavior and population density. Oecologia 129:551–560
Romero GQ, Benson WW (2004) Leaf domatia mediate mutualism between mites and a tropical tree. Oecologia 140:609–616
Romero GQ, Benson WW (2005) Biotic interactions of mites, plants and leaf domatia. Curr Opin Plant Biol 8:436–440
Roshchina VV, Roshchina VD (1993) The excretory function of higher plants. Springer, Heidelberg
Santos M, Almeida SL (1995) Contribuição ao estudo morfológico e anatômico das domácias em espécies de Ocotea Aubl. (Lauraceae) da região sul do Brasil. Insula Rev Bot 24:73–97
Solís S, Ferrucci MS (2006) Comparative leaf morpho-anatomical studies of two South American species of Cardiospermum (Sapindaceae) with special reference to adaxial domatia. Blumea 51:153–164
Solís-Montero L, Rendón-Carmona N, Terrazas T, Ishiki M (2009) Los domacios de Mortoniodendron (Malvaceae s.l.). Brittonia 61:71–84
Souza RCOS, Marquete O (2000) Miconia tristis Spring e Miconia doriana Cogn. (Melastomataceae): anatomia do eixo vegetativo e folhas. Rodriguésia 51:133–142
Tillberg CV (2004) Cordia gerascanthus (Boraginaceae) produces stem domatia. J Trop Ecol 20:355–357
Val ED, Dirzo R (2003) Does ontogeny cause changes in the defensive strategies of themyrmecophyte Cecropia peltata? Plant Ecol 169:35–41
Walter DE, O’Dowd DJ (1992) Leaves with domatia have more mites. Ecology 73:1514–1518
Wiggers MS, Pratt PD, Tipping PW, Welbourn C, Cuda JP (2005) Within-plant distribution and diversity of mites associated with the invasive plant Schinus terebinthifolius (Sapindales: Anacardiaceae) in Florida. Environ Entomol 34:953–962
Acknowledgements
We thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Fundação de Amparo à Pesquisa do Rio de Janeiro (FAPERJ) for financial support; B. F. Ribeiro for technical work in the laboratory of LBCT/CBB/UENF. This study is a part of the MSc degree thesis of S. P. carried out at the Universidade Estadual do Norte Fluminense.
Author information
Authors and Affiliations
Corresponding author
Additional information
The original article has been revised: The figure panels in Figs. 1–34 were incorrectly labelled in the initial online publication. The original article has been corrected.
A correction to this article is available online at https://doi.org/10.1007/s40415-017-0420-1.
Rights and permissions
About this article
Cite this article
Pireda, S., Marques, J.d.B.C., Rabelo, G.R. et al. Structural analysis and developmental stages of domatia of Schinus terebinthifolius Raddi (Anacardiaceae). Braz. J. Bot 40, 1041–1048 (2017). https://doi.org/10.1007/s40415-017-0414-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-017-0414-z