Abstract
The morphological variation among 11 populations belonging to two endemic congener species of Neobuxbaumia, columnar cacti, was studied. One of our hypotheses was that N. mezcalaensis (Bravo) Backeb. with widespread distribution would show a higher variation of morphological characters and geographic–environmental variables compared with N. multiareolata (E.Y. Dawson) Bravo, Scheinvar & Sánchez-Mej. displaying a narrow distribution range, thus N. mezcalaensis will have a higher plasticity in some of its morphological traits. For each population, 41 morphological variables, three geographic, 10 climatic, and six soil properties were generated and analyzed by the simplified relative distance plasticity index, principal component, and regression analyses. The plasticity index across all populations for N. mezcalaensis showed more levels of plasticity than N. multiareolata in 12 variables as we expected. Principal component analysis explained 74% of the total variation. The first principal component, 47.41%, resulted from environmental differences and contributes to species separation. Difference in fruits traits and plant height was correlated with longitude (P < 0.0001) and elevation (P < 0.0001), thereby indicating a steep clinal decrease in fruit size and plant height from east to west as well as with the increase of elevation (P < 0.0001). Moreover, this variation negatively correlated with the mean temperature of the driest quarter (P < 0.0001) and annual precipitation (P < 0.0001), thereby indicating also a steep clinal decrease from east to west in N. mezcalaensis, the species with widespread distribution. The vegetative characters show that plasticity could be influenced by geographic, climatic, and edaphic variables, while the reproductive characters are probably genetically fixed because no significant variation was detected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Phenotypic plasticity is the ability of an organism to change its morphology and physiology in response to environmental variation (Schlichting1986), and perennial plants respond to environmental changes primarily by phenotypic plasticity (Rubio de Casas et al. 2007). Plant morphology variation is frequently associated with a combination of local environmental conditions and intrinsic genetic variation (Ellison et al. 2004). Moreover, this variation is associated with plastic responses to environmental gradients and the biogeographic history of each species (Heslop-Harrison 1964; Briggs 1969; Bruschi et al. 2003; Warren et al. 2005). Such morphological variation seems to be a prerequisite for the formation of new species (Losos and Glor 2003). In several plant species, a significant correlation between morphological characteristics and geographic and climatic variables (Zobel and van Buijtenen 1989; Bruschi et al. 2003; Sugiyama 2003; Ellison et al. 2004; Li et al. 2006; Ruedas et al. 2006; Hernández et al. 2007; Uribe-Salas et al. 2008; Pyakurel and Wang 2013) has been shown. Moreover, latitudinal or elevation gradients, with differences in climatic factors, may result in strong natural selection for local adaptation and ecological specialization for species with a broad distribution (Endler 1977) as compared with those with a narrow distribution (Karron 1987; Waller et al. 1987; Lavergne et al. 2004). However, modification of morphological characters depends on the extent of continuous or discrete variation along geographic range of species, which may result in clinal and ecotypic variation (Briggs 1969). Species that succeed in a rapidly changing climate are likely to have ample genetic variation for traits important in the new environment, broad ecological amplitudes, highly plastic phenotypes, short generation times or adaptations for long-distance seed dispersal (Vitt et al. 2010). The understanding of plasticity in diverse environments has been given importance in forecasting plant response to environmental change caused by global warming or anthropogenic disturbances (Valladares et al. 2007).
In the cactus family, around 1475 (73%) species display a narrow distribution, few populations, small populations size, low population density, habitat specificity or a combination of those factors (Rabinowitz et al. 1986) resulting in a high proportion of local endemism (Hernández and Godínez-Álvarez 1994; Ortega-Baes and Godíınez-Álvarez 2006). Few cacti species have a wide distribution (26%) and are frequently found in many habitats (Ortega-Baes and Godínez-Álvarez 2006). Groups of parental species located in different and distant geographic sites are rare. Although they have a common origin, the divergence among them may have occurred as a consequence of recent evolution of each group separately (Bevill and Louda 1999). Mexico concentrates nearly 45% of all known cactus species, of which around 80% are endemic (Ortega-Baes and Godínez-Álvarez 2006). Thus, Cactaceae is an ideal system to study the morphological variation either in species with wide or narrow distribution as well as for endemic species with high or low population densities. For example, most studies on cactus concern the morphological variation of wild species using univariate and regression analyses (Felger and Lowe 1967; Racine and Downhower 1974; Rundel 1977; Felker et al. 2002) as well as multivariate analyses (Chamberland 1997; Hicks and Mauchamp 2000; Muñoz Urias et al. 2008) have focus on species complexes. Multivariate analyses have primarily been applied to answer taxonomical questions at different hierarchical levels (Chamberland 1997; Baker and Johnson 2000; Schmalzel et al. 2004; Muñoz Urias et al. 2008; Arroyo-Cosultchi et al. 2010; Baker and Butterworth 2013; Sánchez et al. 2013; Vázquez-Benítez et al. 2016). In these studies, the multivariate analyses favor the recognition of few diagnostic morphological characters that allow differentiating species, especially when many variables are analyzed. In addition, PCA is also an appropriate statistical technique to examine the relationships among multiple intercorrelated environmental variables (McCune and Grace 2002). For example, other studies (Parker 1991; Ruedas et al. 2006; Bárcenas-Argüello et al. 2010; Ribeiro-Silva et al. 2016) applying univariate and multivariate analyses and mineralogy assessments were conducted to identify which of the edaphic properties (e.g., soil development, depth, texture, as well as calcium carbonate and phosphorous content) contribute the most to the establishment, early developmental stages, distribution, and abundance of different cactus species. In contrast, the presence/absence of many cactus species is associated with elevation or latitude constraints (Gurvich et al. 2014; Bauk et al. 2015), above which freezing temperatures reach or appear to reach the threshold (Jordan and Nobel 1981).
Neobuxbaumia Backeb. is an endemic genus to Mexico (Guzmán-Cruz et al. 2003; Ortega-Baes and Godínez-Álvarez 2006), which ranges from Tamaulipas in northeastern Mexico to Oaxaca in southwestern Mexico, associated with thorn scrubs and deciduous forests. The genus comprises branched or unbranched trees with stems that are stout, gray–green, and cylindrical. The genus is also characterized by numerous ribs and usually stiff or flexible spines; flowers that are commonly funnel-shaped, covered by small fleshy scales and bristles and mostly open at night; fruits that are globose or ovoid dehiscing by vertical slits; and seeds that are large to extremely large and black–brown with a glossy surface and periphery keeled (Bravo-Hollis 1978; Arroyo-Cosultchi et al. 2007). This genus has nine species with a distribution from broad to narrow (Bravo-Hollis 1978; Guzmán-Cruz et al. 2003). However, species limits have been controversial for some ones as for Neobuxbaumia multiareolata (E.Y. Dawson) Bravo, Scheinvar & Sánchez-Mej (Fig. 1) which was described originally as a variety of N. mezcalaensis (Bravo) Backeb (Fig. 2). Although, Bravo-Hollis et al. (1972) first recognized as a new distinct species, Hunt (2006) does not recognize this independent taxon, so treat it as a subspecies of N. mezcalaensis. However, Arroyo-Cosultchi et al. (2010) and Tapia et al. (2016) proposed that they represent independent species based on morphological evidences such as areole size and form, central spine length and distribution pattern of the radial spines, plus four reproductive characters (size of the flower, pericarpel, fruit, and seed) mentioned previously by other authors. These reproductive characters do not overlap between species and are diagnostic for each one (Arroyo-Cosultchi et al. 2010). One of the questions that arise was what makes that one of these two species to have a wide distribution and the other to be restricted to a few nearby hills at Tierra Colorada and Acahuizotlá at the Costa Chica-Rio Verde hydrological region? The most common species of Neobuxbaumia (N. mezcalaensis, N. polylopha (DC.) Backeb., and N. squamulosa Scheinvar & Sánchez-Mej.) are found in a wide range of habitats, while the endemic species (N. laui (P.V. Heath) D.R. Hunt, N. macrocephala (F.A.C. Weber ex K.Schum.) E.Y. Dawson, and N. multiareolata) have a limited narrow distribution (Guzmán-Cruz et al. 2003; Ruedas et al. 2006; Arroyo-Cosultchi et al. 2010). N. mezcalaensis is a keystone and critical for survival of many other plant and animal species in some communities in states of Guerrero and Puebla (Esparza-Olguín et al. 2005). It is found in a wide range of habitats and exhibits high levels of genetic and phenotypic diversity in most distribution areas (Esparza-Olguín 2005). In contrast, distribution of N. multiareolata is narrow and restricted to specific habitats resulting in a suitable and limited number of sites for species presence and low density.
N. mezcalaensis encounters widespread range of habitats in south of Mexico and different ecological conditions. Due to the heterogeneous nature of N. mezcalaensis habitats, it can therefore be assumed that the species has developed adaptive phenotypic plasticity to enable it to occupy all the natural range opposite to a species with narrow distribution like N. multiareolata. The aim of this study was to elucidate the influence of geographic, climatic, and edaphic gradients on morphological character variation among populations of N. mezcalaensis and N. multiareolata using the simplified relative distance plasticity index, multivariate, and regression analyses. We hypothesized that species with a widespread distribution range would show a higher level of morphological character variation compared with species displaying a narrow distribution range. Moreover, we predicted that vegetative characters would display more variation than reproductive ones that are less plastic. Additionally, we postulated that morphological variations in N. mezcalaensis may be associated with geographic location, soil properties, or climatic variables and that a null overlap exists for the morphological characters between both species.
2 Materials and methods
Species studied
– Neobuxbaumia mezcalaensis is a non-branched columnar cactus than may reach between two and 14 m height. Its flowers emerge along the stem between April and June and are white (occasionally green–red) with nocturnal anthesis; they are pollinated by bats and seeds dispersed by bats and birds between May and June (Valiente-Banuet et al. 1997). Populations are dense, with 1000–17,000 ind. ha−1 (Ruedas et al. 2006). This cactus species inhabits in thorny forest and tropical dry forest, on calcareous soils. It is commonly found between 486 and 2000 m elevation, with a broad geographic distribution range that partially covers the Tehuacán-Cuicatlán valley (Mexican states of Puebla and Oaxaca), as well as the Balsas River Basin (Guerrero and Puebla, southwest of Mexico). Neobuxbaumia multiareolata is an unbranched, columnar cactus that reaches between 2 and 7 m in height in the adult stage. Its red–purple flowers, which bloom between April and May, are borne both along the stem and in crowns near the stem tips. Neobuxbaumia multiareolata inhabits tropical dry forest, growing on cliff faces (Bravo-Hollis 1978; Arroyo-Cosultchi et al. 2010; Ojedzi-Aley and Rodríguez-López 2011), with a narrow elevation distribution between 192 and 450 m. This species is endemic to a small region in the Costa Chica-Rio Verde hydrological region in the state of Guerrero, Mexico.
Study area and fieldwork
– Eleven populations of N. mezcalaensis and N. multiareolata were sampled along their geographic distribution (Table 1; Fig. 3). A total of 20 mature individuals per population were randomly sampled (N = 220 individuals). For each set of 20 plants, we measured the length of 13 vegetative characters. Five areoles were selected, and in each one, the length of the central and lateral spines was counted and measured. Five mature flowers and fruits were collected and fixed (50% ethanol) per individual, and later in the laboratory, 28 reproductive variables were measured and counted (Table 2). The geographic location and elevation of each population site was georeferenced using a handheld GPS (global positioning system) unit accurate to ±50 m (Table 3). Soil properties were characterized from ca. 1 kg of soil collected at each population.
Laboratory procedures
– Climatic variables for the 11 population sites were obtained applying the bioclimatic modeling approach implemented in the BIOCLIM program (Houlder et al. 2000), which uses interpolated climatic surfaces estimated from a standard network of meteorological stations. The climatic surfaces or digital files obtained were generated using the thin-plate smoothing spline methods in the ANUSPLIN package (Hutchinson and Gessler 1994). Derivation of the bioclimatic profiles was based on selected-simple-matching thresholds and the limits throughout a grid of data points for each of the 10 bioclimatic parameters selected (Table 3).
The soil analyses were carried out in the Soil Fertility Laboratory at Colegio de Postgraduados. For each sample, pH and mean electric conductivity (E.C) (1:5 mmhos cmdS-m−1) were measured. Organic matter was assessed by determining the organic carbon decay of animal and vegetal tissues from the humic fraction on the mineralization process and from inert elementary carbon, via K2Cr2O7 oxidation using the Walkley–Black method. The phosphorus (P) (NH4O Ac1 N pH 7 Meq 100 g−1) was assessed using the Olsen method. Soil texture was determined via granulometric analysis based on the dispersion and sedimentation of soil particles, which is known as Bouyoucos’ density method (Etchevers 1988) (Table 3).
Statistical analyses
– A simplified relative distance plasticity index (RDPI) was carried on (Valladares et al. 2007). The RDPI measures the relative distances between mean values of the variables for all pairwise comparisons between populations of each species. The values of RDPIs were estimated after Valladares et al. (2007) and range from 0 (no plasticity) to 1 (maximal plasticity).
A data matrix was generated for the 12 morphometric characters (N = 220 individuals) which showed RDPI ≥ 0.1 (Table 2), 13 climatic and geographic variables, and six edaphic variables; all of them were analyzed using principal component analysis (PCA). Variables were transformed to logarithms (continuous characters), square root (counts), and arcsine (percentage or proportion characters) prior to performing the PCA. From variable loading of the PCA, we identified the most important morphometric and environmental variables given by their highest loading factors, which explained the global variation in the populations of both species. For those morphological variables highlighted by the first three principal components (PC1–PC3) as a result of the PCA, linear regressions of geographic–climatic variables against morphological variables of the PCA scores were made exclusively for the N. mezcalaensis populations. All analyses were performed with SAS (2008).
3 Results
Simplified Relative Distance Plasticity Index
– The RDPIs showed that the 41 morphological variables measured for both species have low plasticity values. RDPI values were lower for N. multiareolata than N. mezcalaensis (Table 2). For N. mezcalaensis seven vegetative variables (height, ribs height, areole length and width, number of central spines, central spine length, number of radial spines), four of fruit (fruit width, fruit volume, fruit spine length and podary width), and one floral variable (flower scales length) reached above 0.1, whereas only three variables (central spine length, fruit volume, and number of fruit areoles) had a value higher than 0.1 for N. multiareolata (Table 2).
Principal component analysis
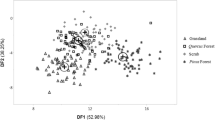
– The PCA including the 12 morphometric variables with RDPI values ≥0.1 (Table 2) plus environmental variables showed that three components accounted for 74.42% of the total variance of N. mezcalaensis and N. multiareolata. PC-1 explained 47.41% of the total variance with 15 variables of high loadings. One of these variables was morphometric (Nr. of radial spines); 12 variables were geographic and climatic (i.e., latitude, longitude, elevation, annual mean temperature, temperature seasonality, mean temperature of wettest quarter, mean temperature of driest quarter, mean temperature of warmest quarter, mean temperature of the coldest quarter, annual precipitation, precipitation of the wettest period, and precipitation seasonality), and two were edaphic (i.e., pH and electric conductivity). PC-2 explained 16.56% of the residual variance with five variables as follows: four morphometric (i.e., plant height, fruit width, spine fruit length, and podary fruit width) plus one edaphic (i.e., phosphorus of the soil). PC-3 explained 10.45% of the residual variance with three variables: areole length and width and mean diurnal range (Table 3). Figure 4 shows that PC-1 contributed mostly to separate the two species mainly due to their geographic distribution and their associated climatic variables, while PC-2 and PC-3 contributed to the separation among populations into N. mezcalaensis due to morphometric variables.
Tri-plot resulting from principal component analysis (1–3 PC) of Neobuxbaumia mezcalaensis (solid symbols, 9 populations) and Neobuxbaumia multiaereolata (open symbols, 2 populations). Las Estacas (filled black up triangle), Colonia San Martín, (filled black down triangle), Santiago Chazumba (filled black square), Atenango del Río (filled black diamond), Petlalcingo (filled black star), Mezcalas (filled black plus), Casa Verde (filled black circle), Xochipala (filled black right pointer) and Zumpango del Río (filled black left pointer), Tierra Colorada (open white circle), and La Venta (open white square)
Regression analyses
– For N. mezcalaensis, a multiple regression analysis between PC-2 (fourth morphological characters) against geographic and climatic variables with higher loading values for PC1 were performed. The morphological variables (associated with fruit size and plant height) corresponding to the second principal component (PC-2) displayed a significant correlation between the population average score and the longitude and elevation of the populations (r = −0.80; P < 0.0001, Fig. 5a; r = 0.58; P < 0.0001, Fig. 5b). This finding suggests that fruit characters and plant height decrease clinally from east–west and positively with elevation. According to these results, the populations from the Tehuacán-Cuicatlán valley would have larger fruits than the western populations (i.e., Balsas River Basin). The PC-2 was also significantly correlated with mean temperature of the driest quarter (r = −0.51; P < 0.0001; Fig. 5c), mean temperature of the coldest quarter (r = −0.46; P < 0.0001; Fig. 5d), and annual precipitation (r = −0.47; P < 0.0001; Fig. 5e).
Linear relationships between geographic locations, climatic variables, and PC2 morphological characters of Neobuxbaumia mezcalaensis. a Longitude, b elevation, c mean temperature of the driest quarter, d mean temperature of the coldest quarter, e annual precipitation. Neobuxbaumia mezcalaensis populations: Las Estacas (filled black up triangle), Colonia San Martín (filled black down triangle), Santiago Chazumba (filled black square), Atenango del Río (filled black diamond), Petlalcingo (filled black star), Mezcala (filled black plus), Casa Verde (filled black circle), Xochipala (filled black right pointer), and Zumpango del Río (filled black left pointer). In all cases, the response variable is the second principal axis (fruit characteristics and plant height) (see Table 3 for all loading)
4 Discussion
Species with wide distribution ranges usually show larger morphological variability compared with species with narrow distribution (Rapson and Maze 1994; Lavergne et al. 2004). This assertion was supported with the RDPI values obtained for N. mezcalaensis. Moreover, most reproductive characters showed less plasticity than vegetative ones and those variables used to distinguish species by Arroyo-Cosultchi et al. (2010) as for flower length, tube length, seed area, and podaria fruit length showed very low RDPI. Abiotic variables may have a significant effect on the phenotype of a species. This work is the first of its kind to study the relationship between the morphological variables and the geographic, edaphic, and climatic variables. The results showed that the environmental variables did contribute to the expressed plasticity of some characters of N. mezcalaensis and N. multiareolata, although they are different between species (see below) and the floral ones showed less variation in both taxa.
This study indicates that individuals of N. mezcalaensis modify their fruit characters and plant height along a longitudinal gradient. The pronounced east–west longitudinal clinal variation in morphological fruit characters indicates a strong phenotypic variation among populations of N. mezcalaensis in response to a climatic gradient of temperature and precipitation (Figs. 2, 3). Three relevant climatic variables: mean temperature of the driest quarter, mean temperature of the coldest quarter, and annual precipitation, showed a significant variability along the longitudinal gradient. The pattern of morphological variation suggests that the fruit characters are larger in the east, where sites are drier and cooler (i.e., Tehuacán-Cuicatlán), whereas the size of fruits and plant height are gradually smaller toward the west, where population sites are wetter and warmer (i.e., Balsas River Basin; Zopilote Canyon). These morphological attributes, which gradually change along with the longitudinal gradient, elevation, and precipitation, also appear to mean the response to other environmental factors such as local rainfall, soil nutrient deficiency, and extreme temperatures. Fruit size is among the reproductive characters that have been found to covary more often with longitudinal, elevation, and environmental variables in several cactus species (Echinocactus polycephalus (Engelm. & Bigelow) complex, Chamberland 1997; Opuntia ficus-indica ((L.) Mill.), Felker et al. 2002; Ferocactus cylindraceus subsp. cylindraceus (Engelm.) Orcutt, Carnegiea gigantea (Engelm.) Britton & Rose, Lophocereus schottii (Engelm.) Britton & Rose, and Stenocereus thurberi (Engelm.) Buxb., Gibson and Nobel 1986; Coryphantha robustispina (Ant.Schott ex Englem.) Britton & Rose, Schmalzel et al. 2004). Therefore, the current results suggest that the local environmental variations play an important role in shaping local plant morphology.
In comparison with previous studies conducted on other cactus species (Felger and Lowe 1967; Rundel 1977; Chamberland 1997), our results reveal that the strong variation in the vegetative characters is a plastic response to the diverse environments where populations of N. mezcalaensis occurred along their natural distribution. Clinal variation may not only result from selection along environmental gradients but may also be influenced by the patterns of gene flow among populations (Endler 1977). For example, high rates of gene flow among populations located in geographic proximity would contribute to a stronger phenotypic similarity than would environmental variables. In contrast, clines may also be the product of historical isolation and the divergence of populations with subsequent expansion and contact (Endler 1977). The results of ongoing population genetic studies of N. mezcalaensis may help to discern between these alternatives. A study of isozyme variation in N. mezcalaensis showed a high and low degree of genetic variation between populations and within populations, respectively (Esparza-Olguín 2005), and no information exists for N. multiareolata, but lower genetic variation is expected.
The higher vegetative and fruit morphological variability were found of N. mezcalaensis than N. multiareolata, the RDPI of both species revealed that some morphological characters showed plasticity values close to zero. Clinal variation appears to be common for columnar cactus, considering that their cross-pollination and seed dispersion occur primarily via bats and birds, agents which are able to move great distances (15 km per night and 200 km per week) (Rojas-Martínez et al. 1999; Valiente-Banuet et al. 2004; Arias-Cóyotl et al. 2006).
As a possible consequence of their distribution range, the results clearly show that the types of habitats in which N. mezcalaensis may be found are more heterogeneous than the habitats in which N. multiareolata are distributed. The PCA allowed us to identify the geographic, climatic, and edaphic variables that are partially responsible for these patterns. In particular, N. mezcalaensis is found in habitats with alkaline water potential (7.8–8.3), lower electric conductivity percentage (15–31%), lower organic matter (4.6–16.6), lower phosphorus content (2–9), and soils with various textures. In contrast, N. multiareolata occupies habitats with acidic water potential (6.6–6.8), high electric conductivity percentage (32–35%), high organic matter (31.1–53.1), high phosphorus content (32–42), and loam soil texture as compared to N. mezcalaensis. Distribution of different cactus species has been associated with the diversity of soil properties, as they are affected by water and nutrient uptake (Parker 1991; Ruedas et al. 2006; Ribeiro-Silva et al. 2016). The populations of N. mezcalaensis in the Tehuacán-Cuicatlán valley inhabit higher elevation sites, with lower precipitation and soil with a higher content of organic matter and phosphorus compared with the populations in the Zopilote Canyon, which are located at lower elevations, higher temperature, and precipitation levels and poorer soils (i.e., low organic matter and phosphorus levels) as other studies have indicated (Ruedas et al. 2006) for the same species. Concerning the soil, most of the sites occupied by N. mezcalaensis are of sandy texture, although no other difference was observed with other columnar cacti (Hernández et al. 2007).
N. multiareolata grows in tropical dry forests, inhabiting cliff faces (Bravo-Hollis 1978; Arroyo-Cosultchi et al. 2010) between elevations of 192 and 450 m, with higher annual precipitation (1218–1400 mm), lower temperature seasonality (29–30), and distinctive soils features (see above) than N. mezcalaensis. The combination of these soil properties and climatic factors contributes to the narrow distribution and endemicity of N. multiareolata to a small region (Tierra Colorada and Acahuizotlá at the Costa Chica-Rio Verde hydrological region) in the state of Guerrero, Mexico. The geographic, climatic, and soil variables are clearly strong determinants of the presence and isolated distribution of both cactus species, thereby partially explaining their lack of sympatric distribution. Recent speciation or multiple origins for N. multiareolata may easily explain the morphological similarity to N. mezcalaensis. It is possible that a recent speciation event and hybridization have occurred (Smith and Pham 1996).
In conclusion, this study suggests that morphological differences between N. mezcalaensis and N. multiareolata across geographic and environmental gradients are the result of a combination of climatic and edaphic variables. It is worth noting that N. multiareolata, the species with the narrow distribution range, shows lower level of morphological character variation than to N. mezcalaensis, the species with the broader distribution range, which is similar with a study in two congenetic bunch grasses Achnatherum (=Oryzopsis) with different distribution (Rapson and Maze 1994). Therefore, we suggest that N. multiareolata could have originated from a recent speciation of another species of wider distribution, in this case N. mezcalaensis.
Both species show geographic–climatic ranges totally contrasting. N. mezcalaensis has greater amplitude for them and does not overlap with those of N. multiareolata. This explains that the first has been adapted to grow in a greater number of environments and shows evidence that both species have well-defined environmental niches.
Environmental factors tend to have greater influence on vegetative characters than on reproductive ones (Jones 1988). For instance, the flowers were consistent with a bat pollination syndrome (i.e., night flowering, nectar production, and large quantities of pollen) (Valiente-Banuet et al. 1997) and can show a slow phenotypic plasticity in response to spatiotemporal variation in both biotic (i.e., visitors assemblage) and abiotic conditions (i.e., resources availability; Herrera 1993; Rojas-Sandoval and Meléndez-Ackerman 2009); floral variation for some characters showed a conservative pattern of evolution between sister branches of the phylogeny (Martínez-Peralta et al. 2014). There is a clinal morphological variation in N. mezcalaensis from east to west associated primarily with longitude and elevation gradient, which mainly impacts fruit characters and plant size.
References
Arias-Cóyotl E, Stoner K, Casas A (2006) Effectiveness of bats as pollinators of Stenocereus stellatus (Cactaceae) in wild, managed in situ, and cultivated populations in La Mixteca Baja, central Mexico. Am J Bot 93:1675–1683. doi:10.3732/ajb.93.11.1675
Arroyo-Cosultchi G, Terrazas T, Arias S, López-Mata L (2007) Seed morphology in Neobuxbaumia (Cactaceae). Bol Soc Bot Mex 81:17–25
Arroyo-Cosultchi G, Terrazas T, Arias S, López-Mata L (2010) Delimitación de Neobuxbaumia mezcalaensis y N. multiareolata (Cactaceae) con base en análisis numéricos. Bol Soc Bot Mex 86:53–64
Baker M, Johnson R (2000) Morphometric analysis of Escobaria sneedii var. sneedii, E-sneedii var. leei, and E-guadalupensis (Cactaceae). Syst Bot 25:577–587. doi:10.2307/2666722
Baker MA, Butterworth CA (2013) Geographic distribution and taxonomic circumscription of populations within Coryphantha section Robustispina (Cactaceae). Am J Bot 100:984–997. doi:10.3732/ajb.1200619
Bárcenas-Argüello ML, Gutiérrez-Castorena MC, Terrazas T, López-Mata L (2010) Rock-soil preferences of three Cephalocereus (Cactaceae) species of tropical dry forests. Soil Sci Soc Am 74:1374–1382. doi:10.2136/sssaj2009.0310
Bauk K, Pérez-Sánchez R, Zeballos SR, Laura Las Peñas M, Flores J, Gurvich DE (2015) Are seed mass and seedling size and shape related to altitude? Evidence in Gymnocalycium monvillei (Cactaceae). Botany 93:529–533. doi:10.1139/cjb-2015-0026
Bevill R, Louda S (1999) Comparisons of related rare and common species in the study of plant rarity. Conserv Biol 13:493–498. doi:10.1046/j.1523-1739.1999.97369.x
Bravo-Hollis H (1978) Las cactáceas de México, vol 1. Universidad Nacional Autónoma de México, México
Bravo-Hollis H, Scheinvar L, Sánchez-Mejorada H (1972) Estudio comparativo del género Neobuxbaumia Backberg. IV Neobuxbaumia multiareolata. Cact Suc Mex 18:59–67
Briggs D (1969) Plant variation and evolution. Mc Graw Hill, New York
Bruschi P, Grossoni P, Bussotti F (2003) Within- and among-tree variation in leaf morphology of Quercus petraea (Matt.) Liebl. natural populations. Trees 17:164–172. doi:10.1007/s00468-002-0218-y
Chamberland M (1997) Systematics of the Echinocactus polycephalus complex (Cactaceae). Syst Bot 22:303–313
Ellison AM, Buckley HL, Miller TE, Gotelli NJ (2004) Morphological variation in Sarracenia purpurea (Sarraceniaceae): geographic, environmental, and taxonomic correlates. Am J Bot 91:1930–1935. doi:10.3732/ajb.91.11.1930
Endler JA (1977) Geographic variation, speciation, and clines. Princeton University Press, Princeton
Esparza-Olguín L (2005) Estudio comparativo de tres especies de cactáceas columnares del género Neobuxbaumia que difieren en su nivel de rareza: un enfoque genético-demográfico. Dissertation Universidad Nacional Autónoma de México, Mexico City
Esparza-Olguín L, Valverde T, Mandujano MC (2005) Comparative demographic analysis of three Neobuxbaumia species (Cactaceae) with differing degree of rarity. Popul Ecol 47:229–245
Etchevers DJ (1988) Diagnóstico de la fertilidad del suelo. Colegio de Postgraduados, Estado de México, Montecillo
Felger RS, Lowe CH (1967) Clinal variation in the surface-volume relationships of the columnar cactus Lophocereus schottii in Northwestern Mexico. Ecology 48:530–536. doi:10.2307/1936495
Felker P, Soulier C, Leguizamon G, Ochoa J (2002) A comparison of the fruit parameters of 12 Opuntia clones grown in Argentina and the United States. J Arid Environ 52:361–370. doi:10.1006/jare.2002.1001
Gibson CA, Nobel PS (1986) The cactus primer. Harvard University Press, Cambridge
Gurvich DE, Zeballos SR, Demaio PH (2014) Diversity and composition of cactus species along an altitudinal gradient in the Sierras del Norte Mountains (Córdoba, Argentina). S Afr J Bot 93:142–147. doi:10.1016/j.sajb.2014.03.018
Guzmán-Cruz U, Arias S, Dávila PD (2003) Catálogo de cactáceas mexicanas. Universidad Nacional Autónoma de México, México
Hernández HM, Godínez-Álvarez H (1994) Contribución al conocimiento de las cactáceas mexicanas amenazadas. Acta Bot Mex 26:33–52
Hernández M, Terrazas T, Delgado-Alvarado A, Luna Cavazos M (2007) Los estomas de Myrtillocactus geometrizans (Mart. ex. Pfeiff.) Console (Cactaceae): variación en su área de distribución. Rev Fitotec Mex 30:235–240
Herrera C (1993) Selection on floral morphology and environmental determinants of fecundity in a hawk moth- pollinated violet. Ecol Mon 63:251–275. doi:10.2307/2937101
Heslop-Harrison J (1964) Forty years of genecology. In: Cragg JB (ed) Advances in ecological research, vol 2. Academic Press, New York, pp 159–247
Hicks D, Mauchamp A (2000) Population structure and growth patterns of Opuntia echios var. gigantea along an elevational gradient in the Galapagos islands. Biotropica 32:235–243. doi:10.1111/j.1744-7429.2000.tb00466.x
Houlder D, Hutchinson ME, Nix HA, McMahon JP, Ord KD (2000) ANUCLIM Users Guide. The Australian National University, Canberra
Hunt DR (2006) The new cactus lexicon. International Cactaceae Systematics Group, Milborne Port
Hutchinson M, Gessler P (1994) Splines-more than just a smooth interpolator. Geoderma 62:45–67
Jones SBJ (1988) Sistemática vegetal. Mc Graw Hill, Mexico City
Jordan PW, Nobel PS (1981) Seed establishment of Ferocactus acanthodes in relation to drought. Ecology 62:901–906. doi:10.2307/1936987
Karron J (1987) A comparison of levels of genetic polymorphism and self-compatibility in geographically restricted and widespread plant congeners. Evol Ecol 1:47–58. doi:10.1007/BF02067268
Lavergne SJ, Thompson JD, Garnier E, Debussche M (2004) The biology and ecology of narrow endemic and widespread plants: a comparative study of trait variation in 20 congeneric pairs. Oikos 107:505–518. doi:10.1111/j.0030-1299.2004.13423.x
Li C, Zhang X, Liu X, Luukkanen O, Berninger F (2006) Leaf morphological and physiological responses of Quercus aquifolioides along an altitudinal gradient. Silva Fenn 40:5–13. doi:10.14214/sf.348
Losos J, Glor R (2003) Phylogenetic comparative methods and the geography of speciation. Trends Ecol Evol 18:220–227. doi:10.1016/S0169-5347(03)00037-5
Martínez-Peralta C, Molina-Freaner F, Golubov J, Vázquez-Lobo A, Mandujano MC (2014) A comparative study of the reproductive traits and floral morphology of a genus of geophytic cacti. Int J Plant Sci 175:663–680. doi:10.1086/676302
McCune B, Grace JB (2002) Analysis of ecological communities. MjM Software Design, Gleneden Beach
Muñoz Urias A, Palomino-Hasbach G, Terrazas T, García-Velázquez A, Pimienta-Barrios E (2008) Variación anatómica y morfológica en especies y entre poblaciones de Opuntia en la porción sur del Desierto Chihuahuense. Bol Soc Bot Mex 83:1–11
Ojedzi-Aley A, Rodríguez-López R (2011) Estudio florístico y vegetación de la localidad de Acahuizotla, Municipio de Chilpancingo de Los Bravo, Guerrero, México. Thesis, Universidad Autónoma de Guerrero, Iguala
Ortega-Baes P, Godínez-Álvarez H (2006) Global diversity and conservation priorities in the Cactaceae. Biodiv Conserv 15:817–827. doi:10.1007/s10531-004-1461-x
Parker K (1991) Topography, substrate, and vegetation patterns in the northern Sonoran desert. J Biogeogr 18:151–163
Pyakurel A, Wang JR (2013) Leaf morphological variation among paper birch (Betula papyrifera Marsh.) genotypes across Canada. Open Ecol J 3:284–295
Rabinowitz D, Cairns S, Dillon T (1986) Seven forms of rarity and their frequency in the flora of the British Isles. In: Soulé ME (ed) Conservation biology: the science of scarcity and diversity. Sinauer Associates, Sunderland, pp 182–204
Racine CH, Downhower JF (1974) Vegetative and reproductive strategies of Opuntia (Cactaceae) in the Galapagos Islands. Biotropica 6:175–186
Rapson GL, Maze J (1994) Variation and integration in the rare grass Achnatherum (Oryzopsis) hendersonii: phenotypic comparison with parapatric common congeners. Can J Bot 72:693–700
Ribeiro-Silva S, Medeiros MB, Lima VVF, Peixoto MR, Aona LYS (2016) Patterns of Cactaceae species distribution in a protected area in the semiarid Caatinga biome of north-eastern Brazil. Edinb J Bot 73:157–170
Rojas-Martínez A, Valiente-Banuet A, Arizmendi MC, Alcántara-Eguren A, Arita H (1999) Seasonal distribution of the long-nosed bat (Leptonycteris curasoae) in North America: Does a generalized migration pattern really exist? J. Biogeogr 26:1065–1077. doi:10.1046/j.1365-2699.1999.00354.x
Rojas-Sandoval J, Meléndez-Ackerman E (2009) Pollination biology of Harrisia portoricensis (Cactaceae), an endangered Caribbean species. Am J Bot 96:2270–2278. doi:10.3732/ajb.0900026
Rubio De Casas R, Vargas P, Pérez-Corona E, Manrique E, Quintana J, García-Verdugo C, Balaguer L (2007) Field patterns of leaf plasticity in adults of the long-lived evergreen Quercus coccifera. Ann Bot 100:325–334. doi:10.1093/aob/mcm112
Ruedas M, Valverde T, Zavala-Hurtado JA (2006) Analysis of the factors that affect the distribution and abundance of three Neobuxbaumia species (Cactaceae) that differ in their degree of rarity. Acta Oecol 29:155–164. doi:10.1016/j.actao.2005.09.002
Rundel P (1977) Population variability in the genus Trichocereus (Cactaceae) in central Chile. Plant Syst Evol 127:1–9
Sánchez D, Arias S, Terrazas T (2013) Análisis morfométrico de las especies de Echinocereus sección Triglochidiati (Cactaceae) en México. Brittonia 65:368–385. doi:10.1007/s12228-012-9285-6
SAS Institute (2008) SAS/STAT User’s Guide. 9.2 edition. SAS Institute Inc, North Carolina
Schlichting C (1986) The evolution of phenotypic plasticity in plants. Annu Rev Ecol Syst 17:667–693. doi:10.1146/annurev.es.17.110186.003315
Schmalzel R, Nixon R, Best A, Tress J Jr (2004) Morphometric variation in Coryphantha robustispina (Cactaceae). Sys Bot 29:553–568
Smith J, Pham T (1996) Genetic diversity of the narrow endemic Allium aaseae (Alliaceae). Am J Bot 83:717–726
Sugiyama S (2003) Geographical distribution and phenotypic differentiation in populations of Dactylis glomerata L. in Japan. Plant Ecol 169:295–305
Tapia H, Arias S, Yañez-Espinosa L, Terrazas T (2016) El uso de espinas del tallo en la identificación de las especies de Neobuxbaumia (Cactaceae). Rev Mex Biodiv 87:288–300. doi:10.1016/j.rmb.2016.04.006
Uribe-Salas D, Sáenz-Romero C, González-Rodríguez A, Téllez-Valdéz O, Oyama K (2008) Foliar morphological variation in the white oak Quercus rugosa Née (Fagaceae) along a latitudinal gradient in Mexico: potential implications for management and conservation. For Ecol Manage 256:2121–2126. doi:10.1016/j.foreco.2008.08.002
Valiente-Banuet A, Rojas-Martíınez A, Arizmendi MC, Dávila P (1997) Pollination biology of two columnar cacti (Neobuxbaumia mezcalaensis and Neobuxbaumia macrocephala) in the Tehuacan Valley, Central Mexico. Am J Bot 84:452–455
Valiente-Banuet A, Molina-Freaner F, Torres A, Arizmendi MC, Casas A (2004) Geographic differentiation in the pollination system of the columnar cactus Pachycereus pecten-aboriginum. Am J Bot 91:850–855. doi:10.3732/ajb.91.6.850
Valladares F, Gianoli E, Gómez J (2007) Ecological limits to plant phenotypic plasticity. New Phytol 176:749–763. doi:10.1111/j.1469-8137.2007.02275.x
Vázquez-Benítez B, Arias S, Cervantes-Sandoval A (2016) Variación morfológica de Coryphantha (Cactaceae): un enfoque multivariado. Acta Bot Mex 116:21–47
Vitt P, Havens K, Kramer A, Sollenberger D, Yates E (2010) Assisted migration of plants: changes in latitudes, changes in attitudes. Biol Conserv 143:18–27. doi:10.1016/j.biocon.2009.08.015
Waller DM, O’Malley DM, Gawler SC (1987) Genetic variation in the extreme endemic Pedicularis furbishiae, (Scrophulariaceae). Conserv Biol 1:335–340. doi:10.1111/j.1523-1739.1987.tb00053.x
Warren C, Tausz M, Adams M (2005) Does rainfall explain variation in leaf morphology and physiology among populations of red ironbark (Eucalyptus sideroxylon subsp. tricarpa) grown in a common garden? Tree Physiol 25:1369–1378. doi:10.1093/treephys/25.11.1369
Zobel BJ, Van Buijtenen P (1989) Wood variation: its causes and control. Springer, New York
Acknowledgements
We thank the Mexican Council of Science and Technology (CONACyT) for supporting this research (33064-V to T.T.) and granting a scholarship (190345 to A.C.G). We would like to thank C. Catalán, L. Sánchez and D. Martínez for technical support and field assistance, Oswaldo Tellez for providing the climatic data, and Julio César Montero Rojas for art work. Two anonymous reviewers are acknowledged for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arroyo-Cosultchi, G., Arias, S., López-Mata, L. et al. Morphological plasticity of an endemic widespread columnar cactus and its congener. Braz. J. Bot 40, 1029–1040 (2017). https://doi.org/10.1007/s40415-017-0399-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-017-0399-7