Abstract
Introduction
The effects of resistance exercise on vascular function are unclear.
Aim
To investigate the acute haemodynamic (blood pressure and augmentation index) and rate of perceived exertion (RPE) response to two types of resistance exercises of equal workload—a set of unilateral 35% of one repetition maximum (1RM) quadriceps extension and a set of unilateral 70% 1RM quadriceps extension.
Methods
Twenty two young healthy males completed both exercises on separate days. Heart rate, central and peripheral systolic and diastolic blood pressure (BP), augmentation pressure, augmentation index (AIx), augmentation index at a heart rate of 75 beats per minute (AIx75), and RPE were measured using applanation tonometry before exercise, immediately after exercise, 5 min after exercise and 15 min after exercise.
Results
AIx75 was significantly lower 5 min after exercising at 35% of 1RM than 70% of 1RM. Systolic blood pressure was significantly lower at 5 min post exercise for both intensities. There was no significant difference in RPE between conditions or time points.
Conclusions
Results suggest that changes in blood pressure and augmentation index vary depending on the intensity of resistance exercise regardless of the volume of exercise carried out. Changes in AIx75 in response to resistance exercise may be independent of changes in BP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Traditionally, brachial systolic blood pressure and pulse pressure have been measured to identify those at risk of CVD, however the focus has now moved towards central aortic blood pressures and indices of arterial stiffness, which are considered more accurate predictors of cardiovascular disease and mortality [1,2,3]. This is reflected in the guidelines devised by the European Hypertension Society which recommend measuring indices of arterial stiffness in adults, adolescents and children [4].
Arterial stiffness is described as the “reduced capability of an artery to expand and contract in response to pressure changes” [5]. Stiffening of the arteries is related to hypertension, atherosclerosis and the ageing process [6]. A decrease in the elastic properties of arteries diminishes their buffering ability, leading to an increase in cardiac workload. Augmentation index (AIx) and augmentation pressure (AP) are indicators of arterial stiffness. AIx is also influenced by several non-stiffness related measurements such as heart rate, height, age, ejection duration and peripheral vascular tone. AIx is a measure of the interaction of the forward and reflected traveling pressure wave arriving in the central arteries, and an independent prognostic indicator of cardiovascular and all cause morbidity and mortality in disease populations [1]. The use of AIx as an indicator of central vascular stiffness has been questioned as it is effected by factors which are independent of vascular compliance and mathematically it’s calculation may not enable changes in pressure waves to be detected where present [7]. Despite these limitations AIx, can still yield information of vascular functioning as a measure of the timing and amplitude of wave reflection. Little is known on whether resistance exercise effects AIx.
The beneficial effects of exercise in the prevention and treatment of CVD are well known. The role of aerobic exercise in the prevention and reduction of arterial stiffness has also been well documented [8,9,10,11,12]. Conversely, there is a lack of clarity on the effect resistance exercise has on vascular function. Resistance exercise has been shown to increase stiffness of the arteries both acutely and chronically [13, 14]. Resistance exercise has also been shown to improve endothelial function [15,16,17] and reduce arterial stiffness in some studies [18, 19]. A review stated that although the effects of resistance training have not been studied as extensively as aerobic exercise, resistance exercise has a role to play in improving endothelial function [11]. Conversely a meta-analysis stated that resistance exercise does not effect PWV or AIx [12]. Arterial stiffness is known to return to baseline shortly after an acute bout of resistance exercise [13], the effects of an acute rise in blood pressure and/or changes in arterial wave reflections, in particular on patient populations is not fully understood. It is therefore prudent to examine the effects of resistance exercise on blood pressure and indices of arterial stiffness such as augmentation index into the immediate recovery phase.
Differing exercise prescriptions between studies examining the effects of resistance exercise on arterial stiffness may explain the lack of consistency in results. There are many methods of resistance exercise prescription. Such prescriptions can target improvements in muscular strength (high intensity low repetition), endurance (low intensity high repetition), or power (increased rate of force development) [20]. There is a paucity of literature comparing the acute haemodynamic and RPE effects of different resistance exercise prescriptions. Much of the research on the effects of resistance exercise on vascular function has examined whole body or high intensity resistance exercise focussing on strength rather than low intensity resistance exercise which focuses on muscular endurance. In hospital and community settings, rehabilitation often aims to increase muscular endurance in order to enable patients to carry out activities of daily living without undue exertion. Rate of perceived exertion (RPE) is often used to determine training intensity and guide exercise prescription. RPE has been shown to be an effective tool to prescribe and monitor exercise prescription in clinical and athlete populations and is related to physiological markers such as heart rate, lactate and oxygen consumption [21, 22]. Beyond exercise prescription and despite its subjectivity, the validity of RPE has been demonstrated in the diagnostic screening of athletes and the general population too. It has shown robust consistency in exercise stress testing and both aerobic and resistance exercises independently of heart rate and exercise intensity [23]. Nevertheless, whether there is a relationship between blood pressure, AIx and RPE is unknown.
The aim of this study was to compare the acute haemodynamic (blood pressure and augmentation index) and perceived exertion response, using the Borg CR-10 scale, to two resistance exercise conditions of the same workload at baseline, immediately after exercise, 5 min into recovery and 15 min into recovery. Three time points were chosen as the body’s response to resistance exercise is acute in nature and changes in the immediate recovery phase [24]. A single leg extension exercise at low to moderate intensity was chosen for examination as a realistic representation of exercise that would form part of a patient rehabilitation program.
2 Methods
The work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. Ethical approval was granted by the relevant hospital Research Ethics Committee. This study used a crossover design. On separate days within a 14 days period, participants completed baseline testing and exercise conditions detailed in Table 1. Although quite a low intensity, unilateral knee extension at 35% of 1RM is considered a “light” resistance exercise rather than aerobic exercise due to the activity being restricted to one muscle group [20]. The order of the exercise conditions was randomised such that at the first visit anthropometric data and RM was measured, at the second visit participants performed either 35% RM or 70% RM, and at the third visit participants performed the remaining exercise condition (i.e., 35% RM or 70% RM). The order of the exercise condition was determined by tossing a coin. This process resulted in a repeated measures counterbalanced design. The work load and time frame for each condition was standardised such that only the intensity (weight lifted) and rate at which the repetitions were carried out changed between conditions (see Table 1). Participants were instructed to complete one set of each condition. The rate was controlled by a research assistant who instructed the participant when to lift the weight. The work load and rate were chosen to ensure that equal total amounts of work were carried out between conditions. The weight was designed to be within a realistic range for patient populations and limited by the exercise equipment available. As seen in Table 1, this led to one condition (35% of 1RM) being clearly within the range of muscular endurance training (or light resistance exercise) while the other (70% of 1RM) was closer to hypertrophy or strength development [20]. Both conditions would be typical of exercises used in clinical rehabilitation settings such as cardiac rehabilitation.
Only males aged between 18 and 35 were eligible to take part in this pilot study. These criteria served to reduce variability between participants. Participants were recruited through posters pinned to university notice boards. To take part in this study participants were required to provide written informed consent, have no contraindications to exercise, and be available for testing on three separate occasions. None of the participants were taking prescribed medications and all were free of known cardiorespiratory disease. The physical activity readiness questionnaire (PAR-Q) was used as a screening tool to ensure all participants were safe to partake in exercise. During the first visit quadriceps strength, resting blood pressure and anthropometric measurements were taken. Participants were also shown how to exhale during muscle contraction in order to avoid the Valsalva manoeuvre. During the subsequent visits participants completed one of the exercise conditions detailed in Table 1.
A leg extension bench was used to determine quadriceps strength. The NSCA protocol was used to determine 1RM of the non-dominant leg with a minimal increment available on the leg extension bench of 5 kg ([25]). Resting measurements were taken following a 10 min seated rest. All blood pressure measurements were taken as per recommended guidelines [26], using an OMRON automated sphygmomanometer. Height and weight were measured using a SECA scale and stadiometer. The Borg scale (original version CR-10) was used to measure rate of perceived exertion (RPE) [21].
Radial applanation tonometry was used to record a 10 s snapshot of the radial arterial pressure wave, with the system software of SphygmoCor® (ArCor Medical Inc). Aortic pressure waveforms and blood pressures were derived from these readings using a validated transfer function. The aortic pressure waveform was in turn used to calculate AP (the pressure difference between the first and second peak (P2-P1) which indicates augmentation of central aortic pressure due to the reflected wave), AIx [the aortic augmentation expressed as a percentage of pulse pressure (PP)], and AIx corrected to a heart rate of 75 bpm (AIx75). The validity and reliability of the SphymoCor has previously been detailed in the literature [27,28,29].
Measurements of central and peripheral blood pressure, indices of arterial stiffness, heart rate and RPE were taken directly before performing each exercise condition, immediately after completing the exercise, 5 min after completion of the exercise and 15 min after completion of the exercise. All measurements with the exception of RPE were taken in triplicate, preprandial, between 18:00 and 20:00 on weekdays in a seated position in a temperature controlled room. Measurements of indices of arterial stiffness were taken by one experienced assessor to reduce measurements error.
Data analysis was carried out using Statistical Package for the Social Sciences (SPSS, version 22, SPSS, Inc., Chicago, IL, USA). Data was checked for normality using the Kolmogorov–Smirnov test. Data quality was also checked manually as per equipment guidelines (Atcor Medical 2008). Measurements taken in triplicate were averaged for analysis. Repeated measures analysis of variance (ANOVA) tests were used for statistical analyses with both exercise intensity (35% and 70% conditions) and time point (baseline, 5 min post exercise and 15 min post exercise) as within subject variables. Posthoc pairwise comparisons were used where applicable. Friedmann’s test was used to analyse difference between Borg RPE scores.
3 Results
Participant characteristics are detailed in Table 2. Haemodynamic results to each exercise condition at each time point are detailed in Table 3. There was no significant difference between baseline haemodynamic measurements taken before each condition. The quality of data obtained from the Sphygmocor was checked as per manufacturer guidelines. Several measurements taken immediately after exercise were of low quality due to a combination of high pulse height variation, time to the first peak being less than 80 ms, or ejection duration being out of the recommended range. Repeated measures ANOVA revealed a significant difference between repeated measurements taken immediately after exercise. Due to poor confidence in the quality of the measurements taken immediately after exercise, this data was not used in analysis (CV > 10%).
3.1 Comparison Between Exercise Conditions
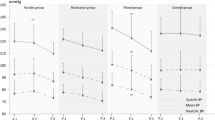
AIx75 was significantly lower for 35% of 1RM than 70% of 1RM (Wilks’ Lambda = 0.76, F (1, 21) = 76.68, p = 0.017, n2 = 0.24). This result is depicted graphically in Figure 1. There was no significant time by condition interaction. Post-hoc pairwise comparisons revealed that Alx75 was significantly lower for 35% than 70% 5 min after exercise (Fig. 1). Although differences in AIx75 at baseline were not significantly different, there was a tendency for AIx75 to be higher prior to the higher exercise intensity. Analysis of covariance was therefore conducted to correct for differences in baseline AIx35. This analysis did not change results seen. There was no significant difference between 35% of 1RM and 70% of 1RM in any other variable measured, including RPE.
3.2 Comparison Between Time Points
There was a significant difference in brachial systolic blood pressure (SBP) across time points [Wilks’ Lambda = 0.71, F (1, 21) = 3.97, p = 0.036, n2 = 0.30]. Post-hoc analysis revealed that SBP was lower at 5 min post exercise than baseline. This is depicted graphically in Fig. 2. There was no significant difference in SBP between baseline and 15 min after exercise, or between 5 min after exercise and 15 min after exercise. There was also no time × condition interaction. There was no significant difference between time points in any other variable measured.
4 Discussion
There is evidence that both acute and chronic aerobic exercise leads to a decrease in central arterial stiffness [10, 12, 19]. Unlike aerobic exercise, the effects of resistance exercise on arterial stiffness are not consistent. As previously stated, both acute and chronic resistance exercise have been associated with an increase [13, 30, 31], a decrease [15, 18, 32] and no change in arterial stiffness [12]. Most studies published on the effects of resistance exercise on arterial stiffness have examined high intensity whole body resistance exercise. Less research has examined the acute effects of low intensity resistance exercise on arterial stiffness, which is arguably more relevant for clinical populations in rehabilitation.
This was the first study to compare the acute effects of short duration resistance exercise of two different intensities on RPE and AIx. Through manipulation of the intensity and number of repetitions, both exercise conditions examined resulted in the same workload. The time taken to carry out each exercise was also standardised. This was intentional since the aim of this study was to investigate the effect of exercise intensity rather than workload on hemodynamic parameters and RPE.
Okamoto et al [18] found reductions in arterial stiffness similar to those expected of aerobic exercise in response to bench press to exhaustion at an intensity of 40% of 1RM [18]. According to Howley [20], aerobic exercise “involves large muscle groups in dynamic activities” [20]. Results of this study along with those of Okamoto [18] indicate that physiologically, the response to a low dose of resistance exercise may mimic that of aerobic exercise. Our study however did not exercise participants to exhaustion, and interestingly there was a difference in AIx75 between conditions without a significant difference in RPE. This finding may reflect the fact that exercise intensities were both low and the sensitivity of the CR-10 may have been too low to detect differences in RPE over one set of a single leg extension. Furthermore, a statistically significant difference in AIx as reported in this study may not reflect a clinically important difference and may be too low to be perceived by participants as exertion. This leads us to question what the change seen in AIx truly represents.
The cohort examined had compliant arteries, as determined by the presence of type C waves [33] and negative Alx and AP values at baseline. There is some evidence to suggest that negative AIx values are not valid measurements of wave reflection, however they are common in younger populations [34]. Disparate results were seen between exercise conditions in AIx75 (Fig. 1), which were pronounced at 5 min post exercise. There was also a significant difference at 5 min post exercise compared to baseline in SBP. Although AIx is closely related to blood pressure, the difference in AIx75 seen between conditions may be independent of changes in SBP since SBP decreased in both exercise conditions, whereas AIx75 decreased in response to light intensity resistance exercise and increased in response to higher intensity exercise. The significant difference in Alx75 between exercise conditions suggests that changes in vascular activity/function after resistance exercise may vary depending on the intensity of the exercise. A plausible mechanisms explaining the decrease in AIx75 following low intensity resistance exercise is the decrease in systolic blood pressure. Since there was no significant change in HR between conditions or testing times in the current study, the change in BP may have been driven by changes in stroke volume. It is also possible that peripheral mechanical compression of the arteries by the muscles caused a degree of hyperemia leading to vasodilation. These results are noteworthy as during resistance exercise peripheral vasoconstriction and vasodilation may limit the use of AIx and AIx75 as an indicator of central arterial stiffness. The current study reveals the possibility of peripheral vessel changes in response to a low dose of resistance exercise, as prescribed in this study, effecting AIx results despite AIx being measured at a non-exercised site (i.e., at the wrist). In this study the exercise carried out involved one muscle group in the lower limb. Regional changes in arterial stiffness were not examined, which could be considered a limitation since previous research has shown significant changes in regional arterial stiffness in response to resistance exercise. Another considerable limitation is that PWV was not measured. Sugaware et al. [35] investigated the effects of single leg low resistance cycling on arterial stiffness among young healthy men and found a regional decrease in pulse wave velocity (PWV) in the exercised leg compared to the non-exercised leg. Similarly Heffernan et al. [19] found regional reductions in arterial stiffness after unilateral lower limb resistance exercise in the exercised leg without changes in arterial stiffness either in the non-exercised leg or centrally. There was a significant difference only in one parameter (Alx75) between exercise conditions in this study. These results suggests that either central changes in arterial stiffness are possible with isolated resistance exercise in the lower limb, and may vary depending on the intensity of exercise, or that peripheral vascular changes may alter AIx values in the post exercise period, despite tonometry measurements taking place in a non-exercised limb. It is possible that an increase in arterial stiffness resulted from an increase in sympathetic tone or a decrease in nitric oxide availability causing an increase in vasoconstrictor tone in smooth muscle [32]. Further studies examining both central and regional changes in haemodynamic variables in response to resistance exercise conditions are warranted.
Post exercise hypotension is commonly reported following resistance exercise [36,37,38]. In this study, despite the low volume of exercise, there was a significant decrease in brachial systolic blood pressure 5 min after exercise. Changes in peripheral and central systolic pressure are believed to influence the vascular response to resistance exercise [13, 39]. In this study the reduction in systolic blood pressure could explain the decrease in AIx75 seen after low intensity resistance exercise but does not account for the difference between exercise intensities seen. These results also indicate that acute changes in AIx in response to high intensity resistance exercise may not be related to systolic blood pressure. Yoon et al [24] reported similar results when they examined the blood pressure and arterial stiffness response to resistance exercises at 60% of 1RM and found changes in arterial stiffness without a significant change in aortic systolic blood pressure. Further studies into the relationship between peripheral blood pressure, central blood pressures, arterial stiffness and resistance exercise are needed to fully appreciate the complex interaction between these variables, and to determine the use of AIx as an indication of arterial stiffness in the context of resistance exercise prescription. Overall, the results of this study need further investigation to understand if, beyond statistical considerations, they have a clinical impact as although they reached statistical significance, changes in variables measured were modest.
The Valsalva manoeuvre, which is often performed during resistance exercise, is known to increase arterial stiffness [40]. For the purpose of this study a physiotherapist demonstrated how to avoid the Valsalva manoeuvre, and instructed participants not to breath hold during the exercises. The same physiotherapist was on site to supervise exercises during measurement and ensure that the Valsalva manoeuvre was avoided. Other mechanisms that have been shown to affect arterial stiffness in response to exercise include baroreceptor sensitivity, autonomic and sympathetic nervous system activation or relaxation, the secretion of vasodilatory substances, baseline levels of physical activity, and a history of resistance training and gender [12, 30, 32, 41, 42]. A potential limitation of this study is that physical activity levels of participants were not assessed. Okomoto et al. [43] have shown that low intensity resistance exercise can suppress increases in plasma noradrenaline concentrations and suppress sympathetic tone whereas high intensity resistance exercise may not. The analysis of blood samples was beyond the scope of this study, however it is possible that differences in the cardiovascular reflex response to high versus low intensity resistance exercise may explain divergent trends in AIx75 seen.
A limitation of this study was the poor quality of results taken immediately after exercise. Readings were not stable enough to secure reliable measurements as participant heart rate and blood pressure were recovering with each beat (CV greater than 10%). The decision not to include measurements taken immediately after exercise in analysis stemmed from the fact that the authors did not have confidence in the quality of these measurements, and preliminary analysis suggested that they were not reliable or comparable. For example, the time taken to capture data using the Spygmocor can be several seconds. Analysis revealed that measurements taken several seconds apart immediately after exercise differed significantly. Since data acquisition was not taken at exactly at the same time for each participant (e.g., the first measurement may not have been of good enough quality to keep) the variance in results was large and measurements between participants rendered non-comparable. Since strict quality assurance measures were adhered to in this study, we can have confidence that results reported at baseline 5 and 15 min after exercise were of high quality. A difficulty with taking measurements immediately after exercise is that some tonometry devices which apply an algorithm to correct AIx for heart rate are only valid below certain heart rates (for example 110 bpm), this limitation renders capturing valid data immediately post exercise, where heart rate would be expected to rise, difficult.
The aim of this study was to compare the haemodynamic and RPE response to a low volume of high intensity versus low intensity resistance exercise. There were no significant differences in RPE between time points or exercise intensities. This could be due to the low volume of exercise carried out. Future studies may wish to examine a greater volume and/or duration of resistance exercise. It is possible that with a greater volume of exercise, the changes seen in indices of arterial stiffness with each individual exercise may be greater. Greater differences in haemodynamic variables may also have been reported if the exercise was carried out across several muscle groups, or on muscles of the upper limbs [44]. Among healthy individuals resistance exercise is often prescribed to exhaustion. Since there is very little research in this area it was considered prudent to examine a small volume of exercise. Furthermore lower volumes of exercise are more representative of daily activities and can translate to functional benefits in clinical populations. Results of this pilot study cannot be projected to clinical populations, or older healthy populations, however future studies may wish to examine the effects of various types of resistance exercise on populations with reduced haemodynamic buffering ability. Finally, results of this study question the use of AIx as an indicator of central arterial stiffness in the context of resistance exercise prescription.
5 Conclusion
In summary, there remains a lack of clarity in the available evidence on the effects of resistance exercise on arterial stiffness and other haemodynamic variables. A significant difference in AIx75 was seen between low and high intensity resistance exercise 5 min after exercise which suggests that intensity has a role to play. The reduction in augmentation index observed with low intensity resistance exercise may be due to a decrease in systolic blood pressure. However the increase seen after the higher intensity appears to be independent of SBP. Further research is needed to understand the complex interactions between variables of exercise prescription, blood pressure and arterial stiffness, in particular among clinical populations, however this study has demonstrated that the haemodynamic response to two exercises of identical workloads differ. By controlling the exercise condition closely we can deduce that the difference stems from either the intensity of exercise or rate at which it was undertaken. Results of this study could be used to inform further study protocols, in particular among those with compromised haemodynamics (e.g., cardiac rehabilitation population). Care should be exercised when using AIx as an indicator of arterial compliance in resistance exercise prescription as changes to this variable could result from peripheral vascular dynamics despite measurements taking place in a non-exercised limb.
References
Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50(1):197–203. https://doi.org/10.1161/HYPERTENSIONAHA.107.089078.
Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37(5):1236–41.
Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39(1):10–5.
Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25(6):1105–87. https://doi.org/10.1097/HJH.0b013e3281fc975a.
Cecelja M, Chowienczyk P. Role of arterial stiffness in cardiovascular disease. JRSM Cardiovasc Dis. 2012. https://doi.org/10.1258/cvd.2012.012016.
Staessen J, Amery A, Fagard R. Isolated systolic hypertension in the elderly. J Hypertens. 1990;8(5):393–405.
Cheng LT, Tang LJ, Cheng L, Huang HY, Wang T. Limitation of the augmentation index for evaluating arterial stiffness. Hypertens Res. 2007;30(8):713–22. https://doi.org/10.1291/hypres.30.713.
Seals DR, Desouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol (1985). 2008;105(4):1323–32. https://doi.org/10.1152/japplphysiol.90553.2008.
Tanaka H, DeSouza CA, Seals DR. Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol. 1998;18(1):127–32.
Schuler G, Adams V, Goto Y. Role of exercise in the prevention of cardiovascular disease: results, mechanisms, and new perspectives. Eur Heart J. 2013;34(24):1790–9. https://doi.org/10.1093/eurheartj/eht111.
Pal S, Radavelli-Bagatini S, Ho S. Potential benefits of exercise on blood pressure and vascular function. J Am Soc Hypertens. 2013;7(6):494–506. https://doi.org/10.1016/j.jash.2013.07.004.
Ashor AW, Lara J, Siervo M, Celis-Morales C, Mathers JC. Effects of exercise modalities on arterial stiffness and wave reflection: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2014;9(10):e110034. https://doi.org/10.1371/journal.pone.0110034.
DeVan AE, Anton MM, Cook JN, Neidre DB, Cortez-Cooper MY, Tanaka H. Acute effects of resistance exercise on arterial compliance. J Appl Physiol (1985). 2005;98(6):2287–91. https://doi.org/10.1152/japplphysiol.00002.2005.
Okamoto T, Masuhara M, Ikuta K. Effects of eccentric and concentric resistance training on arterial stiffness. J Hum Hypertens. 2006;20(5):348–54. https://doi.org/10.1038/sj.jhh.1001979.
Ho SS, Radavelli-Bagatini S, Dhaliwal SS, Hills AP, Pal S. Resistance, aerobic, and combination training on vascular function in overweight and obese adults. J Clin Hypertens (Greenwich). 2012;14(12):848–54. https://doi.org/10.1111/j.1751-7176.2012.00700.x.
Okamoto T, Masuhara M, Ikuta K. Effect of low-intensity resistance training on arterial function. Eur J Appl Physiol. 2011;111(5):743–8. https://doi.org/10.1007/s00421-010-1702-5.
Umpierre D, Stein R. Hemodynamic and vascular effects of resistance training: implications for cardiovascular disease. Arq Bras Cardiol. 2007;89(4):256–62.
Okamoto T, Min S, Sakamaki-Sunaga M. Arterial compliance and stiffness following low-intensity resistance exercise. Eur J Appl Physiol. 2014;114(2):235–41. https://doi.org/10.1007/s00421-013-2770-0.
Heffernan KS, Rossow L, Jae SY, Shokunbi HG, Gibson EM, Fernhall B. Effect of single-leg resistance exercise on regional arterial stiffness. Eur J Appl Physiol. 2006;98(2):185–90. https://doi.org/10.1007/s00421-006-0259-9.
Howley ET. Type of activity: resistance, aerobic and leisure versus occupational physical activity. Med Sci Sports Exerc. 2001;33(6 Suppl):S364–9 (discussion S419–S420).
Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–81.
Mezzani A, Hamm LF, Jones AM, McBride PE, Moholdt T, Stone JA, et al. Aerobic exercise intensity assessment and prescription in cardiac rehabilitation: a joint position statement of the European Association for Cardiovascular Prevention and Rehabilitation, the American Association of Cardiovascular and Pulmonary Rehabilitation and the Canadian Association of Cardiac Rehabilitation. Eur J Prev Cardiol. 2013;20(3):442–67. https://doi.org/10.1177/2047487312460484.
Sirico F, Fernando F, Di Paolo F, Adami PE, Signorello MG, Sannino G, et al. Exercise stress test in apparently healthy individuals—where to place the finish line? The Ferrari corporate wellness programme experience. Eur J Prev Cardiol. 2019;26(7):731–8. https://doi.org/10.1177/2047487318825174.
Yoon ES, Jung SJ, Cheun SK, Oh YS, Kim SH, Jae SY. Effects of acute resistance exercise on arterial stiffness in young men. Korean Circ J. 2010;40(1):16–22. https://doi.org/10.4070/kcj.2010.40.1.16.
Haff GGTN. The Essentials of Strength Training and Conditioning 4th edition. Human Kinetics; 2016.
O’Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31(9):1731–68. https://doi.org/10.1097/HJH.0b013e328363e964.
Adji A, Hirata K, Hoegler S, O’Rourke MF. Noninvasive pulse waveform analysis in clinical trials: similarity of two methods for calculating aortic systolic pressure. Am J Hypertens. 2007;20(8):917–22. https://doi.org/10.1016/j.amjhyper.2007.03.006.
O’Rourke MF, Hashimoto J. Pressure pulse waveform analysis in critical care. Crit Care Med. 2006;34(5):1569–70. https://doi.org/10.1097/01.CCM.0000216193.25486.6A.
Pauca AL, O’Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38(4):932–7.
Miyachi M. Effects of resistance training on arterial stiffness: a meta-analysis. Br J Sports Med. 2013;47(6):393–6. https://doi.org/10.1136/bjsports-2012-090488.
Fernhall and Smith. Advanced cardiovascular exercise physiology. Champaign: Human Kinetics; 2011.
Heffernan KS, Collier SR, Kelly EE, Jae SY, Fernhall B. Arterial stiffness and baroreflex sensitivity following bouts of aerobic and resistance exercise. Int J Sports Med. 2007;28(3):197–203. https://doi.org/10.1055/s-2006-924290.
Murgo JP, Westerhof N, Giolma JP, Altobelli SA. Aortic input impedance in normal man: relationship to pressure wave forms. Circulation. 1980;62(1):105–16.
Hughes AD, Park CM, Davies J, Curtis S, Thom SAM, Mayet J, et al. Augmentation index is not a valid measure of wave reflection when it is negative and this distorts the presumed relationship between aging and wave reflection. Artery Res. 2010;4(4):144. https://doi.org/10.1016/j.artres.2010.10.028.
Sugawara J, Otsuki T, Tanabe T, Maeda S, Kuno S, Ajisaka R, et al. The effects of low-intensity single-leg exercise on regional arterial stiffness. Jpn J Physiol. 2003;53(3):239–41.
Tibana RA, de Sousa NM, da Cunha Nascimento D, Pereira GB, Thomas SG, Balsamo S, et al. Correlation between acute and chronic 24-hour blood pressure response to resistance training in adult women. Int J Sports Med. 2014. https://doi.org/10.1055/s-0034-1382017.
Dos Santos ES, Asano RY, Filho IG, Lopes NL, Panelli P, Nascimento Dda C et al. Acute and chronic cardiovascular response to 16 weeks of combined eccentric or traditional resistance and aerobic training in elderly hypertensive women: a randomized controlled trial. J Strength Cond Res. 2014;28(11):3073–84. https://doi.org/10.1519/JSC.0000000000000537.
Cornelissen VA, Fagard RH, Coeckelberghs E, Vanhees L. Impact of resistance training on blood pressure and other cardiovascular risk factors: a meta-analysis of randomized, controlled trials. Hypertension. 2011;58(5):950–8. https://doi.org/10.1161/HYPERTENSIONAHA.111.177071.
London GM, Guerin AP. Influence of arterial pulse and reflected waves on blood pressure and cardiac function. Am Heart J. 1999;138(3 Pt 2):220–4.
Heffernan KS, Jae SY, Edwards DG, Kelly EE, Fernhall B. Arterial stiffness following repeated Valsalva maneuvers and resistance exercise in young men. Appl Physiol Nutr Metab. 2007;32(2):257–64. https://doi.org/10.1139/h06-107.
Brito LC, Queiroz AC, Forjaz CL. Influence of population and exercise protocol characteristics on hemodynamic determinants of post-aerobic exercise hypotension. Braz J Med Biol Res. 2014;47(8):626–36.
Green DJ, Maiorana A, O’Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561(Pt 1):1–25. https://doi.org/10.1113/jphysiol.2004.068197.
Okamoto T, Masuhara M, Ikuta K. Low-intensity resistance exercise with slow lifting and lowering does not increase noradrenalin and cardiovascular responses. Clin Physiol Funct Imaging. 2009;29(1):32–7. https://doi.org/10.1111/j.1475-097X.2008.00829.x.
Okamoto T, Masuhara M, Ikuta K. Upper but not lower limb resistance training increases arterial stiffness in humans. Eur J Appl Physiol. 2009;107(2):127–34. https://doi.org/10.1007/s00421-009-1110-x.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Nil.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
The work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. Ethical approval was granted by the relevant research ethics committee.
Rights and permissions
About this article
Cite this article
Forde, C., Johnston, M., Haberlin, C. et al. Low Dose Resistance Exercise: A Pilot Study Examining Effects on Blood Pressure and Augmentation Index Between Intensities. High Blood Press Cardiovasc Prev 27, 83–91 (2020). https://doi.org/10.1007/s40292-020-00362-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40292-020-00362-5