Abstract
MicroRNAs (miRNAs) are endogenous noncoding RNAs that mediate the fibrotic process by regulating multiple targets. MicroRNA-based therapy can restore or inhibit miRNA expression and is expected to become an effective approach to prevent and alleviate fibrotic diseases. However, the safe, targeted, and effective delivery of miRNAs is a major challenge in translating miRNA therapy from bench to bedside. In this review, we briefly describe the pathophysiological process of fibrosis and the mechanism by which miRNAs regulate the progression of fibrosis. Additionally, we summarize the miRNA nanodelivery tools for fibrotic diseases, including chemical modifications and polymer-based, lipid-based, and exosome-based delivery systems. Further clarification of the role of miRNAs in fibrosis and the development of a novel nanodelivery system may facilitate the prevention and alleviation of fibrotic diseases in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
MicroRNAs regulate the occurrence of fibrotic disease. |
A nanocarrier can mediate delivery of microRNAs for fibrotic diseases. |
1 Introduction
Fibrotic diseases are mediated by chronic pathophysiological processes in which fibrous connective tissue accumulates excessively during injury or inflammation, leading to scarring or organ failure [1]. Fibrosis is considered to result from abnormal repair after organ damage. A typical repair process involves regeneration, in which damaged tissue is replaced by cells of the same type without evidence of damage. However, uncontrolled injury results in remodeling of the extracellular matrix and the establishment of an abnormal fibrotic state (Fig. 1) [2]. Continued deterioration of the fibrotic state leads to organ failure and can even lead to death in severe cases. Worldwide, organ fibrosis is the leading cause of death and disability resulting from many diseases. Despite the continuous development of medical technology and the decreasing trend in fibrotic diseases, nearly half of the fatalities from diseases in developed countries can be attributed to tissue fiber hyperplasia [3, 4].
Antifibrotic drug treatment and elimination of the cause of injury, for example, by inhibiting inflammation, repairing damage, promoting extracellular matrix degradation, and altering collagenase activity, can effectively alleviate the development of fibrotic diseases [5, 6]. However, the antifibrotic effects of a single-agent treatment is limited owing to the low permeability, poor targeting, and inevitable side effects of the drug [7]. Increasing the drug dose to achieve a therapeutic effect may produce irreversible effects on the target organs, and the single-agent treatment approach does not guarantee specific delivery to the target cells allowing the suppression of disease development [8]. Thus, searching for specific small-molecule drugs that regulate the key signaling pathways of fibrosis may provide the optimal therapeutic option for fibrotic diseases.
2 MicroRNAs
2.1 Biogenesis of MicroRNAs
MicroRNAs (miRNAs) are single-stranded noncoding RNAs that contain approximately 18–25 nucleotides and are encoded by an endogenous gene [9, 10]. They contain a seed sequence complementary to the 3′ untranslated region (3′UTR), the 5′UTR, or the coding sequence of the target messenger RNA (mRNA) and regulate cell proliferation, apoptosis, differentiation, and metabolism by inhibiting the translation process or enhancing mRNA cleavage [11,12,13,14].
After DNA transcription, the primary transcript, called a primary miRNA can be processed to produce a precursor miRNA, which is released into the cytoplasm for cleavage into a double-stranded miRNA by RNase III. Mature single-stranded miRNAs can interact with the RNA-induced silencing complex to regulate mRNA translation [15]. A single miRNA can regulate multiple target genes, and multiple miRNAs can regulate a single target gene. This precise regulatory network diversifies the complement of miRNA regulatory mechanisms [16]. As important components of epigenetics, miRNAs participate in various physiological and pathological processes and regulate the occurrence and progression of fibrosis [17].
2.2 MicroRNAs in Fibrosis
MicroRNAs can mediate the development of fibrotic diseases by regulating the expression of target genes [18, 19]. The transforming growth factor (TGF)-β/Smad signaling pathway can be triggered by miRNAs to mediate the development of liver and lung fibrosis and scleroderma [20]. In addition, miRNAs can participate in the SIRT1/p38 signaling pathway to regulate the process of renal fibrosis and can target the TGF-β/Smad and PI3K/Akt/mammalian target of rapamycin signaling pathways to alleviate myocardial fibrosis and keloids [21,22,23]. The regulatory mechanisms of miRNAs provide a theoretical basis for the treatment of fibrotic diseases [24, 25].
2.3 MicroRNA-Based Therapies
In fibrotic diseases, miRNAs can be upregulated as a fibrosis marker or downregulated as an antifibrotic factor [26,27,28]. When miRNAs are suppressed in fibrotic diseases, alternative therapies are suitable. Synthesized miRNA mimics instead of endogenous miRNA can interact with the RNA-induced silencing complex, which regulates the translation process and restore the expression of profibrotic miRNAs downregulated in fibrotic diseases. If a miRNA is overexpressed in fibrotic diseases, miRNA suppression therapy is used. Therapeutic approaches using miRNA-specific antagonists or synthetic anti-miRNA oligonucleotide chains to bind to the target miRNA can rescue the function of target genes and promote their antifibrotic function in vivo.
Using overexpressed miRNA mimics, anti-miRNA oligonucleotides or miRNA antagonists that modulate the function of miRNAs, has been suggested as a possible effective therapeutic strategy for human fibrotic diseases [29, 30]. Therefore, it is possible that miRNA can be used as a drug and a target to suppress the fibrotic process.
2.4 Major Obstacles in MicroRNA Delivery
MicroRNAs are negatively charged and easily degraded by nucleases; thus, their tissue-specific delivery and passage through the cell membrane are difficult to achieve [31]. The dilemma of targeted delivery involves challenges such as maximizing cell uptake and tissue-specific delivery and minimizing off-target effects [32]. To improve the efficiency of miRNA delivery, viral and nonviral vectors have been developed to deliver genetic material to target cells to perform the corresponding functions [33]. However, because of the low loading capacity of viral vectors, their propensity to induce inflammatory reactions, and their toxicity and side effects that limit their delivery capabilities, the development of liposomes, polymer systems, nanoparticles, and other nonviral vectors is trending [34]. Nanocarriers have the advantages of improved resistance to enzymatic degradation and high affinity and are therefore very promising for application in treating fibrotic diseases.
2.5 Nanocarriers for MicroRNA Delivery
Nanomedicine is expected to play a role in the targeted delivery of miRNAs for treating fibrosis. Nanodrug delivery carriers have high affinity and stability and can increase the solubility and reduce the toxicity and side effects of drugs [35]. Moreover, the addition of targeting groups such as polypeptides can endow nanodrug carriers with active targeting potential [36, 37].
Because the current outcome for patients with fibrotic diseases is not optimistic, developing new treatment strategies with an understanding of the mechanism of fibrosis development is urgently needed. Because of the unique properties of nanomaterials, increasing attention is being paid to the therapeutic application of nanomedicine in fibrotic diseases. Here, we review the miRNA delivery nano-systems useful for the treatment of fibrotic diseases (Table 1).
3 MicroRNA Delivery Approaches
3.1 Chemical Modifications and Oligonucleotide Conjugates
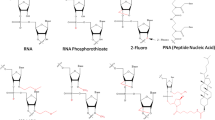
Chemical modifications of miRNA include locked nucleic acids (LNAs), peptide nucleic acids (PNAs), backbone modifications, and ribose 2′-OH group modifications, which can increase the stability and reduce the off-target effects of miRNAs [38,39,40].
3.1.1 Locked Nucleic Acids
Locked nucleic acids are a class of oligonucleotide derivatives that contain a methylene linkage between the 2′-oxygen and the 4′-carbon. Due to their unique structure, LNAs have high binding power. Locked nucleic acid-modified DNAzymes can target regulatory RNAs, and LNAs can also modify small-interfering RNAs (siRNAs) to disrupt gene expression [41]. In addition, LNAs have strong antisense activity and participate in reverse regulation of targeted miRNAs. The target site blockers seal specific binding sites of miRNA to the mRNA, and block the binding of normal miRNA to the target gene mRNA by occupying the binding site in vivo and in vitro [42,43,44,45,46].
MiR-29b1 can regulate liver fibrosis. The hedgehog inhibitor GDC-0449 and miR-29b1 cooperate to inhibit hepatic stellate cell activation and extracellular matrix production in mice subjected to common bile duct ligation [47, 48]. LNA-miR-29b1, phosphorothioate (PS-miR-29b1), 2′-O-methyl-phosphorothioate (OMe-miR-29b1), and N,N′-diethyl-4-(4-nitronaphthalen-1-ylazo)-phenylamine (ZEN-miR-29b1) can be used to chemically modify the antisense strand of miR-29b1. These chemical modifications can significantly improve the stability of miR-29b1 in medium containing 50% fetal bovine serum. However, among the modified miRNAs tested, LNA-miR-29b1 was less stable than OMe-PS-miR-29b1 [47]. Therefore, the delivery of LNA-miR-29b1 needs further optimization as a promising therapeutic strategy for liver fibrosis.
Several siRNAs corresponding to miRNAs modified by LNA have been shown to alleviate organ fibrosis in mouse models. LNA-anti-miR-132 was promising for the treatment of liver fibrosis [49]. The LNA-modified anti-miR-34a, miR-21, and miR-320 can prevent heart enlargement and fibrosis [50,51,52,53]. LNA-anti-miR-150 reduces pro-inflammatory M1 and M2 macrophages polarization via the SOCS 1/JAK 1/STAT 1 pathway[54,55,56]. The target site blockers were used to block miR-9 binding to the 3′UTR of anoctamin 1 to increase its activity, thus compensating for the lack of transmembrane conductance modulators in cystic fibrosis (CF) [57].
3.1.2 Peptide Nucleic Acids
Peptide nucleic acids are negatively charged nucleic acid analogs whose sugar-phosphate backbone is replaced by a polypeptide backbone. Peptide nucleic acids are very stable structural analogs of delivered miRNAs [58,59,60].
Cystic fibrosis is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene that result in reduced or altered CFTR functions [61]. Several miRNAs downregulate the expression of CFTR, thereby causing or aggravating the symptoms of CF [62]. Modification of miRNAs with PNAs facilitates the protection of specific sequences in the 3′UTR of the CFTR mRNA [63, 64]. PNAs synthesized by the addition of two tetrapeptides (Gly-SerP-SerP-Gly) at the two C-termini can specifically bind to miR-509-3p as a target miRNA-binding agent, blocking the effect of this miRNA on CFTR mRNA activity [65]. In A549 cells co-transfected with the pLuc-CFTR-3′UTR vector and different combinations of PNAs, longer PNA constructs restored up to 70% of the luciferase activity, suggesting that the appropriate use of PNAs can counteract the decrease in CFTR expression and is expected to alleviate the symptoms of CF [5].
MiR-145 is another miRNA that targets and inhibits CFTR expression [66]. Conjugation of the octoglycine (R8) carrier peptide at the N-terminus of the PNA chain can block the regulatory effect of miR-145-5p and miR-101-3p, enhance the expression of the CFTR gene, and suppress the development of CF [60, 67]. Another study suggested that inhibition of miR-33 in macrophages by administration of anti-miR-33 PNA attenuates fibrosis in mouse pulmonary fibrosis models in vivo and ex vivo [68]. Because PNAs have high affinity for nucleic acid targets, they regulate gene expression with outstanding efficacy and are an effective means of inducing pharmacologically mediated changes in gene expression in vivo and in vitro.
Abnormal fatty acid oxidation may have an important effect on the progression of kidney disease. The lack of miR-33 as an important regulator of lipid metabolism can partially prevent fatty acid oxidation in fibrotic kidneys and reduce lipid accumulation. Using low pH insertion peptides as a carrier can allow the delivery of PNA miR-33 inhibitors to the kidney and other acidic microenvironments, which can effectively promote the expression of fatty acid oxidation mediators and reduce the development of fibrosis [69]. These findings suggest that delivery of PNA miR-33 inhibitors may be an attractive therapeutic approach for chronic kidney disease.
3.1.3 Oligonucleotide Conjugate Modifications
MiR-29 is downregulated in multiple fibrotic organs, including the skin and lungs, and negatively regulates fibrosis [70]. A miR-29b oligonucleotide was synthesized via standard phosphoramidite solid-phase synthesis, and the sense strand of the miR-29b oligonucleotide mimic was conjugated to cholesterol at the 3′ end. Intravenous injection of this synthetic oligonucleotide increased miR-29 levels in vivo. In bleomycin-induced pulmonary fibrosis in mice, treatment with a miR-29b oligonucleotide mimic restored the function of endogenous miR-29, thereby reducing collagen expression and in turn blocking and reversing pulmonary fibrosis [71]. Another trial was conducted to evaluate the pharmacodynamic activity of a second development-stage miR-29b mimic, remlarsen (also called MRG-201) [72]. Remlarsen was shown to regulate the expression of miR-29b in skin wounds of mice, rats, and rabbits, as well as in cultured human skin fibroblasts. In this intrasubject controlled clinical trial (ClinicalTrials.gov ID NCT02603224), remlarsen inhibited the expression of collagen and the development of fibrosis in incised skin wounds. Currently, a carbohydrate-conjugated miRNA oligonucleotide drug, RCS-21, which is a miR-21 inhibitor, is being developed to deliver inhaled oligonucleotides efficiently and selectively to lung macrophages. RCS-21 reverses the pathological activation of macrophages and prevents lung dysfunction and fibrosis after acute lung injury in mice. RCS-21 effectively prevents exaggerated inflammatory responses in human lung tissue infected with SARS-CoV-2 in vitro [73]. These results suggest that miR-29b oligonucleotide mimics may effectively prevent fibrotic pulmonary and skin conditions such as hypertrophic scars, keloids, and scleroderma.
3.2 Inorganic Delivery Systems
Compared with conventional drugs, chemotherapy, and radiotherapy, inorganic nanomaterials used as drug carriers can enhance the targeted transport, controlled release, and sustained release of drugs [74]. Common inorganic nanomaterials include gold nanoparticles (AuNPs), mesoporous silica nanoparticles, carbon nanomaterials, and magnetic nanoparticles, but the use of these nanomaterials in miRNA delivery to treat fibrotic diseases needs to be further explored [75].
Gold nanoparticles are precious metal colloids with a particle diameter that ranges from 1 to 100 nm. These colloids exhibit secondary electron emission, and an ion resonance effect can be produced through the interaction of incident light with free electrons in AuNPs to achieve drug delivery (Fig. 2) [76]. They are excellent carriers for delivering small molecule drugs and biomolecules. MicroRNAs delivered into cells via AuNPs can specifically bind to mRNA sequences to exert inhibitory effects on their target genes [77]. For example, miR-133b can inhibit the transformation of myofibroblasts [78]. A nanocomposite made of AuNPs and miR-133b was loaded onto the surface of a corneal collagen membrane and on the inside of the collagen membrane [79]. The properties of the collagen membrane did not change, although the cornea was rapidly epithelialized and the corneal transparency remained constant. In addition, only a low level of fibrosis was observed in the corneal stroma. These results suggest that the AuNP/miR-133b complex can achieve rapid corneal repair and inhibit scarring.
Diabetic cardiomyopathy is a common disease in postmenopausal women, in whom the lack of estrogen aggravates its pathology. Compared with diabetic mice, ovariectomized diabetic mice exhibited increased ROS accumulation, apoptosis, myocardial hypertrophy, and fibrosis. miR-155 is a potentially effective promoter of type 1 proinflammatory (M1) macrophage polarization, and its expression can be further enhanced in macrophages and heart tissue by ovariectomy [80, 81]. miR-155 AuNPs were injected into the tail vein, the thiol-modified antagomiR-155 was covalently bound to AuNPs, and the nucleic acid was preferentially delivered to macrophages via phagocytosis [80]. By increasing the proportion of anti-inflammatory type 2 (M2) macrophages and decreasing inflammation, in vivo administration of antagomiR-155 reduced apoptosis and restored cardiac function. The recovery effect of miR-155-AuNP was far superior to that of general depletion of macrophage clones. Obviously, an imbalance in the M1/M2 ratio led to aggravation of cardiomyopathy in ovariectomized diabetic mice. Thus, AuNP-mediated inhibition of miR-155 in macrophages is a promising strategy for improving cardiac function.
Another non-viral biomimetic system was constructed by coating the FH peptide-modified neutrophil membrane on mesoporous silicon nanoparticles loaded with miR Combo (miR-1, 133, 208, and 499). In a mouse model of myocardial ischemia/reperfusion injury, intravenous injection of nanoparticles successfully delivered miRCombo into fibroblasts, which was used to reprogram cardiac fibroblasts for cardiac regeneration after myocardial injury, thereby reducing fibrosis and improving cardiac function[82].
3.3 Polymer-Based Delivery Systems
Polymers have good biosimulation characteristics, biocompatibility, and a wide range of structural changes therefore they are widely used in drug-delivery systems [81]. Polymer nanodrugs are nanopreparations that connect polymers and drugs through chemical bonds. Upon entry into the body, the conjugate responds to exogenous or endogenous changes to break the chemical bond and release the drug at the target site. Polymers are classified as synthetic or natural. Synthetic polymers are composed of mainly polyethyleneimines (PEIs) and poly (lactic-glycolic acid), while natural polymers include peptides, proteins, and polysaccharides [83].
3.3.1 Synthetic Polymers
3.3.1.1 Polyethyleneimines
Polyethyleneimines are a class of cationic synthetic polymers that contain multiple amino groups within their linear or branched structure [84]. The positively charged amino groups in PEIs and the negatively charged phosphate groups in nucleic acids undergo polycondensation through electrostatic interactions to form nanoparticles, not only preventing the degradation of nucleic acids during delivery but also improving the cell uptake efficiency because of their high transfection efficiency [85]. Polyethyleneimines are considered the gold standard for nonviral vectors. Currently, studies based on PEIs as delivery vehicles are increasing; moreover, PEI-based delivery of miRNA is being explored for the treatment of fibrotic diseases.
Polyethyleneimine nanoparticles were shown to effectively deliver miR-146a and significantly enhance its expression in obstructive kidney disease while reducing the area of renal fibrosis, the expression of alpha-smooth muscle actin and the infiltration of F4/80-positive macrophages into the obstructed area [86]. These effects may occur because miR-146a polyethyleneimine nanoparticles can inhibit the TGF-β/Smad and tumor necrosis factor receptor-associated factor 6/nuclear factor kappa B signaling pathways. These results indicate that miR-146a delivery alleviates renal fibrosis by inhibiting profibrotic and inflammatory signaling pathways.
Polyethyleneimine-based nanoparticles also significantly promote miR-126 entry into human F508del CF transmembrane conductance regulator bronchial epithelial (CFBE41o) cells [87]. The low nitrogen/phosphate ratio of PEI-premiR-126 nanoparticles resulted in significant knockdown of target of Myb1 (TOM1), a known target of miR-126. The reduction in TOM1 expression was most pronounced (66% reduction) in CFBE41o cells with an nitrogen/phosphate ratio of 1:1.
Polyethyleneimine forms a stable PEI-miRNA complex via electrostatic interactions. These complexes are immobilized on an electrospun smooth porous scaffold to achieve continuous delivery of two muscle-specific miRNAs (miR-1 and miR-133a). These dual miRNA scaffold systems proved to be a good formulation, and the delivered dual miRNAs contributed to the precise control of the cardiac fibroblast fate to alleviate myocardial fibrosis [88].
However, the main limitations of PEIs as nanocarriers are their low biodegradability inside cells and their propensity to form aggregates with negatively charged proteins in cells, resulting in dose-dependent cytotoxicity. To reduce their cytotoxicity and improve their transfection and targeting efficiency, chemical structural modifications of PEIs have been widely studied, yielding constructs such as polyurethane-grafted PEI, poly-L-lysine-modified PEI co-polymers, poly(1,8-octanediol-citric acid)-grafted PEI, and poly(lactic acid)-grafted PEI copolymers [89]. These PEIs with modified chemical structures and loaded with miRNAs constitute a future therapeutic option for fibrotic diseases.
3.3.1.2 Poly (Lactic-Co-Glycolic Acid)
Poly(lactic-co-glycolic acid) [PLGA] is a safe, biocompatible, and biodegradable polymer. Poly(lactic-co-glycolic acid) can be hydrolyzed into non-toxic lactic acid and glycolic acid monomers, which are metabolized by the body without any side effects [90]. Nanoparticles prepared from PLGA polymers can capture biologically active molecules and escape from the lysosome into the cytoplasm, inducing sustained release of the transported substance in the cell and achieving the goal of slow release. The PLGA delivery system can be subjected to a variety of surface modifications and is currently used for miRNA delivery to treat fibrotic diseases (Fig. 3) [91, 92].
miR-17 mimics were encapsulated in poly(lactic acid) graft-based particles, which were phagocytosed by bronchial epithelial cells, and possibly improved CF [93]. The PLGA microparticle system encapsulating premiR-19b-3p can deliver mature miR-19b-3p to macrophages in vitro with high efficiency. Indeed, the level of secretion leucoprotease inhibitor, the target gene of miR-19b-3p, was still observed to be significantly reduced 72 hours after delivery [94]. As macrophages are key inflammatory cells, they are essential mediators of chronic inflammatory lung diseases such as CF. MiRNA-coated PLGA particles may be delivered to CF tissues by inhalation, thus providing a new treatment paradigm for delivery to macrophages.
However, the ability of anionic PLGA nanoparticles to transfect genetic materials into cells is poor, and their encapsulation efficiency is low, which limits their application. To overcome these limitations, positively charged low-molecular-weight chitosan can be added to the PLGA system. Low-molecular-weight chitosan may facilitate the encapsulation of negatively charged substances such as nucleic acids, thereby improving the packaging efficiency of PLGA. By coupling miRi (a miR-21 inhibitor) with PLGA and low-molecular-weight chitosan, Geng et al. prepared small cationic miRi-low-molecular-weight chitosan-modified PLGA nanoparticles [95]. The easily degradable miRi was encapsulated in these PCNPs, thereby preventing its degradation by nucleases. In vitro and in vivo assays showed that PCNPs have good biocompatibility, a high cell uptake efficiency, and selective kidney-targeting ability. Moreover, the therapeutic effect of miRi-PCNPs on renal fibrosis was much higher than that of miRi alone. The tubule injury index and tubulointerstitial fibrosis area in the miRi-PCNP group were 2.5 times lower than those in the saline and miRi groups. miRi-PCNPs mainly suppress the TGF-β1/Smad3 and extracellular signal-regulated kinase/mitogen-activated protein kinase signaling pathways by inhibiting the expression of miR-21 [95]. Therefore, miRi-PCNPs with specific renal targeting and strong antifibrotic therapeutic effects may constitute a promising basis for the design and development of treatments for renal fibrosis.
Porous polymer particles made from PLGA satisfy unique characteristics required for drug delivery to the lungs, such as aerodynamic density, an adaptive aerodynamic diameter, a porous surface, sustainable release, and enhanced lung deposition effects, which can promote access by or weaken the phagocytosis of lung macrophages. Their good atomization characteristics in the lungs have been exploited to deliver miRNAs to the lungs [96, 97]. Doxorubicin and miR-519c were encapsulated in porous PLGA particles via the water-oil-water emulsion solvent evaporation method and were used for pulmonary administration [98]. These two-component porous PLGA particles had a stronger inhibitory effect on cell proliferation than porous PLGA particles containing a single component (doxorubicin or miR-519c). Therefore, porous PLGA particles can enhance antifibrotic effects and reduce side effects and can be used as sustained-release carriers for the treatment of idiopathic pulmonary fibrosis.
3.3.2 Natural polymers
3.3.2.1 Chitosan
Chitosans (CS) are a family of biodegradable, nontoxic, linear cationic polysaccharides consisting of repeating D-glucosamine and N-acetyl-D-glucosamine units connected by 1,4-glycosidic bonds. They are produced by partial deacetylation of chitin isolated from crustacean shells [99]. Chitosans alone can prevent tendon adhesion during tendon healing. In addition, Chen et al. found that CS can increase the sliding distance of repaired tendons and reduce the content of collagen fibers [100]. This effect of CS may occur through the promotion of miR-29b and P21 expression in fibroblasts with a concurrent reduction in the levels of TGF-β1 and Smad3.
MiR-21 promotes fibroblast proliferation and collagen formation in tissue fibrosis, and CS/TPP/hyaluronic acid (HA) nanoparticles are coated with a smooth Ti surface, which promotes gingival fibroblast adhesion, proliferation, and increased expression of genes related to the extracellular matrix [101]. Chitosans were used to prepare CS-tripolyphosphate-miR-126 (CS-TPP-miRNA) nanocomposites, and the nanoparticles showed relative non-toxicity. However, compared with that of the PEI-miRNA nanodelivery system, the ability of the CS-TPP-miRNA system to deliver miR-126 was poor (Fig. 3) [87]. Therefore, the CS-loaded miRNA system needs further optimization.
3.3.2.2 Hyaluronic Acid
Hyaluronic acid sulfate is an anionic nanocarrier that can deliver the reversible complex of miRNA or siRNA with calcium ions in solution to produce an anionic complex [102]. Compared with unmodified HA, sulfated HA is not easily degraded, has enhanced stability during transport, and contains multiple functional groups that can be used for targeted ligand binding and other functions [103]. Hyaluronic acid sulfate can deliver miRNA to inhibit the development of fibrosis. Nanoparticles assembled from miR-21 mimics, calcium bridge channels, and HA sulfate can be delivered to cardiac macrophages after a myocardial infarction (Fig. 3) [104]. MiR-24-3p-rich exosomes functionalized di(ethylene glycol) monomethyl ether methacrylate-modified HA hydrogels reduces corneal stromal fibrosis and activation of macrophages activation, suggesting its potential utility in cell-free therapy for corneal epithelial regeneration [105]. These nanoparticles can induce a switch from a proinflammatory to a reparative phenotype, regulate angiogenesis, and reduce fibrosis in the distal myocardium.
3.4 Lipid-Based Delivery Systems
Lipids, including cationic lipids, ionizable lipids, and auxiliary lipids, are one of the most widely used vehicles for nucleic acid delivery [106]. Cationic lipids have been developed for use in lipid-based nanoparticles to deliver siRNA and miRNA [107]. However, less attention has been devoted to “helper lipids”. The addition of the unsaturated fatty acid oleic acid to LNP formulations significantly improved the mRNA delivery efficiency [108]. MiR-122 is a biospecific miRNA associated with many liver diseases, including liver fibrosis [109]. Lipid-based nanoparticles containing oleic acid delivered miR-122 more effectively than the commercial transfection agent Lipofectamine 2000. The expression of mature miR-122 increased 1.8-fold, and the target of miR-122, Bcl-w, was significantly downregulated. Compared with Invivofectamine®, another commercial transfection agent designed specifically for liver delivery, lipid-based nanoparticles containing oleic acid showed considerable liver accumulation and delivery efficiency in vivo. Yan et al. designed a lung-targeted cationic liposome preparation to encapsulate anti-miR-21, and cationic liposome-miR-21 was delivered to inhibit myofibroblast differentiation, which reduced extracellular matrix synthesis, and inhibited fibrotic progression [110]. These findings demonstrate the importance of the “helper lipid” component in LNP preparations for enhancing the miRNA uptake and transfection efficiency [111]. Lipid-based nanoparticles containing OA are promising nanocarriers for miRNA-based therapy in liver fibrosis.
3.5 Exosome-Based Delivery Systems
Exosomes are nanosized (40–100 nm) vesicular particles secreted by cells and exist in various body fluids, such as plasma, saliva, and urine [112]. The membrane is composed of lipid raft domains containing proteins and lipids, which protects the cell-specific proteins, lipids, and nucleic acids (mRNAs, miRNAs, and lncRNAs) carried within from RNase-mediated degradation [113]. Exosomes can participate in immune regulation, cell migration and differentiation, angiogenesis, and proteolysis. Cells can selectively transport noncoding RNA-containing exosomes to adjacent or distant cells, and exosomes have an active sorting mechanism, which plays an important role in cellular information exchange [114]. Interestingly, stem cell-derived exosomes have been found to alleviate fibrosis by delivering miRNAs (Fig. 2) [115].
Cy3-labeled let-7 mimics and antagomiR-let-7 were shown to be delivered to alveolar epithelial cells and lung tissue through menstrual blood-derived, stem-cell secreted exosomes. Let-7 in exosomes can reduce reactive oxygen species levels, alleviate mitochondrial DNA damage, and activate NLRP3 to relieve pulmonary fibrosis [116]. Exosomes secreted from placenta-derived mesenchymal stem cells can express high levels of miR-29c, and were shown to deliver miR-29c from exosomes to myofibroblasts in a co-culture system [117]. Placental-derived mesenchymal stem cells were found to reduce the degree of fibrosis in the myocardium and diaphragm [118]. Adipose stem cell-derived exosomes contain high levels of miRNA, and exosomal miRNA can reduce the expression of fibrosis-related proteins and relieve myocardial fibrosis after an acute myocardial infarction [119,120,121,122]. Exosomes derived from adipose-derived mesenchymal stem cells inhibit the proliferation of keloid fibroblasts and promote angiogenesis through miR-181a and miR-7846-3p [123, 124]. Bone marrow mesenchymal stem cell-derived exosomal miR-21a-5p attenuates glycolysis by targeting ATP-dependent 6-phosphofructokinase, thereby alleviating renal fibrosis [125]. Inhibiting the interleukin-33/ST2 axis by delivering miR-214 thereby relieves skin fibrosis in systemic sclerosis [126]. Exosomal miR-17 derived from human embryonic stem cells prevents pulmonary fibrosis by targeting thrombospondin-2 [127].
In addition to stem cells, exosomes secreted by differentiated cells also mediate the progression of fibrosis. Hepatocyte-derived exosomal miR-146a-5p suppresses the epithelial–mesenchymal transition process in hepatic stellate cells [128]. The exosome-associated miR-99a-5p targeting BMPR 2 promotes hepatocyte apoptosis during liver fibrosis [129]. Satellite cell-derived exosome-mediated delivery of the miR-23a/27a/26a cluster ameliorates renal tubulointerstitial fibrosis in diabetic nephropathy in mice [130]. Exosomal miR-381 derived from M2-polarized macrophages attenuates the activation of urethral fibroblasts through YAP/gls 1-regulated glutaminolysis [131]. Urinary exosomal miR-615-3p and miR-3147 are highly expressed and associated with inflammation and fibrosis in diabetic nephropathy [132]. Plasma exosomal miR-125a-5p regulates T-lymphocyte subsets, promoting silica-induced pulmonary fibrosis by targeting tumor necrosis factor receptor-associated factor 6 [133].
Obviously, stem cell-derived exosomes are very promising for the development of new materials for miRNA nanodelivery systems; more importantly, the development of artificial exosomes for miRNA delivery has been attempted [134,135,136]. The miR-141-3p-functionalized exosomes were loaded in a soluble microneedle array for the treatment of hypertrophic scars on rabbit ears [137]. Microneedle patches loaded with exosomes containing miR-29b prevented cardiac fibrosis in a mouse myocardial infarction model [138]. The delivery of miRNA via exosomes may prevent and alleviate fibrotic diseases.
4 Prospects and Conclusion
RNA interference-based drugs have been used in therapy, and oligonucleotide mimics constructed using miRNAs have been clinically tested in fibrotic diseases [72]. Various nanomaterials as new vehicles for miRNA delivery, offer a potentially effective therapeutic strategy for fibrotic diseases and combination drug therapy. Various miRNA delivery pathways have been explored and are showing promise. The preparation method of liposomes is simple, safe, and non-toxic. Unfortunately, the synthesis of liposome molecules is complex and expensive. Polymer nanodelivery systems have significant advantages, such as high therapeutic efficiency and good biocompatibility; however, designing multifunctional nanodelivery systems still faces many challenges. Inorganic nanoparticles can protect miRNA from nuclease degradation and regulate the expression of cellular target genes, which is promising, but their biodegradable properties need to be further improved. Stem cell-derived exosomes provide new strategies and methods for tissue repair and antifibrosis of miRNA; however, the methods to isolate and purify exosomes needs improvement [139,140,141].
However, the delivery of miRNAs to specifically regulate target genes is still the greatest challenge to be overcome [142] and to achieve this, two details need to be clarified. First, miRNAs undoubtedly have high specificity and low immunogenicity, but their biological function and the mechanism by which they regulate fibrosis still need to be further understood. Second, the liposome molecular synthesis process is still relatively complex; moreover, although the inorganic nanoparticle preparation method is simple and these nanoparticles have good biocompatibility and stability, their biodegradability remains an issue, and their metabolic kinetics still need to be clarified in vivo [143, 144].
With the developments in nanomaterials and biomedicine, as well as the clarification of the mechanism underlying miRNA-mediated fibrosis, the development of nanocarriers with different structures and functions is expected to solve the problems of effective miRNA delivery. The safety, efficiency, and stability of miRNA nanodelivery for fibrosis treatment is expected to be improved.
References
Rosenbloom J, Castro SV, Jimenez SA. Narrative review: fibrotic diseases: cellular and molecular mechanisms and novel therapies. Ann Intern Med. 2010;152(3):159–66.
Gerarduzzi C, Di Battista JA. Myofibroblast repair mechanisms post-inflammatory response: a fibrotic perspective. Inflamm Res. 2017;66(6):451–65.
Cvjeticanin B, Prutki M, Dumic-Cule I, Veir Z, Grgurevic L, Vukicevic S. Possible target for preventing fibrotic scar formation following acute myocardial infarction. Med Hypotheses. 2014;83(6):656–8.
Song K, Li Q, Yin XY, Lu Y, Liu CF, Hu LF. Hydrogen sulfide: a therapeutic candidate for fibrotic disease? Oxid Med Cell Longev. 2015;2015: 458720.
Lemoinne S, Friedman SL. New and emerging anti-fibrotic therapeutics entering or already in clinical trials in chronic liver diseases. Curr Opin Pharmacol. 2019;49:60–70.
Zhao Y, Shi J, Lyu L. Critical role and potential therapeutic efficacy of interleukin-37 in the pathogenesis of keloid scarring. J Cosmet Dermatol. 2020;19(7):1805–6.
Rockel JS, Rabani R, Viswanathan S. Anti-fibrotic mechanisms of exogenously-expanded mesenchymal stromal cells for fibrotic diseases. Semin Cell Dev Biol. 2020;101:87–103.
Ulukan B, SilaOzkaya Y, Zeybel M. Advances in the epigenetics of fibroblast biology and fibrotic diseases. Curr Opin Pharmacol. 2019;49:102–9.
Kranick JC, Chadalavada DM, Sahu D, Showalter SA. Engineering double-stranded RNA binding activity into the Drosha double-stranded RNA binding domain results in a loss of microRNA processing function. PLoS One. 2017;12(8): e0182445.
Lee D, Shin C. Emerging roles of DROSHA beyond primary microRNA processing. RNA Biol. 2018;15(2):186–93.
McGeary SE, Lin KS, Shi CY, Pham TM, Bisaria N, Kelley GM, et al. The biochemical basis of microRNA targeting efficacy. Science. 2019;366(6472): eaav1741.
Brummer A, Hausser J. MicroRNA binding sites in the coding region of mRNAs: extending the repertoire of post-transcriptional gene regulation. BioEssays. 2014;36(6):617–26.
Hausser J, Syed AP, Bilen B, Zavolan M. Analysis of CDS-located miRNA target sites suggests that they can effectively inhibit translation. Genome Res. 2013;23(4):604–15.
Fang Z, Rajewsky N. The impact of miRNA target sites in coding sequences and in 3’UTRs. PLoS One. 2011;6(3): e18067.
Xie M, Li M, Vilborg A, Lee N, Shu MD, Yartseva V, et al. Mammalian 5′-capped microRNA precursors that generate a single microRNA. Cell. 2013;155(7):1568–80.
Wang Z, Ma Z, Castillo-Gonzalez C, Sun D, Li Y, Yu B, et al. SWI2/SNF2 ATPase CHR2 remodels pri-miRNAs via Serrate to impede miRNA production. Nature. 2018;557(7706):516–21.
He Y, Deng Z, Alghamdi M, Lu L, Fear MW, He L. From genetics to epigenetics: new insights into keloid scarring. Cell Prolif. 2017;50(2): e12326.
Shi J, Yao S, Chen P, Yang Y, Qian M, Han Y, et al. The integrative regulatory network of circRNA and microRNA in keloid scarring. Mol Biol Rep. 2020;47(1):201–9.
Zhang J, Xu D, Li N, Li Y, He Y, Hu X, et al. Downregulation of microRNA-31 inhibits proliferation and induces apoptosis by targeting HIF1AN in human keloid. Oncotarget. 2017;8(43):74623–34.
Wolska-Gawron K, Bartosinska J, Krasowska D. MicroRNA in localized scleroderma: a review of literature. Arch Dermatol Res. 2020;312(5):317–24.
Gabisonia K, Prosdocimo G, Aquaro GD, Carlucci L, Zentilin L, Secco I, et al. MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature. 2019;569(7756):418–22.
Lyu L, Zhao Y, Lu H, Liu Z, Guo J, Lu D, et al. Integrated interaction network of microRNA target genes in keloid scarring. Mol Diagn Ther. 2019;23(1):53–63.
Zhong L, Bian L, Lyu J, Jin H, Liu Z, Lyu L, et al. Identification and integrated analysis of microRNA expression profiles in keloid. J Cosmet Dermatol. 2018;17(5):917–24.
Ghafouri-Fard S, Abak A, Talebi SF, Shoorei H, Branicki W, Taheri M, et al. Role of miRNA and lncRNAs in organ fibrosis and aging. Biomed Pharmacother. 2021;143: 112132.
Zhao H, Feng YL, Liu T, Wang JJ, Yu J. MicroRNAs in organ fibrosis: From molecular mechanisms to potential therapeutic targets. Pathol Res Pract. 2021;225: 153588.
Henry TW, Mendoza FA, Jimenez SA. Role of microRNA in the pathogenesis of systemic sclerosis tissue fibrosis and vasculopathy. Autoimmun Rev. 2019;18(11): 102396.
Li Y, Zhang J, Lei Y, Lyu L, Zuo R, Chen T. MicroRNA-21 in skin fibrosis: potential for diagnosis and treatment. Mol Diagn Ther. 2017;21(6):633–42.
Suzuki HI. MicroRNA control of TGF-beta signaling. Int J Mol Sci. 2018;19(7):1901.
Jakob P, Landmesser U. Current status of cell-based therapy for heart failure. Curr Heart Fail Rep. 2013;10(2):165–76.
Kamps JA, Krenning G. Micromanaging cardiac regeneration: targeted delivery of microRNAs for cardiac repair and regeneration. World J Cardiol. 2016;8(2):163–79.
Gurbuz N, Ozpolat B. MicroRNA-based targeted therapeutics in pancreatic cancer. Anticancer Res. 2019;39(2):529–32.
Landmesser U, Poller W, Tsimikas S, Most P, Paneni F, Luscher TF. From traditional pharmacological towards nucleic acid-based therapies for cardiovascular diseases. Eur Heart J. 2020;41(40):3884–99.
Miniarikova J, Evers MM, Konstantinova P. Translation of microRNA-based Huntingtin-lowering therapies from preclinical studies to the clinic. Mol Ther. 2018;26(4):947–62.
Shatsberg Z, Zhang X, Ofek P, Malhotra S, Krivitsky A, Scomparin A, et al. Functionalized nanogels carrying an anticancer microRNA for glioblastoma therapy. J Control Release. 2016;10(239):159–68.
Ruman U, Fakurazi S, Masarudin MJ, Hussein MZ. Nanocarrier-based therapeutics and theranostics drug delivery systems for next generation of liver cancer nanodrug modalities. Int J Nanomed. 2020;15:1437–56.
Ma G, Lin W, Wang Z, Zhang J, Qian H, Xu L, et al. Development of polypeptide-based zwitterionic amphiphilic micelles for nanodrug delivery. J Mater Chem B. 2016;4(31):5256–64.
Wang Q, Jiang N, Fu B, Huang F, Liu J. Self-assembling peptide-based nanodrug delivery systems. Biomater Sci. 2019;7(12):4888–911.
Ghobadi AF, Jayaraman A. Effect of backbone chemistry on hybridization thermodynamics of oligonucleic acids: a coarse-grained molecular dynamics simulation study. Soft Matter. 2016;12(8):2276–87.
Jing Z, Qi R, Thibonnier M, Ren P. Molecular dynamics study of the hybridization between RNA and modified oligonucleotides. J Chem Theory Comput. 2019;15(11):6422–32.
Manicardi A, Gambari R, de Cola L, Corradini R. Preparation of anti-miR PNAs for drug development and nanomedicine. Methods Mol Biol. 2018;1811:49–63.
Kauppinen S, Vester B, Wengel J. Locked nucleic acid: high-affinity targeting of complementary RNA for RNomics. Handb Exp Pharmacol. 2006;173(173):405–22.
Gallant-Behm CL, Piper J, Dickinson BA, Dalby CM, Pestano LA, Jackson AL. A synthetic microRNA-92a inhibitor (MRG-110) accelerates angiogenesis and wound healing in diabetic and nondiabetic wounds. Wound Repair Regen. 2018;26(4):311–23.
Sanghvi YS, Schulte M. Therapeutic oligonucleotides: the state-of-the-art in purification technologies. Curr Opin Drug Discov Devel. 2004;7(6):765–76.
Smith CIE, Zain R. Therapeutic oligonucleotides: state of the art. Annu Rev Pharmacol Toxicol. 2019;6(59):605–30.
Fernandez Fernandez E, Santos-Carballal B, de Santi C, Ramsey JM, MacLoughlin R, Cryan SA, et al. Biopolymer-based nanoparticles for cystic fibrosis lung gene therapy studies. Materials (Basel). 2018;11(1):122.
De Santi C, Nally FK, Afzal R, Duffy CP, Fitzsimons S, Annett SL, et al. Enhancing arginase 2 expression using target site blockers as a strategy to modulate macrophage phenotype. Mol Ther Nucleic Acids. 2022;13(29):643–55.
Kumar V, Kumar V, Luo J, Mahato RI. Therapeutic potential of OMe-PS-miR-29b1 for treating liver fibrosis. Mol Ther. 2018;26(12):2798–811.
Kumar V, Mondal G, Dutta R, Mahato RI. Co-delivery of small molecule Hedgehog inhibitor and miRNA for treating liver fibrosis. Biomaterials. 2016;76:144–56.
Momen-Heravi F, Catalano D, Talis A, Szabo G, Bala S. Protective effect of LNA-anti-miR-132 therapy on liver fibrosis in mice. Mol Ther Nucleic Acids. 2021;3(25):155–67.
Ghosh N, Fenton S, van Hout I, Jones GT, Coffey S, Williams MJA, et al. Therapeutic knockdown of miR-320 improves deteriorated cardiac function in a pre-clinical model of non-ischemic diabetic heart disease. Mol Ther Nucleic Acids. 2022;13(29):330–42.
Nonaka CKV, Sampaio GL, Silva KN, Khouri R, Macedo CT, Chagas Translational Research Consortium, et al. Therapeutic miR-21 silencing reduces cardiac fibrosis and modulates inflammatory response in chronic Chagas disease. Int J Mol Sci. 2021;22(7):3307.
Zhang XL, Zhang G, Bai ZH. miR-34a attenuates myocardial fibrosis in diabetic cardiomyopathy mice via targeting Pin-1. Cell Biol Int. 2021;45(3):642–53.
Hinkel R, Ramanujam D, Kaczmarek V, Howe A, Klett K, Beck C, et al. AntimiR-21 prevents myocardial dysfunction in a pig model of ischemia/reperfusion injury. J Am Coll Cardiol. 2020;75(15):1788–800.
Hao X, Luan J, Jiao C, Ma C, Feng Z, Zhu L, et al. LNA-anti-miR-150 alleviates renal interstitial fibrosis by reducing pro-inflammatory M1/M2 macrophage polarization. Front Immunol. 2022;13: 913007.
Luan J, Fu J, Wang D, Jiao C, Cui X, Chen C, et al. miR-150-Based RNA interference attenuates tubulointerstitial fibrosis through the SOCS1/JAK/STAT pathway in vivo and in vitro. Mol Ther Nucleic Acids. 2020;4(22):871–84.
Fluitt MB, Shivapurkar N, Kumari M, Singh S, Li L, Tiwari S, et al. Systemic inhibition of miR-451 increases fibrotic signaling and diminishes autophagic response to exacerbate renal damage in Tallyho/Jng mice. Am J Physiol Renal Physiol. 2020;319(3):F476–86.
Sonneville F, Ruffin M, Coraux C, Rousselet N, Le Rouzic P, Blouquit-Laye S, et al. MicroRNA-9 downregulates the ANO1 chloride channel and contributes to cystic fibrosis lung pathology. Nat Commun. 2017;8(1):710.
Fabbri E, Tamanini A, Jakova T, Gasparello J, Manicardi A, Corradini R, et al. Treatment of human airway epithelial Calu-3 cells with a peptide-nucleic acid (PNA) targeting the microRNA miR-101-3p is associated with increased expression of the cystic fibrosis transmembrane conductance regulator () gene. Eur J Med Chem. 2021;1(209): 112876.
Sultan S, Rozzi A, Gasparello J, Manicardi A, Corradini R, Papi C, et al. A peptide nucleic acid (PNA) masking the miR-145-5p binding site of the 3′UTR of the cystic fibrosis transmembrane conductance regulator (CFTR) mRNA enhances CFTR expression in Calu-3 cells. Molecules. 2020;25(7):1677.
Fabbri E, Tamanini A, Jakova T, Gasparello J, Manicardi A, Corradini R, et al. A peptide nucleic acid against microRNA miR-145-5p enhances the expression of the cystic fibrosis transmembrane conductance regulator (CFTR) in Calu-3 cells. Molecules. 2017;23(1):71.
Fernandez Fernandez E, De Santi C, De Rose V, Greene CM. CFTR dysfunction in cystic fibrosis and chronic obstructive pulmonary disease. Expert Rev Respir Med. 2018;12(6):483–92.
McKiernan PJ, Greene CM. MicroRNA dysregulation in cystic fibrosis. Mediators Inflamm. 2015;2015: 529642.
Ramachandran S, Karp PH, Osterhaus SR, Jiang P, Wohlford-Lenane C, Lennox KA, et al. Post-transcriptional regulation of cystic fibrosis transmembrane conductance regulator expression and function by microRNAs. Am J Respir Cell Mol Biol. 2013;49(4):544–51.
Zarrilli F, Amato F, Morgillo CM, Pinto B, Santarpia G, Borbone N, et al. Peptide nucleic acids as miRNA target protectors for the treatment of cystic fibrosis. Molecules. 2017;22(7):1144.
Amato F, Tomaiuolo R, Nici F, Borbone N, Elce A, Catalanotti B, et al. Exploitation of a very small peptide nucleic acid as a new inhibitor of miR-509-3p involved in the regulation of cystic fibrosis disease-gene expression. Biomed Res Int. 2014;2014: 610718.
Megiorni F, Cialfi S, Cimino G, De Biase RV, Dominici C, Quattrucci S, et al. Elevated levels of miR-145 correlate with SMAD3 down-regulation in cystic fibrosis patients. J Cyst Fibros. 2013;12(6):797–802.
Papi C, Gasparello J, Zurlo M, Manicardi A, Corradini R, Cabrini G, et al. Combined treatment of bronchial epithelial Calu-3 cells with peptide nucleic acids targeting miR-145-5p and miR-101-3p: synergistic enhancement of the expression of the cystic fibrosis transmembrane conductance regulator (CFTR) gene. Int J Mol Sci. 2022;23(16):9348.
Ahangari F, Price NL, Malik S, Chioccioli M, Barnthaler T, Adams TS, et al. microRNA-33 deficiency in macrophages enhances autophagy, improves mitochondrial homeostasis, and protects against lung fibrosis. JCI Insight. 2023;8(4): e158100.
Price NL, Miguel V, Ding W, Singh AK, Malik S, Rotllan N, et al. Genetic deficiency or pharmacological inhibition of miR-33 protects from kidney fibrosis. JCI Insight. 2019;4(22): e131102.
Deng Z, He Y, Yang X, Shi H, Shi A, Lu L, et al. MicroRNA-29: a crucial player in fibrotic disease. Mol Diagn Ther. 2017;21(3):285–94.
Montgomery RL, Yu G, Latimer PA, Stack C, Robinson K, Dalby CM, et al. MicroRNA mimicry blocks pulmonary fibrosis. EMBO Mol Med. 2014;6(10):1347–56.
Gallant-Behm CL, Piper J, Lynch JM, Seto AG, Hong SJ, Mustoe TA, et al. A microRNA-29 mimic (Remlarsen) represses extracellular matrix expression and fibroplasia in the skin. J Invest Dermatol. 2019;139(5):1073–81.
Beck C, Ramanujam D, Vaccarello P, Widenmeyer F, Feuerherd M, Cheng CC, et al. Trimannose-coupled antimiR-21 for macrophage-targeted inhalation treatment of acute inflammatory lung damage. Nature Commun. 2023;14(1):4564.
Ma C, Gerhard E, Lu D, Yang J. Citrate chemistry and biology for biomaterials design. Biomaterials. 2018;178:383–400.
Huang Y, Lu X, Qu Y, Yang Y, Wu S. MicroRNA sequencing and molecular mechanisms analysis of the effects of gold nanoparticles on human dermal fibroblasts. Biomaterials. 2015;37:13–24.
Chan MY, Vikesland PJ. Porous media-induced aggregation of protein-stabilized gold nanoparticles. Environ Sci Technol. 2014;48(3):1532–40.
Mohammadniaei M, Koyappayil A, Sun Y, Min J, Lee MH. Gold nanoparticle/MXene for multiple and sensitive detection of oncomiRs based on synergetic signal amplification. Biosens Bioelectron. 2020;1(159): 112208.
Robinson PM, Chuang TD, Sriram S, Pi L, Luo XP, Petersen BE, et al. MicroRNA signature in wound healing following excimer laser ablation: role of miR-133b on TGFbeta1, CTGF, SMA, and COL1A1 expression levels in rabbit corneal fibroblasts. Invest Ophthalmol Vis Sci. 2013;54(10):6944–51.
Zhao X, Song W, Chen Y, Liu S, Ren L. Collagen-based materials combined with microRNA for repairing cornea wounds and inhibiting scar formation. Biomater Sci. 2018;7(1):51–62.
Jia C, Chen H, Wei M, Chen X, Zhang Y, Cao L, et al. Gold nanoparticle-based miR155 antagonist macrophage delivery restores the cardiac function in ovariectomized diabetic mouse model. Int J Nanomedicine. 2017;12:4963–79.
Paoletti A, Rohmer J, Ly B, Pascaud J, Riviere E, Seror R, et al. Monocyte/macrophage abnormalities specific to rheumatoid arthritis are linked to miR-155 and are differentially modulated by different TNF inhibitors. J Immunol. 2019;203(7):1766–75.
Wang Q, Song Y, Chen J, Li Q, Gao J, Tan H, et al. Direct in vivo reprogramming with non-viral sequential targeting nanoparticles promotes cardiac regeneration. Biomaterials. 2021;276: 121028.
Bharadwaz A, Jayasuriya AC. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2020;110: 110698.
Wang J, Zhang L, Wang X, Fu S, Yan G. Acid-labile poly(amino alcohol ortho ester) based on low molecular weight polyethyleneimine for gene delivery. J Biomater Appl. 2017;32(3):349–61.
Wang X, Niu D, Hu C, Li P. Polyethyleneimine-based nanocarriers for gene delivery. Curr Pharm Des. 2015;21(42):6140–56.
Morishita Y, Imai T, Yoshizawa H, Watanabe M, Ishibashi K, Muto S, et al. Delivery of microRNA-146a with polyethylenimine nanoparticles inhibits renal fibrosis in vivo. Int J Nanomedicine. 2015;10:3475–88.
McKiernan PJ, Cunningham O, Greene CM, Cryan SA. Targeting miRNA-based medicines to cystic fibrosis airway epithelial cells using nanotechnology. Int J Nanomedine. 2013;8:3907–15.
Muniyandi P, Palaninathan V, Mizuki T, Mohamed MS, Hanajiri T, Maekawa T. Scaffold mediated delivery of dual miRNAs to transdifferentiate cardiac fibroblasts. Mater Sci Eng C Mater Biol Appl. 2021;128: 112323.
Li Q, Li L, Yu M, Zheng M, Li Y, Yang J, et al. Elastomeric polyurethane porous film functionalized with gastrodin for peripheral nerve regeneration. J Biomed Mater Res A. 2020;108(8):1713–25.
Gong JH, Wang Y, Xing L, Cui PF, Qiao JB, He YJ, et al. Biocompatible fluorinated poly(beta-amino ester)s for safe and efficient gene therapy. Int J Pharm. 2018;535(1–2):180–93.
Yu Q, Xiong X, Zhao L, Xu T, Wang Q. Antifibrotic effects of specific siRNA targeting connective tissue growth factor delivered by polyethyleneimine-functionalized magnetic iron oxide nanoparticles on LX-2 cells. Mol Med Rep. 2020;21(1):181–90.
De Santi C, Fernandez Fernandez E, Gaul R, Vencken S, Glasgow A, Oglesby IK, et al. Precise targeting of miRNA sites restores CFTR activity in CF bronchial epithelial cells. Mol Ther. 2020;28(4):1190–9.
Vencken S, Foged C, Ramsey JM, Sweeney L, Cryan SA, MacLoughlin RJ, et al. Nebulised lipid-polymer hybrid nanoparticles for the delivery of a therapeutic anti-inflammatory microRNA to bronchial epithelial cells. ERJ Open Res. 2019;5(2):00161–2018.
McKiernan PJ, Lynch P, Ramsey JM, Cryan SA, Greene CM. Knockdown of gene expression in macrophages by microRNA mimic-containing poly(lactic-co-glycolic acid) microparticles. Medicines (Basel). 2018;5(4):133.
Geng X, Zhang M, Lai X, Tan L, Liu J, Yu M, et al. Small-sized cationic miRi-PCNPs selectively target the kidneys for high-efficiency antifibrosis treatment. Adv Healthc Mater. 2018;7(21): e1800558.
Patel B, Gupta V, Ahsan F. PEG-PLGA based large porous particles for pulmonary delivery of a highly soluble drug, low molecular weight heparin. J Control Release. 2012;162(2):310–20.
Meenach SA, Kim YJ, Kauffman KJ, Kanthamneni N, Bachelder EM, Ainslie KM. Synthesis, optimization, and characterization of camptothecin-loaded acetalated dextran porous microparticles for pulmonary delivery. Mol Pharm. 2012;9(2):290–8.
Wu D, Wang C, Yang J, Wang H, Han H, Zhang A, et al. Improving the intracellular drug concentration in lung cancer treatment through the codelivery of doxorubicin and miR-519c mediated by porous PLGA microparticle. Mol Pharm. 2016;13(11):3925–33.
Roy H, Rahaman SA, Kumar TV, Nandi S. Current development on chitosan-based antimicrobial drug formulations for the wound healing. Curr Drug Discov Technol. 2020;17(4):534–41.
Chen Q, Lu H, Yang H. Chitosan inhibits fibroblasts growth in Achilles tendon via TGF-β1/Smad3 pathway by miR-29b. Int J Clin Exp Pathol. 2014;7(12):8462–70.
Wang Z, Wu G, Yang Z, Li X, Feng Z, Zhao Y. Chitosan/hyaluronic acid/microRNA-21 nanoparticle-coated smooth titanium surfaces promote the functionality of human gingival fibroblasts. Int J Nanomed. 2022;17:3793–807.
Hwang DW, Kim HY, Li F, Park JY, Kim D, Park JH, et al. In vivo visualization of endogenous miR-21 using hyaluronic acid-coated graphene oxide for targeted cancer therapy. Biomaterials. 2017;121:144–54.
Wang Z, Zang A, Wei Y, An L, Hong D, Shi Y, et al. Hyaluronic acid capped, irinotecan and gene co-loaded lipid-polymer hybrid nanocarrier-based combination therapy platform for colorectal cancer. Drug Des Devel Ther. 2020;14:1095–105.
Bejerano T, Etzion S, Elyagon S, Etzion Y, Cohen S. Nanoparticle delivery of miRNA-21 mimic to cardiac macrophages improves myocardial remodeling after myocardial infarction. Nano Lett. 2018;18(9):5885–91.
Sun X, Song W, Teng L, Huang Y, Liu J, Peng Y, et al. MiRNA 24–3p-rich exosomes functionalized DEGMA-modified hyaluronic acid hydrogels for corneal epithelial healing. Bioactive Mater. 2023;25:640–56.
Wahane A, Waghmode A, Kapphahn A, Dhuri K, Gupta A, Bahal R. Role of lipid-based and polymer-based non-viral vectors in nucleic acid delivery for next-generation gene therapy. Molecules. 2020;25(12):2866.
Scheideler M, Vidakovic I, Prassl R. Lipid nanocarriers for microRNA delivery. Chem Phys Lipids. 2020;226: 104837.
Sosnowska K, Szymanska E, Winnicka K. Nanoemulsion with clotrimazole: design and optimalization of mean droplet size using microfluidization technique. Acta Pol Pharm. 2017;74(2):519–26.
Halasz T, Horvath G, Par G, Werling K, Kiss A, Schaff Z, et al. miR-122 negatively correlates with liver fibrosis as detected by histology and FibroScan. World J Gastroenterol. 2015;21(25):7814–23.
Yan L, Su Y, Hsia I, Xu Y, Vincent-Chong VK, Mojica W, et al. Delivery of anti-microRNA-21 by lung-targeted liposomes for pulmonary fibrosis treatment. Mol Ther Nucleic Acids. 2023;13(32):36–47.
Wang X, Yu B, Ren W, Mo X, Zhou C, He H, et al. Enhanced hepatic delivery of siRNA and microRNA using oleic acid based lipid nanoparticle formulations. J Control Release. 2013;172(3):690–8.
Moon S, Shin DW, Kim S, Lee YS, Mankhong S, Yang SW, et al. Enrichment of exosome-like extracellular vesicles from plasma suitable for clinical vesicular miRNA biomarker research. J Clin Med. 2019;8(11):1995.
Ohno S, Kuroda M. Exosome-mediated targeted delivery of miRNAs. Methods Mol Biol. 2016;1448:261–70.
Mobergslien A, Sioud M. Exosome-derived miRNAs and cellular miRNAs activate innate immunity. J Innate Immun. 2014;6(1):105–10.
Qin XJ, Zhang JX, Wang RL. Exosomes as mediators and biomarkers in fibrosis. Biomark Med. 2020;14(8):697–712.
Sun L, Zhu M, Feng W, Lin Y, Yin J, Jin J, et al. Exosomal miRNA Let-7 from menstrual blood-derived endometrial stem cells alleviates pulmonary fibrosis through regulating mitochondrial DNA damage. Oxid Med Cell Longev. 2019;2019:4506303.
Bier A, Berenstein P, Kronfeld N, Morgoulis D, Ziv-Av A, Goldstein H, et al. Placenta-derived mesenchymal stromal cells and their exosomes exert therapeutic effects in Duchenne muscular dystrophy. Biomaterials. 2018;174:67–78.
Yang L, Wang T, Zhang X, Zhang H, Yan N, Zhang G, et al. Exosomes derived from human placental mesenchymal stem cells ameliorate myocardial infarction via anti-inflammation and restoring gut dysbiosis. BMC Cardiovasc Disord. 2022;22(1):61.
Venkat P, Cui C, Chen Z, Chopp M, Zacharek A, Landschoot-Ward J, et al. CD133+exosome treatment improves cardiac function after stroke in type 2 diabetic mice. Transl Stroke Res. 2021;12(1):112–24.
Luo Q, Guo D, Liu G, Chen G, Hang M, Jin M. Exosomes from MiR-126-overexpressing Adscs are therapeutic in relieving acute myocardial ischaemic injury. Cell Physiol Biochem. 2017;44(6):2105–16.
Wang X, Zhu Y, Wu C, Liu W, He Y, Yang Q. Adipose-derived mesenchymal stem cells-derived exosomes carry microRNA-671 to alleviate myocardial infarction through inactivating the TGFBR2/Smad2 axis. Inflammation. 2021;44(5):1815–30.
Pan J, Alimujiang M, Chen Q, Shi H, Luo X. Exosomes derived from miR-146a-modified adipose-derived stem cells attenuate acute myocardial infarction-induced myocardial damage via downregulation of early growth response factor 1. J Cell Biochem. 2019;120(3):4433–43.
Zhao Y, Du L, Sun J, Wang X, Cong Z, Chen S, et al. Exosomal miR-218 derived from mesenchymal stem cells inhibits endothelial-to-mesenchymal transition by epigenetically modulating of BMP2 in pulmonary fibrosis. Cell Biol Toxicol. 2023. https://doi.org/10.1007/s10565-023-09810-z.
Wu D, Liu X, Jin Z. Adipose-derived mesenchymal stem cells-sourced exosomal microRNA-7846-3p suppresses proliferation and pro-angiogenic role of keloid fibroblasts by suppressing neuropilin 2. J Cosmet Dermatol. 2023;22(8):2333–42.
Xu S, Cheuk YC, Jia Y, Chen T, Chen J, Luo Y, et al. Bone marrow mesenchymal stem cell-derived exosomal miR-21a-5p alleviates renal fibrosis by attenuating glycolysis by targeting PFKM. Cell Death Dis. 2022;13(10):876.
Xie L, Long X, Mo M, Jiang J, Zhang Q, Long M, et al. Bone marrow mesenchymal stem cell-derived exosomes alleviate skin fibrosis in systemic sclerosis by inhibiting the IL-33/ST2 axis via the delivery of microRNA-214. Mol Immunol. 2023;157:146–57.
Liu Q, Bi Y, Song S, Zhu K, Qiao X, Wang H, et al. Exosomal miR-17-5p from human embryonic stem cells prevents pulmonary fibrosis by targeting thrombospondin-2. Stem Cell Res Ther. 2023;14(1):234.
Lang Z, Li Y, Lin L, Li X, Tao Q, Hu Y, et al. Hepatocyte-derived exosomal miR-146a-5p inhibits hepatic stellate cell EMT process: a crosstalk between hepatocytes and hepatic stellate cells. Cell Death Discov. 2023;9(1):304.
Li F, Yan T, Wang S, Wen X. Exosome-associated miRNA-99a-5p targeting BMPR2 promotes hepatocyte apoptosis during the process of hepatic fibrosis. Clin Exp Med. 2023. https://doi.org/10.1007/s10238-023-01122-0.
Ji JL, Shi HM, Li ZL, Jin R, Qu GT, Zheng H, et al. Satellite cell-derived exosome-mediated delivery of microRNA-23a/27a/26a cluster ameliorates the renal tubulointerstitial fibrosis in mouse diabetic nephropathy. Acta Pharmacol Sin. 2023. https://doi.org/10.1038/s41401-023-01140-4.
Chen YH, Xu YC, Lin TT, Chen H, Dong RN, Cai FP, et al. Exosomal MiR-381 from M2-polarized macrophages attenuates urethral fibroblasts activation through YAP/GLS1-regulated glutaminolysis. Inflamm Res. 2023;72(7):1359–73.
Wang J, Tao Y, Zhao F, Liu T, Shen X, Zhou L. Expression of urinary exosomal miRNA-615-3p and miRNA-3147 in diabetic kidney disease and their association with inflammation and fibrosis. Ren Fail. 2023;45(1):2121929.
Ding M, Pei Y, Zhang C, Qi Y, Xia J, Hao C, et al. Exosomal miR-125a-5p regulates T lymphocyte subsets to promote silica-induced pulmonary fibrosis by targeting TRAF6. Ecotoxicol Environ Saf. 2023;1(249): 114401.
Zhang KL, Wang YJ, Sun J, Zhou J, Xing C, Huang G, et al. Artificial chimeric exosomes for anti-phagocytosis and targeted cancer therapy. Chem Sci. 2019;10(5):1555–61.
Zhang Z, Dombroski JA, King MR. Engineering of exosomes to target cancer metastasis. Cell Mol Bioeng. 2020;13(1):1–16.
Zhu L, Bao L, Zhang X, Xia X, Sun H. Inhibition of porcine reproductive and respiratory syndrome virus replication with exosome-transferred artificial microRNA targeting the 3’ untranslated region. J Virol Methods. 2015;223:61–8.
Meng S, Wei Q, Chen S, Liu X, Cui S, Huang Q, et al. MiR-141-3p-functionalized exosomes loaded in dissolvable microneedle arrays for hypertrophic scar treatment. Small. 2023;18: e2305374.
Yuan J, Yang H, Liu C, Shao L, Zhang H, Lu K, et al. Microneedle patch loaded with exosomes containing microRNA-29b prevents cardiac fibrosis after myocardial infarction. Adv Healthc Mater. 2023;12(13): e2202959.
Wei J, Han X, Zhang C, Liao W, Qin X, Li L, et al. Intracellular delivery of microRNA therapeutics based on nanocarriers: current status and future perspective. Mater Rep. 2019;33(1):16–26.
Bai Z, Wei J, Yu C, Han X, Qin X, Zhang C, et al. Non-viral nanocarriers for intracellular delivery of microRNA therapeutics. J Mater Chem B. 2019;7(8):1209–25.
Fernandez-Pineiro I, Badiola I, Sanchez A. Nanocarriers for microRNA delivery in cancer medicine. Biotechnol Adv. 2017;35(3):350–60.
Meng Z, Zhou D, Gao Y, Zeng M, Wang W. miRNA delivery for skin wound healing. Adv Drug Deliv Rev. 2018;129:308–18.
Hashemi A, Gorji-Bahri G. MicroRNA: promising roles in cancer therapy. Curr Pharm Biotechnol. 2020;21(12):1186–203.
Zhang D, Lee H, Jin Y. Delivery of functional small RNAs via extracellular vesicles in vitro and in vivo. Methods Mol Biol. 2020;2115:107–17.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This article was supported by grants from the National Natural Science Foundation of China (grant nos. 81960354, 82360447), the Joint Fund for Basic Research of Yunnan Provincial Department of Science and Technology and Kunming Medical University (202101AY070001-012, 202001AY070001-014), Kunming Science and Technology Plan Project (2019-1-N-25318000003496) and Bai Xiaochun expert workstation (YSZJGZZ-2020040).
Conflicts of Interest
Yanfang Guo, Hanying Wang, Rumin Lyu, Juan Wang, Ting Wang, Jingpei Shi and Lechun Lyu have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Authors’ Contributions
LL and JS contributed to the conception. YG, HW, RL, JW, and TW performed the experiments. LL and JS wrote the original draft. All the authors have reviewed the final manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, Y., Wang, H., Lyu, R. et al. Nanocarrier-Mediated Delivery of MicroRNAs for Fibrotic Diseases. Mol Diagn Ther 28, 53–67 (2024). https://doi.org/10.1007/s40291-023-00681-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-023-00681-y