Abstract
Background
Physical activity has been associated with reduced oxidative stress (OS) in observational studies and clinical trials.
Objective
The purpose of this systematic review and meta-analysis of controlled trials was to determine the effect of physical exercise on OS parameters.
Methods
We conducted a systematic review of the literature up to March 2016 that included the following databases: PubMed, SCOPUS, and Web of Science. A keyword combination referring to exercise training and OS was included as part of a more thorough search process. We also manually searched the reference lists of the articles. From an initial 1573 references, we included 30 controlled trials (1346 participants) in the qualitative analysis, 19 of which were included in the meta-analysis. All trials were conducted in humans and had at least one exercise intervention and a paired control group. Using a standardized protocol, two investigators independently abstracted data on study design, sample size, participant characteristics, intervention, follow-up duration, outcomes, and quantitative data for the meta-analysis. Thus, the investigators independently assigned quality scores with a methodological quality assessment (MQA).
Results
The agreement level between the reviewers was 85.3 %. Discrepancies were solved in a consensus meeting. The MQA showed a total score in the quality index between 40 and 90 % and a mean quality of 55 %. Further, in a random-effects model, data from each trial were pooled and weighted by the inverse of the total variance. Physical training was associated with a significant reduction in pro-oxidant parameters (standard mean difference [SMD] –1.08; 95 % confidence interval [CI] –1.57 to –0.58; p < 0.001) and an increase in antioxidant capacity (SMD 1.45; 95 % CI 0.83–2.06; p < 0.001).
Conclusion
The pooled analysis revealed that regardless of intensity, volume, type of exercise, and studied population, the antioxidant indicators tended to increase and pro-oxidant indicators tended to decrease after training. Therefore, we conclude that exercise training seems to induce an antioxidant effect. Thus, it is suggested that people practice some kind of exercise to balance the redox state, regardless of their health status, to improve health-related outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Regardless of intensity, volume, type of exercise and studied population, antioxidant parameters seem to increase and pro-oxidant indicators to decrease after physical training. |
While data relating to redox balance after exercise training for specific populations are sparse, the available evidence suggests that the general population can undertake any kind of physical training to decrease oxidative stress and prevent and treat metabolic diseases. |
1 Introduction
Worldwide, about 30 % of adults are insufficiently active [1], which is a greater risk factor than smoking, high cholesterol, and elevated blood pressure for cardiovascular disease and all-cause mortality [2, 3]. A sedentary lifestyle is associated with several diseases, such as obesity [4], type 2 diabetes mellitus (T2DM) [5], hypertension [6], coronary artery disease [7], and many others. Thus, physical fitness components have been frequently used in clinical and scientific practice as markers of health status [8, 9], highlighting the importance of regular physical exercise for health [10].
The conditions by which a sedentary lifestyle may lead to diseases are diverse, but usually are related to metabolic and immune disorders [11], which may be both preceded by [12] and/or result in degenerative processes such as oxidative stress (OS) and chronic inflammation [13] that in turn may accelerate the aging process. OS is the imbalance between reactive oxygen species (ROS), which are byproducts of aerobic metabolism, and the mechanisms of defense and repair, known widely as antioxidants [14]. Although ROS may trigger fundamental signaling pathways in skeletal muscle [15], its exacerbated production may lead to intra- and extra-cellular damage-induced malfunction of molecular mechanisms and chronic inflammatory conditions [16–19]. OS is associated with the development of various diseases, including atherosclerosis [20], cardiovascular diseases [21], T2DM [22], cancer [23, 24], and neurological diseases [25].
On the other hand, it is well-established that physical exercise may reduce inflammation in the long term [26–30]. Today, the scientific evidence is strong regarding how a physically active lifestyle attenuates OS. This attenuation may be one of the mechanisms responsible for the improvement in several clinical aspects, such as attenuated cellular aging [31], increased insulin sensitivity and lipid profile regulation [32], and reduced endothelial dysfunction [33] after exercise training.
Traditionally, OS is quantified by pro-oxidant parameters, which may indicate DNA damage, lipid peroxidation, or protein oxidation. On the other hand, these effects seem to be attenuated through the activity of antioxidants, which may be enzymatic or non-enzymatic, and reflect the total antioxidant capacity [34].
Regarding the OS response to physical exercise, it seems that exercise acutely increases ROS production followed by antioxidant compensation [35]. Longitudinally, studies have suggested that the effect on OS of physical exercise may vary according to the exercise mode, volume, intensity, and population, making it difficult to reach a consensus about the effects of exercise on oxidative balance. Vincent et al. [4] suggested that physical training would be the only method capable of reducing OS independently of body adiposity, when compared with other interventions such as caloric restriction, bariatric surgery, and pharmacotherapy or antioxidant supplementation. However, comparisons are quite difficult, as studies have been conducted using a wide variety of experimental designs, e.g., studied samples, interventions, and outcomes [36, 37]. Moreover, although several narrative reviews and different clinical trials have investigated the effects of exercise training on OS parameters, a pooled analysis may add some weight to the conclusions of individual studies. Therefore, to contribute to the literature, this review aimed to summarize the effects of regular physical exercise on pro- and antioxidant parameters through a systematic review and meta-analysis of controlled clinical trials. To the best of our knowledge, this is the first time such a review and analysis has been undertaken.
2 Methods
2.1 Search Strategy
We conducted a systematic review of the literature up to March 2016, including the following databases: PubMed, SCOPUS, and Web of Science. We also manually searched the reference lists of the articles. We chose the date of the first article published in 2004 [38] that related to exercise training effect on OS markers as the initial date of the search.
2.2 Inclusion/Exclusion Criteria
Studies proposed to be included in the review were checked for the following criteria: (1) the study was a full report published in a peer-reviewed journal; (2) the study assessed humans; (3) one or more exercise programs were performed; (4) the study was a randomized or non-randomized clinical/controlled trial; and (5) the keyword combination referred to exercise training. We included OS as part of a thorough and exhaustive search process.
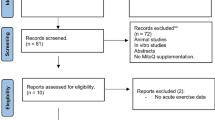
Articles were excluded if (1) we could not obtain the full-text article; (2) exercise was not performed; (3) the study did not include a non-exercise control group; (4) articles were not written in English, Spanish, or Portuguese; and (5) the study used drug administration or nutritional supplementation before or after the exercise (Fig. 1).
2.3 Identification of Eligible Studies
Eligible studies were longitudinal experimental interventions executed in humans that analyzed the effects of exercise training on OS parameters and did not administer any drug or nutritional supplementation. At least two independent researchers read and evaluated the abstracts of all articles identified through the search and selected potentially eligible articles. They also carefully checked the reference list of every article to identify other potentially eligible studies.
2.4 Quality Assessment
The selected studies were subsequently analyzed using a methodological quality assessment (MQA) adapted from the Downs and Black Quality Index [39] and Quality Assessment for Longitudinal Studies [40]. This modified version consists of eight objective questions (Table 1). Each study was allocated a “1” for “yes” or a “0” for “no” for each question, as previously described [36], and responses were summed for a total of eight. A total score ≥6 indicated a high-quality study, a total score of 3–5 indicated moderate quality, and a score <3 indicated low quality.
2.5 Data Extraction
We extracted all relevant data from studies that met the eligibility criteria: characteristics of the sample, methodological design, exercise modality, training protocol (volume/intensity), method of measuring OS, and the OS outcomes after exercise training. For this analysis, the number of subjects (n) in each study was only considered for the intervention groups and their paired non-exercise group(s).
2.6 Statistical Analysis
We performed the meta-analysis by computing the standardized mean difference (SMD) (Hedges’ g) and using the random-effects model, and performed quantitative analyses on the confined data derived from the last measure of the intervention and control groups of each study. As previously suggested [41], we combined the outcomes for trials with more than one parameter (pro- or antioxidant). For studies with more than one intervention group (i.e., exercise training), we considered each comparison (treated vs. control) as an independent study. We assessed heterogeneity with the Cochran’s Q test and tau-squared (τ 2) and measured inconsistency (the percentage of total variation across studies due to heterogeneity) of effects across exercise interventions using the I 2 statistic proposed by Higgins et al. [42]. We visually and objectively assessed the risk of publication bias with Egger’s test, a funnel plot symmetry. The significance level was set at p < 0.05 for all analyses, except for Egger’s regression to assess funnel plot asymmetry, which was set at p < 0.1, as suggested [43]. We used the aforementioned procedures for two independent analyses, one for pro-oxidant and another for antioxidant parameters. To assess the statistical power of the included studies, we considered the F statistic for analysis of variance (ANOVA) within and between factors according to the number of groups (i.e., experimental 1 + experimental 2 + control) and moments (baseline vs. post-training) of each study. The statistical power was calculated considering an alpha of 5 % and effect size of 0.25 to reach at least 80 % (1−β = 0.8), to assume that the sample size was sufficient to avoid type 1 or 2 error [44, 45]. We used the software Comprehensive Meta-Analysis (CMA 3.0) and G*power (v. 3.0.10) to carry out all procedures.
3 Results
3.1 Search Results
The search of PubMed, SCOPUS, and Web of Science provided a total of 1573 citations and one identified through a reverse search. After adjusting for duplicates, 1086 articles remained. Of these, we discarded 1020 after abstract review because they did not meet the inclusion criteria. We examined the full text of the remaining 66 citations in more detail; 36 appeared to not meet the inclusion criteria as described. A total of 30 studies met all of the criteria and were therefore included in the systematic review. A further 11 studies were excluded from the meta-analysis because they did not provide enough data for quantitative analysis (Fig. 1).
3.2 Study Characteristics
All studies selected for this review were controlled trials published in English (n = 29) or Spanish (n = 1). The duration of the interventions ranged from 8 to 48 weeks, with two to seven exercise sessions per week. A total of 20 trials had supervised sessions, two were non-supervised, and eight did not report on this. The 30 studies included in the present review tested 38 interventions and involved 1346 participants. The interventions included aerobic training (n = 23), resistance training (n = 8), aerobic + resistance training combined (n = 3), and others (n = 5). A more explicit description of each exercise intervention is available in Table 2. The studies used 12 OS parameters, including seven pro-oxidants and five antioxidants (Table 3). All studies measured OS in the blood, except for one [46] that measured OS via muscle biopsy.
3.3 Methodological Quality Assessment
Table 4 shows the list of included studies with quality scores. The agreement between the two reviewers was 85.3 %. Disagreement was solved in a consensus meeting. We defined five studies as high quality (score ≥6), 24 as moderate quality (3 ≤ score ≤ 5), and one trial as low quality (score <3). Questions 5 and 7 had the lowest score ratio, with only 19.9 % and 26.7 % of the trials scoring ‘yes’ on these questions, respectively.
3.4 Meta-Analysis
Individual and summary effects of the studies included in the meta-analysis (n = 19) are presented in the forest plot (Fig. 2). In the pooled analysis, exercise seems to reduce pro-oxidant parameters (SMD –1.08; 95 % confidence interval [CI] –1.57 to –0.58; p < 0.001) and increase antioxidants (SMD 1.45; 95 % CI 0.83–2.06; p < 0.001). Some evidence of heterogeneity and inconsistency was found for both pro-oxidant (Q = 23.5; τ 2 = 1.09 ± 0.49; I 2 = 18.96 %) and antioxidant (Q = 19.4; τ 2 = 1.83 ± 0.76; I 2 = 2.11 %) analyses [42].
3.5 Risk of Bias
The funnel plot (Fig. 3) shows evidence of possible asymmetry. The pro-oxidant analysis involved 15 trials, 19 interventions, and 747 individuals, and it achieved an objective asymmetry significance (p = 0.80). On the other hand, the antioxidant analysis involved 14 trials, 19 interventions, and 699 individuals, and it failed to achieve an objective asymmetry (p = 0.21).
4 Discussion
4.1 Summary of Evidence
In general, the presented body of evidence so far seems to be robust and shows a reduction in pro-oxidant parameters and an increase in antioxidant parameters as a result of physical exercise. The meta-analysis results also corroborate these outcomes. However, only 39.9 % of the trials reached adequate statistical power (1−β ≥ 0.8), making this a methodological problem, given that small sample sizes may increase the chance of type 2 errors, i.e., no differences could be detected even when they may exist. Moreover, a small sample size may increase dispersion measures, such as the standard error. These prevalent elevated dispersion measures may have been responsible for the risk of bias assessed in the funnel plot analysis (Fig. 3). Additionally, the trials varied among themselves in the studied sample, in the volume, intensity, and type of exercise, and in the OS markers used.
4.2 Population
4.2.1 Children
Three of the selected studies investigated the effect of exercise on the OS parameters in children [47–49]; one was classified as high quality [49] and two as moderate quality [47, 48]. Onur et al. [48] followed 15 children with asthma and 15 controls who took part in 8 weeks of aerobic training; they reported an improvement (p < 0.05) in the redox state (decrease in pro-oxidant parameters and increase in antioxidant parameters). Kelly et al. [47] and Dennis et al. [49] investigated overweight/obese children who undertook aerobic training and intermittent running games, respectively, and reported unchanged pro-oxidant parameters.
In summary, whether physical training can reduce OS in children is inconclusive. Additionally, exercise training may not be effective for reducing OS in the young and relatively healthy, whose physiological and antioxidant functions are well-preserved. However, it is noteworthy that this population could benefit from other aspects of physical exercise, such as improvement in physical fitness, oxygen consumption, body composition, lipid profile, and fasting blood glucose [49]. Further studies should better describe the possible adverse events or sample losses during the trials and adjust the results for confounders. Although only one study showed improvement in redox state after a period of physical training, other studies showed no worsening. Thus, as a practical application, physical exercise programs for children can reduce OS and, in turn, delay the onset of age-related diseases as well as treat diseases common in this age group (i.e., asthma) that are associated with an imbalance in oxidant/antioxidant status.
4.2.2 Age
Among the six studies that addressed this topic, five were classified as of moderate quality [50–54]. One study compared two groups that differed only by age, preventing a more accurate analysis of the subject. Garcia-Lopez et al. [50] followed 23 healthy middle-aged men (mean age 53.9 ± 2.3 years) who undertook 21 weeks of two different training modes (resistance and aerobic) and reported no improvement in antioxidant markers. Conversely, Karabolut et al. [53] investigated healthy men with a mean age of 40.3 ± 6.2 years who underwent 12 weeks of aerobic training; reported pro-oxidant and antioxidant improvements (p < 0.05) compared with baseline values.
Similarly, Fatouros et al. [51] and Beltran Valls et al. [54] studied elderly individuals (mean age 71.5 ± 6.5 and 72 ± 1 years, respectively) who took part in aerobic and resistance training and reported improvements (p < 0.05) in both antioxidant and pro-oxidant parameters, respectively. Furthermore, Azizbeigi et al. [52] tested healthy young men (mean age 22.3 ± 2.3 years) who underwent 8 weeks of resistance training and also reported improvement (p < 0.05) in pro-oxidant and antioxidant markers.
These results provide strong evidence that physical training may reduce OS and improve health status, which can be considered key to preventing several chronic diseases [4]. Moreover, exercise training seems to counteract the deleterious effects of aging, not only by combating the major triggers of OS but also by exerting additional antioxidant actions [55]. However, more studies are warranted to compare these effects in aged and young adults. It is noteworthy that older adults generally present a higher level of OS and other deleterious health markers such as inflammation [56, 57], which could make them not only more vulnerable to chronic diseases but also more able to benefit from the health-enhancing effects of physical training [58, 59]. On the other hand, although young adults are generally healthier and possibly exhibit lesser magnitude improvements [60], their healthier status also allows them to achieve higher internal and external outputs, which could lead to an elevated physiological adaptation to physical training. The main concerns for future trials are to adjust the results for confounders and statistical power.
In practical terms, adults engaged in physical exercise programs can reduce OS and, in turn, delay the onset of diseases related to aging. Similarly, elderly individuals who practice regular exercise may show improvement in their redox state and can thereby not only reduce the deleterious effects of chronic diseases already conferred but also prevent the onset of other age-related diseases.
4.2.3 Obesity, Type 2 Diabetes, and Metabolic Syndrome
Only one study [61], classified as moderate quality, investigated the effects of physical training on OS parameters in the context of body composition. These authors followed ten normal-weight men (mean body fat 25.9 ± 3.5 %) and 19 overweight/obese men (mean body fat 33.0 ± 1.4 %) who undertook 24 weeks of resistance training and reported a decrease (p < 0.05) in pro-oxidant indicators in both groups compared with the respective baseline values and their controls. The comparison between experimental groups revealed no significant differences. Moreover, the data were reported only graphically, precluding us from calculating the effect size and quantifying the response of both groups to the same stimulus. Thus, evidence is insufficient to verify the role of body composition on OS as related to physical training.
T2DM is a metabolic disease associated with elevated OS. The pathophysiological processes of T2DM may disrupt natural antioxidant defenses and increase ROS production [62, 63]. One high-quality [64] and three moderate-quality [65–67] studies investigated the effects of physical training on OS parameters in T2DM. Two moderate-quality studies [68, 69] described their samples as adults with metabolic syndrome. de Oliveira et al. [67] studied 31 individuals with T2DM who underwent 12 weeks of three different types of physical training (aerobic, resistance, and combined training); they reported unchanged antioxidant indicators. Furthermore, aerobic and combined training were effective in promoting an increase in sulfhydryl compared with baseline values (p < 0.05), showing a possible decrease of the pro-oxidant state. On the other hand, Mitranun et al. [64] followed 28 individuals with T2DM who undertook 12 weeks of two different types of aerobic training (continuous vs. intermittent) and reported an improvement in redox state for the intermittent group. Similarly, Kurban et al. [65] tested 12 weeks of power walking in 30 individuals with T2DM and reported an increase in total antioxidant status compared with baseline values. In subjects with metabolic syndrome, Gomes et al. [68] and Rosety-Rodríguez et al. [69] also reported an improvement in pro- and antioxidant indicators, respectively, after 12 weeks of aerobic training.
In summary, moderate evidence suggests that individuals with T2DM exhibit increased antioxidant defenses and lowered OS after physical training, which could be reflected in decreased ROS production and less activation of many detrimental pathways, including hexosamine pathways, formation of advanced glycation end products (AGEs), and protein kinase C β1/2 (PKCβ1/2) [70]. Furthermore, β cells have lower antioxidant enzyme levels (superoxide dismutase, catalase, and glutathione) and higher sensitivity to OS. β-cell exposure to OS may lead to increased p21 cyclin-dependent kinase inhibitor production, decreased insulin messenger RNA (mRNA), and adenosine triphosphate (ATP) and calcium flux reductions in mitochondria and cytosol-causing apoptosis [71, 72]. Therefore, in practical terms, an attenuation of OS in individuals with T2DM may play a fundamental role in the treatment and prevention of this disease.
4.2.4 Cardiovascular Diseases and Heart Transplant Recipients
Five studies addressed the effects of exercise on OS parameters in patients with cardiovascular diseases and in heart transplant recipients. Four were classified as moderate quality [38, 46, 73, 74] and one as high quality [75]. Beck et al. [74] investigated the effects of 8 weeks of resistance and aerobic training on OS parameters in pre-hypertensive young adults (mean age 20.9 ± 0.7 years) and reported an improvement in total antioxidant capacity for both groups (aerobic and resistance), a decrease in F2-isoprostanes (8-iso-F2: pro-oxidant indicator) for the resistance training group, and a curious increase in 8-iso-F2 in the aerobic group. Braith et al. [73] investigated 12 weeks of aerobic training in nine heart transplant recipients. The pro-oxidant marker (8-iso-F2) did not change, but the individuals who undertook aerobic training had an attenuated decrease in the diameter of the brachial artery and maintained flow-mediated dilation compared with the controls. On the other hand, Linke et al. [46] tested 24 weeks of aerobic training in 12 men with chronic heart failure and reported a decrease in pro-oxidant status (lipid peroxidation [LPx]) and an increase in antioxidant indicators (catalase [CAT] and glutathione peroxidase [GPx]) compared with baseline values.
In addition, Edwards et al. [38] followed nine men with coronary artery disease who underwent 12 weeks of aerobic training; they reported an improvement in redox state. However, in the high-quality study conducted by Luk et al. [75], 32 adults with this same pathology underwent 8 weeks of combined aerobic + resistance training, but the authors observed no improvements in pro-oxidant or antioxidant status.
In combination, these results suggest that exercise training improves redox status. However, some results seem to be contradictory, probably because cardiovascular diseases are often preceded by different metabolic dysfunctions, such as dyslipidemia and chronic hyperglycemia, which may increase extracellular ROS production [76, 77] and overwhelm the antioxidant defensive system, leading to greater metabolic dysfunction [78]. Because cardiovascular diseases are usually multifactorial, a population with this disease may be very heterogeneous [79], which in turn may be a confounding factor that does not allow solid conclusions regarding exercise and OS to be drawn in this group. Further, we suggest that future clinical trials report any adverse events, even if none occurred, especially in these at-risk populations. In practice, although results are conflicting, individuals with cardiovascular disease and those who have received a heart transplant who are engaged in physical exercise programs appear to have reduced OS, which may therefore mitigate the deleterious effects of these diseases.
4.2.5 Spinal Cord Injury and Rheumatoid Arthritis
People with chronic spinal cord injury seem to have higher levels of OS and may be more likely to have metabolic and cardiovascular complications than the general population [80], possibly because of their low levels of physical activity [81]. A moderate-quality study from Ordonez et al. [82] investigated the effects of physical training in nine men versus eight controls with spinal cord injury. The training consisted of 12 weeks of arm-cranking exercise, 30 min at 65 % of heart rate reserve three times per week, and resulted in a decrease in pro-oxidant markers (thiobarbituric acid reactive substances [TBARS] and protein carbonyls [pCarb]) and an improvement in antioxidant status (trolox equivalent antioxidant capacity [TEAC] and GPx). Therefore, physical training seems to reduce OS in men with spinal cord injury. Nevertheless, this particular topic is scarcely explored in this population, and more evidence is needed to provide reliable conclusions.
Wadley et al. [83] conducted a moderate-quality study with 19 middle-aged adults with rheumatoid arthritis; after 12 weeks of aerobic training, pro-oxidant parameters (LPx and 3-nitrotyrosine [3-NT]) reduced, but antioxidant status (CAT and TEAC) did not change. Stiffness, swelling, and progressive destruction of the joints are the primary characteristics of rheumatoid arthritis [84] and may be induced by chronic systemic inflammation and exacerbated ROS production [85]. Therefore, physical exercise, besides inducing an improvement in joint function [86], may also play an important role in the pathogenesis of this disease by balancing the redox state. Nevertheless, the evidence is insufficient to determine the effects of physical training on OS markers in this population.
4.2.6 Severe Depression and Parkinson’s Disease
The human brain is considered highly sensitive to oxidative damage because it possesses high levels of phospholipids and polyunsaturated fatty acids, both of which are very susceptible to oxidants and have high oxygen consumption and low levels of antioxidant enzymes [87, 88]. Therefore, some neurological disorders, such as severe depression and Parkinson’s disease, may be triggered by a constant exposure to OS [87, 89]. A thorough review by Camiletti-Moirón et al. [36] suggests that physical exercise may be a powerful tool to treat such diseases.
In this regard, Schuch et al. [90] conducted a high-quality study with severely depressed men: 15 patients and 11 controls underwent 12 weeks of aerobic training. The results showed no time effect in the within-group comparison, but a significant difference (p < 0.05) between groups (experimental vs. control) in pro-oxidant status (TBARS). This could indicate that, if not a reduction, at least an attenuation of OS may play a fundamental role in the pathogenesis of this disease [89]. However, the evidence is insufficient to determine the effects of physical training on OS markers in severely depressed men. Further, a moderate-quality study [91] investigated the effects of 8 weeks of resistance training on pro-oxidant and antioxidant indicators in 16 middle-aged adults with Parkinson’s disease. The authors reported no effect of exercise training (p > 0.05) for either the within-group or the between-group comparisons. Therefore, although the studies are of high quality, the data are too sparse to make any qualified conclusion about the effect of exercise training on OS in individuals with Parkinson’s disease. In practical terms, although literature are scarce, it does seem that physical training reduces OS in these health conditions, suggesting that regular physical exercise can prevent progression of these diseases.
4.3 Exercise Training
4.3.1 Aerobic Training
Aerobic exercise is the most frequently used type of training in research protocols. Studies included in this review investigated a variety of stimuli (e.g., continuous or intermittent), volume, intensity, and training equipment (e.g., treadmill, cycle-ergometer). A more detailed discussion on the particulars of each of these factors on the outcomes of aerobic training is beyond the scope of this review.
We found 19 studies (21 interventions) that used aerobic training, three of which were classed as high quality [64, 90, 92], and 16 were of moderate quality [38, 46–48, 50, 51, 53, 65–69, 73, 74, 82, 83]. The high-quality studies were consistent regarding the effects of aerobic training on OS parameters. One study [90] reported an unchanged (p > 0.05) redox status after intervention, and the other two [64, 92] reported an improvement (p < 0.05) in pro-oxidant and/or antioxidant parameters.
Taken together, strong evidence supports a positive effect of aerobic training on redox balance. The training-induced adaptations of OS increase the efficiency of the enzymatic and non-enzymatic antioxidant defense systems, leading to a greater mitochondrial capacity to scavenge free radicals [93] and a reduction in ROS production by the mitochondrial membrane potential at basal conditions [37, 94]. Therefore, aerobic training seems to be a suitable intervention to improve redox balance. Future trials should ensure statistical power is sufficient (1−β ≥ 0.8). In practice, the available literature, almost in its entirety, indicates that aerobic training can be a powerful strategy to reduce OS and therefore delay the onset of non-communicable chronic diseases.
4.3.2 Resistance Training
Resistance training is the exercise mode with the largest annual increase in prevalence among experimental studies on health and performance in exercise physiology-related publications in the last decade. Thus, resistance training has been postulated as an important tool to control metabolic and cardiovascular variables, and it has been tested and used for the treatment and prevention of several diseases, such as T2DM and hypertension [95]. Therefore, it is reasonable to infer that resistance training may promote beneficial exercise-induced adaptations in physiological systems that play a role in the pathogenesis of these diseases, such as chronic inflammation and OS [11, 12].
In that regard, six studies were classified as of moderate quality [50, 52, 61, 67, 74, 91] and showed the effects of resistance training on OS parameters. Three studies [50, 67, 91] indicated an improvement in the redox balance, and three [52, 61, 74] reported no differences after resistance training interventions. Unfortunately, the complexity of this training method and the heterogeneity of protocols may produce conflicting results. While researchers using aerobic training sessions usually take into account the total volume (e.g., running distance) and intensity (e.g., % maximal oxygen uptake [VO2], % heart rate reserve, or % of anaerobic threshold intensity), those using resistance training must consider the number of sets and repetitions until exhaustion, the total amount of exercise, and the recovery intervals. Therefore, although the results are not unanimous in showing improvements in redox balance, at least an attenuation was observed. More homogeneous protocols are needed to reach a consistent conclusion, as previously stated [37].
4.3.3 Combined Aerobic and Resistance Training
Combined aerobic and resistance training may be the most comprehensive approach, promoting broad adaptations, such as increased cardiopulmonary fitness and strength, respectively [96]. Nevertheless, few studies have investigated its effects on OS parameters. Only two included studies tested this method, one of moderate quality [67] and one of high quality [75]; both reported that redox status after training was no different from baseline values or that of the control group. Therefore, the findings are sparse, and more studies are needed to confer valid conclusions.
4.3.4 Tai Chi and Yoga
Yoga and tai chi have become increasingly popular in Western cultures as a method of exercise training for several populations [97, 98] and may be effective in health maintenance. Tai chi or yoga sessions are typically performed at around 40 % of VO2, which is below the recommended intensity to promote cardiovascular adaptations [99], but they may be effective for improving balance, lower-body flexibility, and muscular strength [97, 98]. Their effects on OS are poorly investigated and not adequately substantiated, since it has been suggested that chronic adaptations in redox balance are dependent on the intensity and type of exercise utilized. Therefore, exercise performed at very low intensities may not cause enough physiological stress to generate adaptation [37].
Despite this, moderate-quality studies [66, 100] and a low-quality study by Rosado-Pérez et al. [101] investigated the effects of tai chi [100, 101] and yoga [66] on OS parameters, and all three reported an improvement both in pro-oxidant and antioxidant indicators. These results may be partially explained by an additional adaptation besides the physiological effect by itself, since these exercise modes may help with adherence to training and better emotional control, thus helping practitioners to become more resilient and improve their overall health [102, 103], such as through a reduction in OS. Thus, yoga and tai chi are suggested as an alternative to reduce OS. Future studies should better describe the intervention and more rigorously control the intervention’s intensity to improve reproducibility and help experts infer the possible physiological mechanisms involved in the health-enhancing effects of tai chi and yoga. To the best of our knowledge, only three studies have investigated the effects of tai chi on OS indicators [66, 100, 101]. However, they all highlight that their practice seems to improve redox state. It is noteworthy that this modality is commonly practiced by older people who generally have a higher OS [57, 58] than young individuals. Thus, in practical terms, it is suggested that older people who wish to improve or preserve their redox state and therefore treat or delay the onset of chronic degenerative diseases use tai chi for both.
4.4 Perspectives and Limitations
The heterogeneity of training protocols, such as different volumes and intensities, precluded us from definitively identifying the most appropriate and effective exercise mode, duration, and intensity to reduce OS. Furthermore, the studied populations were not homogeneous. Regardless, a general analysis of the quantitative and qualitative results seems to point in the same direction, i.e., that redox state may be balanced by exercise training.
The wide range of pro-oxidant and antioxidant markers used in the studies may also influence the results. An attenuation of OS may indicate a reduction of lipid peroxidation, protein oxidation, and/or free radical production and an increase in antioxidant capacity, which may be caused by an increased amount and/or activity of several proteins, enzymatic or non-enzymatic, that operate in different steps of ROS neutralization [104]. Moreover, substances that have no direct antioxidant mechanism, such as repair enzymes, may also play an important role in OS after oxidative damage. Thus, any identified changes in the redox balance would be quite varied, and a detailed and specific discussion on this subject goes far beyond our scope, which may also be a limitation of this review. However, regardless of the OS markers under consideration, their response to exercise training seems to be similar. Future research should evaluate the minimum intervention required to promote benefits in redox state.
5 Conclusion
The effects of exercise training on OS parameters were analyzed in 30 trials that met our inclusion/exclusion criteria. The studies involved 38 different exercise interventions and 1346 participants. Most studies were of moderate quality (score ≥3 and ≤5) and not reach an adequate statistical power for the analysis (80 %). Given the wide heterogeneity of protocols, we are unable to indicate a specific intensity, volume, or exercise mode that is more effective in decreasing OS. Furthermore, the trials involved a wide variety of populations (e.g., children, obese and healthy adults, and individuals with T2DM, Parkinson’s disease, severe depression, and other diseases caused by immune or metabolic disorders), which in turn precluded us from making any specific conclusion. Conversely, the pooled analysis revealed that, regardless of intensity, volume, type of exercise, and studied population, the antioxidant indicators tended to increase and pro-oxidant indicators tended to decrease after training. In other words, exercise training seems to induce an antioxidant effect. Therefore, we recommend that people should perform some kind of exercise to balance the redox state, regardless of their health status, to improve health-related outcomes.
References
Hallal PC, Andersen LB, Bull FC, et al. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380(9838):247–57. doi:10.1016/S0140-6736(12)60646-1.
Blair SN, Kampert JB, Kohl HW 3rd, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276(3):205–10.
Kokkinos P, Myers J. Exercise and physical activity: clinical outcomes and applications. Circulation. 2010;122(16):1637–48. doi:10.1161/CIRCULATIONAHA.110.948349.
Vincent HK, Innes KE, Vincent KR. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes Obes Metab. 2007;9(6):813–39. doi:10.1111/j.1463-1326.2007.00692.x.
Larsen BA, Martin L, Strong DR. Sedentary behavior and prevalent diabetes in Non-Latino Whites, Non-Latino Blacks and Latinos: findings from the National Health Interview Survey. J Public Health (Oxf). 2015;37(4):634–40. doi:10.1093/pubmed/fdu103.
Beunza JJ, Martinez-Gonzalez MA, Ebrahim S, et al. Sedentary behaviors and the risk of incident hypertension: the SUN Cohort. Am J Hypertens. 2007;20(11):1156–62. doi:10.1016/j.amjhyper.2007.06.007.
Hamer M, Venuraju SM, Urbanova L, et al. Physical activity, sedentary time, and pericardial fat in healthy older adults. Obesity (Silver Spring). 2012;20(10):2113–7. doi:10.1038/oby.2012.61.
Myers J, Prakash M, Froelicher V, et al. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793–801.
Metter EJ, Talbot LA, Schrager M, et al. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci. 2002;57(10):B359–65.
Blair SN, Cheng Y, Holder JS. Is physical activity or physical fitness more important in defining health benefits? Med Sci Sports Exerc. 2001;33(6):379–99.
Mathis D, Shoelson SE. Immunometabolism: an emerging frontier. Nat Rev Immunol. 2011;11(2):81–3.
Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–7. doi:10.1038/nature05485.
Bonnard C, Durand A, Peyrol S, et al. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest. 2008;118(2):789–800. doi:10.1172/JCI32601.
Radak Z, Chung HY, Goto S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic Biol Med. 2008;44(2):153–9. doi:10.1016/j.freeradbiomed.2007.01.029.
Powers SK, Talbert EE, Adhihetty PJ. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J Physiol. 2011;589(9):2129–38.
Fischer R, Maier O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF. Oxid Med Cell Longev. 2015;2015:610813. doi:10.1155/2015/610813.
Siti HN, Kamisah Y, Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vascul Pharmacol. 2015;71:40–56. doi:10.1016/j.vph.2015.03.005.
Sarmiento D, Montorfano I, Cerda O, et al. Increases in reactive oxygen species enhance vascular endothelial cell migration through a mechanism dependent on the transient receptor potential melastatin 4 ion channel. Microvasc Res. 2015;98:187–96. doi:10.1016/j.mvr.2014.02.001.
Schepers E, Glorieux G, Dhondt A, et al. Role of symmetric dimethylarginine in vascular damage by increasing ROS via store-operated calcium influx in monocytes. Nephrol Dial Transplant. 2009;24(5):1429–35. doi:10.1093/ndt/gfn670.
Pratico D, Iuliano L, Mauriello A, et al. Localization of distinct F2-isoprostanes in human atherosclerotic lesions. J Clin Invest. 1997;100(8):2028–34. doi:10.1172/JCI119735.
Yla-Herttuala S, Palinski W, Rosenfeld ME, et al. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989;84(4):1086–95. doi:10.1172/JCI114271.
Maritim AC, Sanders RA, Watkins JB 3rd. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17(1):24–38. doi:10.1002/jbt.10058.
Totter JR. Spontaneous cancer and its possible relationship to oxygen metabolism. Proc Natl Acad Sci. 1980;77(4):1763–7.
Wu JD, Lin DW, Page ST, et al. Oxidative DNA damage in the prostate may predispose men to a higher risk of prostate cancer. Transl Oncol. 2009;2(1):39–45.
Christen Y. Oxidative stress and Alzheimer disease. Am J Clin Nutr. 2000;71(2):621S–9S.
Gjevestad GO, Holven KB, Ulven SM. Effects of exercise on gene expression of inflammatory markers in human peripheral blood cells: a systematic review. Curr Cardiovasc Risk Rep. 2015;9(7):34. doi:10.1007/s12170-015-0463-4.
Gleeson M, Bishop NC, Stensel DJ, et al. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607–15. doi:10.1038/nri3041.
Dias RG, Silva MS, Duarte NE, et al. PBMCs express a transcriptome signature predictor of oxygen uptake responsiveness to endurance exercise training in men. Physiol Genomics. 2015;47(2):13–23. doi:10.1152/physiolgenomics.00072.2014.
Radom-Aizik S, Zaldivar FP Jr, Haddad F, et al. Impact of brief exercise on circulating monocyte gene and microRNA expression: implications for atherosclerotic vascular disease. Brain Behav Immun. 2014;39:121–9. doi:10.1016/j.bbi.2014.01.003.
Fernandez-Gonzalo R, De Paz JA, Rodriguez-Miguelez P, et al. Effects of eccentric exercise on toll-like receptor 4 signaling pathway in peripheral blood mononuclear cells. J Appl Physiol (1985). 2012;112(12):2011–8. doi:10.1152/japplphysiol.01499.2011.
Puterman E, Lin J, Blackburn E, et al. The power of exercise: buffering the effect of chronic stress on telomere length. PLoS One. 2010;5(5):e10837. doi:10.1371/journal.pone.0010837.
Gordon B, Chen S, Durstine JL. The effects of exercise training on the traditional lipid profile and beyond. Curr Sports Med Rep. 2014;13(4):253–9. doi:10.1249/JSR.0000000000000073.
Roque FR, Hernanz R, Salaices M, et al. Exercise training and cardiometabolic diseases: focus on the vascular system. Curr Hypertens Rep. 2013;15(3):204–14. doi:10.1007/s11906-013-0336-5.
Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142(2):231–55. doi:10.1038/sj.bjp.0705776.
Fisher-Wellman K, Bloomer RJ. Acute exercise and oxidative stress: a 30 year history. Dyn Med. 2009;8:1. doi:10.1186/1476-5918-8-1.
Camiletti-Moirón D, Aparicio VA, Aranda P, et al. Does exercise reduce brain oxidative stress? A systematic review. Scand J Med Sci Sports. 2013;23(4):e202–12.
Bouzid MA, Filaire E, McCall A, et al. Radical oxygen species, exercise and aging: an update. Sports Med. 2015;45(9):1245–61. doi:10.1007/s40279-015-0348-1.
Edwards DG, Schofield RS, Lennon SL, et al. Effect of exercise training on endothelial function in men with coronary artery disease. Am J Cardiol. 2004;93(5):617–20. doi:10.1016/j.amjcard.2003.11.032.
Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–84.
Ruiz JR, Castro-Pinero J, Artero EG, et al. Predictive validity of health-related fitness in youth: a systematic review. Br J Sports Med. 2009;43(12):909–23. doi:10.1136/bjsm.2008.056499.
Borenstein M, Hedges LV, Higgins JPT, et al. Introduction to meta-analysis. West Sussex: Wiley; 2011.
Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi:10.1136/bmj.327.7414.557.
Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Cohen J. Statistical power analysis for the behavioral sciences. Cambridge: Academic Press; 2013.
Faul F, Erdfelder E, Lang AG, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91.
Linke A, Adams V, Schulze PC, et al. Antioxidative effects of exercise training in patients with chronic heart failure: increase in radical scavenger enzyme activity in skeletal muscle. Circulation. 2005;111(14):1763–70. doi:10.1161/01.CIR.0000165503.08661.E5.
Kelly AS, Steinberger J, Olson TP, et al. In the absence of weight loss, exercise training does not improve adipokines or oxidative stress in overweight children. Metabolism. 2007;56(7):1005–9. doi:10.1016/j.metabol.2007.03.009.
Onur E, Kabaroglu C, Gunay O, et al. The beneficial effects of physical exercise on antioxidant status in asthmatic children. Allergol Immunopathol (Madr). 2011;39(2):90–5. doi:10.1016/j.aller.2010.04.006.
Dennis BA, Ergul A, Gower BA, et al. Oxidative stress and cardiovascular risk in overweight children in an exercise intervention program. Child Obes. 2013;9(1):15–21. doi:10.1089/chi.2011.0092.
Garcia-Lopez D, Hakkinen K, Cuevas MJ, et al. Effects of strength and endurance training on antioxidant enzyme gene expression and activity in middle-aged men. Scand J Med Sci Sports. 2007;17(5):595–604. doi:10.1111/j.1600-0838.2006.00620.x.
Fatouros IG, Jamurtas AZ, Villiotou V, et al. Oxidative stress responses in older men during endurance training and detraining. Med Sci Sports Exerc. 2004;36(12):2065–72.
Azizbeigi K, Azarbayjani MA, Peeri M, et al. The effect of progressive resistance training on oxidative stress and antioxidant enzyme activity in erythrocytes in untrained men. Int J Sport Nutr Exerc Metab. 2013;23(3):230–8.
Karabolut AB, Kafkas ME, Kafkas AS, et al. The effect of regular exercise and massage on oxidant and antioxidant parameters. Indian J Physiol Pharmacol. 2013;57(4):6.
Beltran Valls MR, Dimauro I, Brunelli A, et al. Explosive type of moderate-resistance training induces functional, cardiovascular, and molecular adaptations in the elderly. Age (Dordr). 2014;36(2):759–72. doi:10.1007/s11357-013-9584-1.
Sallam N, Laher I. Exercise modulates oxidative stress and inflammation in aging and cardiovascular diseases. Oxid Med Cell Longev. 2016;2016:7239639. doi:10.1155/2016/7239639.
Konopka AR, Sreekumaran Nair K. Mitochondrial and skeletal muscle health with advancing age. Mol Cell Endocrinol. 2013;379(1–2):19–29. doi:10.1016/j.mce.2013.05.008.
Bejma J, Ji LL. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J Appl Physiol (1985). 1999;87(1):465–70.
Malbut KE, Dinan S, Young A. Aerobic training in the ‘oldest old’: the effect of 24 weeks of training. Age Ageing. 2002;31(4):255–60.
Kallinen M, Sipila S, Alen M, et al. Improving cardiovascular fitness by strength or endurance training in women aged 76–78 years. A population-based, randomized controlled trial. Age Ageing. 2002;31(4):247–54.
Bacon AP, Carter RE, Ogle EA, et al. VO2max trainability and high intensity interval training in humans: a meta-analysis. PLoS One. 2013;8(9):e73182. doi:10.1371/journal.pone.0073182.
Vincent HK, Bourguignon C, Vincent KR. Resistance training lowers exercise-induced oxidative stress and homocysteine levels in overweight and obese older adults. Obesity (Silver Spring). 2006;14(11):1921–30. doi:10.1038/oby.2006.224.
Jain SK, McVie R. Effect of glycemic control, race (white versus black), and duration of diabetes on reduced glutathione content in erythrocytes of diabetic patients. Metabolism. 1994;43(3):306–9.
Obrosova IG, Van Huysen C, Fathallah L, et al. An aldose reductase inhibitor reverses early diabetes-induced changes in peripheral nerve function, metabolism, and antioxidative defense. FASEB J. 2002;16(1):123–5. doi:10.1096/fj.01-0603fje.
Mitranun W, Deerochanawong C, Tanaka H, et al. Continuous vs interval training on glycemic control and macro- and microvascular reactivity in type 2 diabetic patients. Scand J Med Sci Sports. 2014;24(2):e69–76. doi:10.1111/sms.12112.
Kurban S, Mehmetoglu I, Yerlikaya HF, et al. Effect of chronic regular exercise on serum ischemia-modified albumin levels and oxidative stress in type 2 diabetes mellitus. Endocr Res. 2011;36(3):116–23. doi:10.3109/07435800.2011.566236.
Gordon LA, Morrison EY, McGrowder DA, et al. Effect of exercise therapy on lipid profile and oxidative stress indicators in patients with type 2 diabetes. BMC Complement Altern Med. 2008;8:21. doi:10.1186/1472-6882-8-21.
de Oliveira VN, Bessa A, Jorge ML, et al. The effect of different training programs on antioxidant status, oxidative stress, and metabolic control in type 2 diabetes. Appl Physiol Nutr Metab. 2012;37(2):334–44. doi:10.1139/h2012-004.
Gomes VA, Casella-Filho A, Chagas AC, et al. Enhanced concentrations of relevant markers of nitric oxide formation after exercise training in patients with metabolic syndrome. Nitric Oxide. 2008;19(4):345–50. doi:10.1016/j.niox.2008.08.005.
Rosety-Rodríguez M, Díaz-Ordonez A, Rosety I, et al. Mejora de defensas antioxidantes mediante ejercicio aeróbico en mujeres con síndrome metabólico [Aerobic training improves antioxidant defense system in women with metabolic syndrome]. Medicina. 2012;72(1):4.
Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–25.
Maechler P, Jornot L, Wollheim CB. Hydrogen peroxide alters mitochondrial activation and insulin secretion in pancreatic beta cells. J Biol Chem. 1999;274(39):27905–13.
Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015;6(3):456–80. doi:10.4239/wjd.v6.i3.456.
Braith RW, Schofield RS, Hill JA, et al. Exercise training attenuates progressive decline in brachial artery reactivity in heart transplant recipients. J Heart Lung Transplant. 2008;27(1):52–9. doi:10.1016/j.healun.2007.09.032.
Beck DT, Martin JS, Casey DP, et al. Exercise training improves endothelial function in resistance arteries of young prehypertensives. J Hum Hypertens. 2014;28(5):303–9. doi:10.1038/jhh.2013.109.
Luk TH, Dai YL, Siu CW, et al. Effect of exercise training on vascular endothelial function in patients with stable coronary artery disease: a randomized controlled trial. Eur J Prev Cardiol. 2012;19(4):830–9. doi:10.1177/1741826711415679.
Grossman E. Does increased oxidative stress cause hypertension? Diabetes Care. 2008;31(Suppl 2):S185–9. doi:10.2337/dc08-s246.
Ghoreishian H, Tohidi M, Derakhshan A, et al. Presence of hypertension modifies the impact of insulin resistance on incident cardiovascular disease in a Middle Eastern population: the Tehran Lipid and Glucose Study. Diabet Med. 2015;32(10):1311–8. doi:10.1111/dme.12733.
Schiffrin EL, Canadian Institutes of Health Research Multidisciplinary Research Group on Hypertension. Beyond blood pressure: the endothelium and atherosclerosis progression. Am J Hypertens. 2002;15(10 Pt 2):115S–22S.
López-Suárez A, Bascuñana-Quirell A, Beltrán-Robles M, et al. Metabolic syndrome does not improve the prediction of 5-year cardiovascular disease and total mortality over standard risk markers. Prospective population based study. Medicine. 2014;93(27):e212.
Bastani NE, Kostovski E, Sakhi AK, et al. Reduced antioxidant defense and increased oxidative stress in spinal cord injured patients. Arch Phys Med Rehabil. 2012;93(12):2223–8 e2. doi:10.1016/j.apmr.2012.06.021.
LaVela SL, Evans CT, Prohaska TR, et al. Males aging with a spinal cord injury: prevalence of cardiovascular and metabolic conditions. Arch Phys Med Rehabil. 2012;93(1):90–5. doi:10.1016/j.apmr.2011.07.201.
Ordonez FJ, Rosety MA, Camacho A, et al. Arm-cranking exercise reduced oxidative damage in adults with chronic spinal cord injury. Arch Phys Med Rehabil. 2013;94(12):2336–41. doi:10.1016/j.apmr.2013.05.029.
Wadley AJ, Veldhuijzen van Zanten JJ, Stavropoulos-Kalinoglou A, et al. Three months of moderate-intensity exercise reduced plasma 3-nitrotyrosine in rheumatoid arthritis patients. Eur J Appl Physiol. 2014;114(7):1483–92. doi:10.1007/s00421-014-2877-y.
Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358(9285):903–11. doi:10.1016/S0140-6736(01)06075-5.
Wadley AJ, Veldhuijzen van Zanten JJ, Aldred S. The interactions of oxidative stress and inflammation with vascular dysfunction in ageing: the vascular health triad. Age (Dordr). 2013;35(3):705–18. doi:10.1007/s11357-012-9402-1.
Hakkinen A, Sokka T, Kautiainen H, et al. Sustained maintenance of exercise induced muscle strength gains and normal bone mineral density in patients with early rheumatoid arthritis: a 5 year follow up. Ann Rheum Dis. 2004;63(8):910–6. doi:10.1136/ard.2003.013003.
Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003;53(Suppl 3):S26–36. doi:10.1002/ana.10483 (discussion S-8).
Tuon T, Valvassori SS, Lopes-Borges J, et al. Physical training exerts neuroprotective effects in the regulation of neurochemical factors in an animal model of Parkinson’s disease. Neuroscience. 2012;227:305–12. doi:10.1016/j.neuroscience.2012.09.063.
Maes M, Galecki P, Chang YS, et al. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):676–92. doi:10.1016/j.pnpbp.2010.05.004.
Schuch FB, Vasconcelos-Moreno MP, Borowsky C, et al. The effects of exercise on oxidative stress (TBARS) and BDNF in severely depressed inpatients. Eur Arch Psychiatry Clin Neurosci. 2014;264(7):605–13. doi:10.1007/s00406-014-0489-5.
Bloomer RJ, Schilling BK, Karlage RE, et al. Effect of resistance training on blood oxidative stress in Parkinson disease. Med Sci Sports Exerc. 2008;40(8):1385–9. doi:10.1249/MSS.0b013e31816f1550.
Arikawa AY, Thomas W, Gross M, et al. Aerobic training reduces systemic oxidative stress in young women with elevated levels of F2-isoprostanes. Contemp Clin Trials. 2013;34(2):212–7. doi:10.1016/j.cct.2012.11.003.
Chandwaney R, Leichtweis S, Leeuwenburgh C, et al. Oxidative stress and mitochondrial function in skeletal muscle: effects of aging and exercise training. Age (Omaha). 1998;21(3):109–17. doi:10.1007/s11357-998-0017-5.
Daussin FN, Rasseneur L, Bouitbir J, et al. Different timing of changes in mitochondrial functions following endurance training. Med Sci Sports Exerc. 2012;44(2):217–24. doi:10.1249/MSS.0b013e31822b0bd4.
Phillips SM, Winett RA. Uncomplicated resistance training and health-related outcomes: evidence for a public health mandate. Curr Sports Med Rep. 2010;9(4):208–13. doi:10.1249/JSR.0b013e3181e7da73.
Holviala J, Kraemer WJ, Sillanpaa E, et al. Effects of strength, endurance and combined training on muscle strength, walking speed and dynamic balance in aging men. Eur J Appl Physiol. 2012;112(4):1335–47. doi:10.1007/s00421-011-2089-7.
Li JX, Hong Y, Chan KM. Tai chi: physiological characteristics and beneficial effects on health. Br J Sports Med. 2001;35(3):148–56.
Elwy AR, Groessl EJ, Eisen SV, et al. A systematic scoping review of yoga intervention components and study quality. Am J Prev Med. 2014;47(2):220–32. doi:10.1016/j.amepre.2014.03.012.
Pescatello LS, Arena R, Riebe D, Thompson PD, editors. ACSM’s guidelines for exercise testing and prescription. American College of Sports Medicine. Baltimore: Lippincott Williams & Wilkins; 2013.
Goon JA, Aini AH, Musalmah M, et al. Effect of tai chi exercise on DNA damage, antioxidant enzymes, and oxidative stress in middle-age adults. J Phys Act Health. 2009;6(1):43–54.
Rosado-Pérez J, Santiago-Osorio E, Ortiz R, et al. Tai chi diminishes oxidative stress in Mexican older adults. J Nutr Health Aging. 2012;16(7):5.
Buttle H. Measuring a journey without goal: meditation, spirituality, and physiology. Biomed Res Int. 2015;2015:891671. doi:10.1155/2015/891671.
Kabat-Zinn J, Massion AO, Kristeller J, et al. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am J Psychiatry. 1992;149(7):936–43.
Rahal A, Kumar A, Singh V, et al. Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res Int. 2014;2014:761264. doi:10.1155/2014/761264.
Hamberg-van Reenen HH, Ariëns GAM, Blatter BM, et al. A systematic review of the relation between physical capacity and future low back and neck/shoulder pain. Pain. 2007;130(1):93–107.
Vinetti G, Mozzini C, Desenzani P, et al. Supervised exercise training reduces oxidative stress and cardiometabolic risk in adults with type 2 diabetes: a randomized controlled trial. Sci Rep. 2015;5:9238. doi:10.1038/srep09238.
Soares JP, Silva AM, Oliveira MM, et al. Effects of combined physical exercise training on DNA damage and repair capacity: role of oxidative stress changes. Age (Dordr). 2015;37(3):9799. doi:10.1007/s11357-015-9799-4.
Johnson ML, Irving BA, Lanza IR, et al. Differential effect of endurance training on mitochondrial protein damage, degradation, and acetylation in the context of aging. J Gerontol A Biol Sci Med Sci. 2015;70(11):1386–93. doi:10.1093/gerona/glu221.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors are thankful to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for granting scholarships at undergraduate research (CNPq), MSc (CAPES), and PhD (CAPES) levels. No specific sources of funding were used to assist in the preparation of this article.
Conflict of interest
Caio Victor de Sousa, Marcelo Magalhães Sales, Thiago Santos Rosa, John Eugene Lewis, Rosangela Vieira de Andrade, and Herbert Gustavo Simões have no conflicts of interest relevant to the content of this review.
Additional information
C. V. de Sousa and M. M. Sales contributed equally to this work.
Rights and permissions
About this article
Cite this article
de Sousa, C.V., Sales, M.M., Rosa, T.S. et al. The Antioxidant Effect of Exercise: A Systematic Review and Meta-Analysis. Sports Med 47, 277–293 (2017). https://doi.org/10.1007/s40279-016-0566-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-016-0566-1