Abstract
Gout is common in the elderly, affecting an estimated 4.7 million people aged > 60 years in the USA alone. The incidence and prevalence of gout increases, and male predisposition to gout reduces, with increasing age. The elderly have more comorbidities, and gout manifests differently, with more frequent involvement of knees, ankles, and wrists at disease onset, systemic upset, and tophi. Comorbidities and polypharmacy make the management of gout flares challenging in this population. Intra-articular corticosteroid injection remains the treatment of choice for accessible joints, oral prednisolone is preferred over low-dose colchicine, and non-steroidal anti-inflammatory drugs (NSAIDs) are best avoided. Xanthine oxidase inhibitors (XOI) remain the first-line treatment for hyperuricemia in the elderly. Arhalofenate, an emerging uricosuric anti-inflammatory drug, prevents gout flares while reducing serum urate. It may be particularly relevant in the treatment of gout in the elderly as they are unable to tolerate long-term colchicine for flare prophylaxis and frequently have contraindications to corticosteroids and NSAIDs. However, given its modest urate-lowering effect, it can only be used in combination with an XOI, and the safety and efficacy of this drug has not been examined in the elderly or in those with chronic kidney disease. Diuretics and beta-blockers should be discontinued where feasible, whereas low-dose aspirin can be continued if otherwise indicated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gout flares are best treated with intra-articular or oral corticosteroids in the elderly. |
Colchicine should be used with caution to treat gout flares in the elderly and as a prophylaxis when initiating urate-lowering treatment. |
Arhalofenate has anti-flare and urate-lowering effects and therefore may be relevant in the elderly if initial findings are confirmed in studies in this population. |
1 Introduction
Gout is the commonest inflammatory arthritis, affecting 2.5–3.9% of the general population in the Western world [1, 2]. It results from sustained serum urate elevation, which causes intra- and periarticular monosodium urate (MSU) crystal deposition [3]. These crystals cause gout flares and chronic gouty arthritis. Gout flare is a dramatic illness that may increase in frequency and eventually result in chronic gouty arthritis if potentially curative long-term urate-lowering treatment (ULT) is not initiated [4]. This review describes the epidemiology, clinical features, and management of gout flares in the elderly and provides an overview of arhalofenate, an emerging anti-inflammatory uricosuric agent [5, 6] that may be relevant in the treatment of gout in the elderly.
2 Epidemiology
Gout is common in the elderly, affecting 4.7 million people aged > 60 years in the USA alone [2].
2.1 Age
The prevalence of gout increases with age. For instance, the prevalence of gout increases from 3.3% in those aged 40–49 years to 8.0, 9.3, and 12.6% in those aged 60–69, 70–79 years, and ≥ 80 years, respectively, in the USA [2]. Similar findings have been reported from the UK and Sweden [1, 7].
2.2 Sex
The striking male preponderance for developing gout reduces with increasing age. For instance, in those aged 35–39 years, men are 11.2 times more likely to have gout than women, whereas this reduces to a 2.5-fold greater likelihood in those aged > 90 years [1]. A recent study using data from the western Swedish healthcare region also reported a reduction in male preponderance for gout after the age of 70 years [7]. This occurs because the uricosuric effect of estrogen and progesterone reduces after menopause [8, 9], and the incidence of gout subsequently increases [10]. A cross-sectional study [4] and another prospective cohort study [11] suggested that increasing age itself does not affect the frequency of acute attacks in patients with gout.
2.3 Comorbidities
Older people with gout are more likely to have comorbidities. For instance, a French study using data from the primary care-based CACTUS cohort reported that patients with gout and coronary heart disease, heart failure, or chronic kidney disease (CKD) were, on average, 10 years older than those without these comorbidities, did not drink alcohol, and only 5% drank sugar-sweetened fructose-rich soft drinks [12]. This suggests that gout in the elderly is less likely to be amenable to lifestyle modifications and may be part of the metabolic syndrome or a consequence of CKD.
3 Clinical Features
3.1 Gout Flare
Gout flares may manifest differently in the elderly than in the young. In the elderly, there is a greater involvement of joints other than the first metatarsophalangeal joint (MTPJ) at disease onset, including involvement of the upper limb joints and polyarticular presentation [13]. The latter could be a consequence of increasing disease duration [14]. Furthermore, gout in the elderly can present with systemic upset, fever, and delirium [15, 16]. People with disease onset after the age of 50 years are also less likely to self-report identifiable triggers of gout flares [17]. Older people with gout flares are more severely affected by their symptoms and are more likely to be hospitalized and discharged to intermediate-care facilities than those aged < 50 years [18]. Thus, it is important to take gout seriously in this population.
3.2 Tophaceous Gout
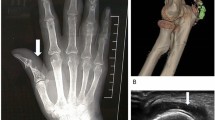
Older people are more likely to have tophaceous gout (Fig. 1) [19]. Tophi typically appear at the hand interphalangeal joints, metacarpophalangeal joints, olecranon bursa, knee, and the Achilles tendon as subcutaneous white chalky deposits.
There is some evidence that elderly women are more likely to develop tophi in their finger interphalangeal joints, especially women with diuretic-induced gout [20]. However, a more recent larger study from New Zealand [21] did not confirm the previously reported association between diuretic-induced gout and tophi.
4 Differential Diagnosis and Investigations
4.1 Investigations
4.1.1 Arthrocentesis
The definite diagnosis of gout requires joint aspiration and polarized light microscopy of the aspirated synovial fluid [3]. The synovial fluid is frequently turbid with low viscosity, and the leukocyte count is often > 10,000/mm3. The presence of strongly birefringent needle-shaped MSU crystals confirms the diagnosis [3] (Fig. 2). The aspirated synovial fluid should also undergo gram stain and be cultured to exclude septic arthritis, which is an important differential diagnosis. However, a confident clinical diagnosis of gout can be made in those with typical features of crystal-induced inflammation affecting the first MTPJ without recourse to joint aspiration [23].

Reproduced from Abhishek et al. [22] with permission
Polarized light microscopy showing needle-shaped negatively birefringent monosodium urate crystal. Note, change in color of the monosodium urate crystal on employment of the polarizing lens. Figure courtesy of the Department of Microbiology, Addenbrookes Hospital, Cambridge, UK.
4.1.2 Blood Tests
Neutrophilia and raised inflammatory markers are common during both gout flares and other forms of acute arthritis so does not help in the differential diagnosis of these conditions. Uric acid is a negative acute-phase reactant that is reduced during a gout flare so should be rechecked 1–2 weeks after flare resolution [24].
4.1.3 Imaging in Gout
Plain radiographs are normal in early gout [25]. Soft tissue swelling may be seen during gout flares, and large joint effusions can sometimes be visualized when they displace fat planes, but these are non-specific findings. People with advanced gout may have juxta-articular punched out erosions with overhanging edges and sclerotic margins (Fig. 3).
Radiographs showing changes in long-standing untreated gouty arthritis. The left panel shows soft-tissue swelling at the thumb inter-phalangeal joint and the index finger distal inter-phalangeal joint in tophaceous gout. The middle panel shows a punched out lesion in the proximal phalanx of the big toe with sclerotic margin. The right panel shows typical juxta-articular punched out erosion in the first metatarsophalangeal joint
Other imaging techniques—such as ultrasonography and dual-energy computerized tomography (CT)—aid in the diagnosis of gout and have acceptable sensitivity and specificity [26]. Ultrasonographic findings of gout flares include effusion and power Doppler changes, as in any acute arthritis. Other changes, such as the double-contour sign (MSU crystal deposition on the surface of hyaline cartilage), intra-articular and intra-bursal tophi, and hyper-echoic aggregates, may be present in both acute and inter-critical gout (Fig. 4). However, these findings are not 100% specific to gout and may be present in joints with calcium pyrophosphate deposition disease (CPPD) [27]. Dual-energy CT has restricted availability, which limits its utility.

Reproduced from Abhishek et al. [22] with permission
Ultrasound scan of the first metatarsophalangeal joint showing double contour sign and tophus in the dorsal synovial recess.
4.2 Differential Diagnosis
Acute CPP crystal arthritis (previously ‘pseudogout’) is an important differential diagnosis of gout flares in the elderly [28]. Acute CPP crystal arthritis can affect any joint but commonly affects the knee, wrist, ankle, elbow, and—rarely—the first MTPJ [29]. Diagnosis requires polarized-light microscopy of the aspirated synovial fluid, which demonstrates weakly positively birefringent or non-refringent rhomboid or squat CPP crystals [30]. Gram stain and culture of the aspirated synovial fluid also excludes septic arthritis, which is an important differential diagnosis of a gout flare.
Apatite-associated destructive arthritis is another differential diagnosis of gout flare. It frequently presents with acute shoulder or hip pain and large joint effusions in the elderly on a background of advanced osteoarthritis [31]. The presence of advanced osteoarthritis and the pattern of joint involvement helps differentiate this condition from acute gout flare.
Gouty tophi can sometimes be confused with rheumatoid nodules. However, the latter are usually homogeneous, not white or chalky, and occur in patients with rheumatoid factor or anti-CCP antibodies.
5 Managing Gout Flares in the Elderly
The American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) gout treatment guidelines [32, 33] all recommend non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, and low-dose colchicine for the treatment of gout flare. Combination treatment with NSAIDs or low-dose colchicine is recommended in those with severe polyarticular gout [32, 33]. The management of gout flare in the elderly can be challenging for several reasons. For instance, comorbidities that are common in the elderly, such as peptic ulcer disease and CKD, mean NSAIDs and colchicine are fraught with danger, and corticosteroids can worsen congestive cardiac failure. Similarly, co-prescription of nephrotoxins such as diuretics, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin II receptor blockers (ARBs) increases the risk of acute kidney injury from NSAIDs. The co-prescription of moderate- to high-potency inhibitors of cytochrome P450 3A4 (responsible for colchicine metabolism) and P-glycoprotein (responsible for the efflux of colchicine from intracellular stores) can result in troublesome and potentially life-threatening adverse events [34]. Thus, medicines such as clarithromycin, erythromycin, cyclosporine, diltiazem, verapamil, ketoconazole, and fluconazole either should not be co-prescribed with colchicine or the dose of colchicine should be reduced [34]. Given the findings of the AGREE study, most guidelines recommend low-dose colchicine to treat gout flares [35]. The ACR guidelines recommend a loading dose of colchicine 1.2 mg, followed by colchicine 0.6 mg within 1 h, and 0.6 mg at 12- and 24-h intervals as required until the gout flare fully resolves [32]. However, the safety profile of this dosing regimen has not been examined in the elderly, and it may be reasonable to avoid the initial loading dose and prescribe colchicine 0.6 mg (or 0.5 mg in countries in which the 0.6 mg dose is not available) twice a day for the duration of the gout flare, with suitable dose reduction for comorbidities and drug interactions. The EULAR gout treatment guidelines recommend this approach. However, the safe use of colchicine in people with CKD-4 (estimated glomerular filtration rate [eGFR] < 30 ml/min) has not been established. Because colchicine clearance is decreased in CKD-4, with a doubling of plasma levels, the EULAR guidelines recommend avoiding colchicine in these patients [33, 36]. Although high-quality trials are lacking, intra-articular corticosteroid injections are recommended for treating mono- or oligoarticular gout flares affecting joints accessible for injections, and this should be the first-line approach, regardless of age [33, 37]. Given the potential for drug interactions and side effects with colchicine and NSAIDs, it may be preferable to prescribe corticosteroids for the treatment of gout flares in the elderly. The recommendation to prescribe oral prednisolone for the treatment of gout flare is based on two double-blind randomized non-inferiority studies investigating its efficacy compared with naproxen or indomethacin [38, 39]. Two dosing regimens with similar results are recommended:
-
prednisolone 30–35 mg/day for 5 days [33]
-
prednisolone at a starting dosage of at least 0.5 mg/kg per day for 5–10 days, or alternatively, 2–5 days at the full dose, followed by tapering for 7–10 days, and then discontinuation [32].
The EULAR gout treatment guidelines recommend an anti-interleukin-1 agent (e.g. subcutaneous anakinra 100 mg/day for 3 days) for the treatment of gout flares, especially in patients who are unable to tolerate or have contraindications to NSAIDs, colchicine, or corticosteroids. Both the ACR and EULAR gout treatment guidelines recommend that pharmacotherapy for gout flares should be started as close to onset of gout flare as possible [32, 33]. Thus, it is desirable that suitably educated patients be prescribed a pack of rescue medications to keep at home to be used at the onset of acute attacks of gout [32, 33].
6 Managing Hyperuricemia with Urate-Lowering Treatment (ULT) in the Elderly
ULT is recommended for people with recurrent acute attacks of gout [32, 33]. Sub-analysis of data from the CONFIRMS study indicates that both allopurinol and febuxostat are equally safe and effective in those aged > 65 years compared with a younger population [40]. This study also found that febuxostat 40 or 80 mg/day was more effective in lowering serum urate than was renal dose-limited fixed-dose allopurinol 200/300 mg/day in those aged > 65 years with CKD, suggesting the superiority of febuxostat in this scenario [40]. However, recent trials suggest that the dose of allopurinol can be cautiously and safely increased beyond the recommended creatinine clearance-based doses [41, 42]. As ULT can trigger acute attacks of gout, it is recommended that people with gout who commence treatment with such medicines are also started on prophylactic drugs such as colchicine, corticosteroids (e.g., prednisolone 7.5 mg/day), and NSAIDs for at least 6 months [32, 33]. This can be challenging for the elderly as they frequently have contraindications to long-term prescription of these medicines.
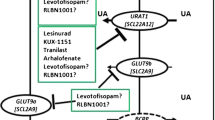
7 Arhalofenate: An Emerging Anti-inflammatory ULT
Arhalofenate is a uricosuric drug that blocks uric acid reabsorption by URAT-1 and inhibits MSU crystal-induced inflammation by inhibiting the NALP-3 inflammasome. It has been shown to prevent gout flares while simultaneously lowering serum urate levels [5, 43,44,45]. If these findings are confirmed in further studies, arhalofenate may be relevant in the treatment of gout in the elderly as they are unable to tolerate long-term colchicine for flare prophylaxis and frequently have contraindications to corticosteroids and NSAIDs.
However, it has a modest urate-lowering effect on its own and only reduces serum urate levels by 12.5–19 and 16–24% at a dose of 600 and 800 mg/day, respectively [5, 6]. As expected, combination arhalofenate and febuxostat reduces serum urate to a greater degree, and all participants receiving a combination of arhalofenate 800 mg/day and febuxostat 80 mg/day achieved a serum urate < 360 µmol/l in a small study (NCT ID 02252835) [6]. Overall, arhalofenate appeared to be safe, with no serious adverse events or elevated serum creatinine [5, 6].
The anti-flare effect of arhalofenate was demonstrated in a randomized double-blind active- and placebo-controlled 12-week study in which participants were randomized to either arhalofenate 600 mg/day, arhalofenate 800 mg/day, allopurinol 300 mg/day plus colchicine 0.6 mg/day, allopurinol 300 mg/day, or placebo (NCT 02063997) [5]. During the 12-week study, those receiving arhalofenate 800 mg/day experienced significantly fewer flares than those receiving allopurinol 300 mg/day or placebo (mean number of gout flares 0.66 vs. 1.24, p = 0.0056; and 0.66 vs. 1.13, p = 0.049). However, participants randomized to allopurinol 300 mg/day and colchicine 0.6 mg/day had even fewer flares (mean 0.4).
7.1 Role of Arhalofenate
Uptitrated allopurinol, or febuxostat if the former is contraindicated, remains the first-line treatment for hyperuricemia. Given the modest urate-lowering effect of arhalofenate, it could be used as add-on therapy to a xanthine oxidase inhibitor (XOI) if the maximum tolerated dose of the latter does not reduce serum urate to < 360 µmol/l. Alternatively, it could be used as a first-line ULT for its combined anti-flare urate-lowering effect, with an added XOI. This treatment strategy is particularly attractive for elderly patients who are either intolerant of or have contraindications to the drugs recommended for prophylaxis of gout flares when starting ULT. However, additional data about the safety and efficacy of arhalofenate in the elderly and in those with CKD are needed, as studies of arhalofenate have been restricted to a relatively young population with preserved renal function.
Lesinurad, another uricosuric drug with a more potent urate-lowering effect than arhalofenate, should be preferred as add-on therapy if the reason for adding arhalofenate is solely to lower the serum urate [46].
8 Managing Comorbidities
Gout is associated with cardiovascular disease, which mandates prescription of aspirin for primary or secondary prophylaxis of myocardial infarction. Even low-dose aspirin, e.g., 75–100 mg/day, increases serum urate by reducing urinary uric acid excretion [47, 48]. However, short-term studies in the elderly suggest this increase is small, at around 20 µmol/l [47, 48]. The cardiovascular benefit from low-dose aspirin outweighs the insignificant risk of hyperuricemia. Thus, patients with gout should continue to receive aspirin if it is otherwise indicated and the modest increase in serum urate levels can be managed with ULT [32, 33].
Similarly, both thiazide and loop diuretics and beta-blockers, which are used in the management of hypertension and congestive cardiac failure, cause hyperuricaemia and are associated with incident gout [49, 50]. Diuretics should be discontinued where feasible. Conversely, the ARB losartan and calcium channel blockers such as amlodipine as well as atorvastatin and fenofibrate reduce the risk of incident gout and should be preferred in the management of comorbidities in patients with gout and hypertension or hyperlipidemia, respectively [49]. The uricosuric effect of losartan is drug specific, and other ARBs and ACE inhibitors do not have this property.
9 Summary
Gout is common in the elderly and affects elderly women more often than younger women. Tophi, systemic upset, and polyarticular involvement are common, and the presence of comorbidities and polypharmacy frequently contraindicates the use of colchicine. Treatments of choice include intra-articular corticosteroid injections into accessible joints and a short course of oral corticosteroids. The presence of comorbidities can make effective prophylaxis of gout flares difficult when starting ULT, and lower doses of colchicine (e.g., 0.6 mg/day) or low-dose prednisolone should be used. Arhalofenate is an emerging anti-inflammatory uricosuric drug that has potential for use in the elderly, but further studies in patients with CKD and in elderly populations are needed before it can be recommended for use in the elderly.
References
Kuo C-F, Grainge MJ, Mallen C, Zhang W, Doherty M. Rising burden of gout in the UK but continuing suboptimal management: a nationwide population study. Ann Rheum Dis. 2014. https://doi.org/10.1136/annrheumdis-2013-204463.
Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63(10):3136–41. https://doi.org/10.1002/art.30520.
McCarty DJ, Hollander JL. Identification of urate crystals in gouty synovial fluid. Ann Intern Med. 1961;54:452–60.
Abhishek A, Valdes AM, Zhang W, Doherty M. Association of serum uric acid and disease duration with frequent gout attacks: a case-control study. Arthritis Care Res. 2016;68(10):1573–7. https://doi.org/10.1002/acr.22855.
Poiley J, Steinberg AS, Choi YJ, Davis CS, Martin RL, McWherter CA, et al. A Randomized, double-blind, active- and placebo-controlled efficacy and safety study of arhalofenate for reducing flare in patients with gout. Arthritis Rheumatol (Hoboken, NJ). 2016;68(8):2027–34. https://doi.org/10.1002/art.39684.
Steinberg AS, Vince BD, Choi YJ, Martin RL, McWherter CA, Boudes PF. The pharmacodynamics, pharmacokinetics, and safety of arhalofenate in combination with febuxostat when treating hyperuricemia associated with gout. J Rheumatol. 2017;44(3):374–9. https://doi.org/10.3899/jrheum.161062.
Dehlin M, Drivelegka P, Sigurdardottir V, Svärd A, Jacobsson LTH. Incidence and prevalence of gout in Western Sweden. Arthritis Res Ther. 2016. https://doi.org/10.1186/s13075-016-1062-6.
Atallah AN, Guimaraes JA, Gebara M, Sustovich DR, Martinez TR, Camano L. Progesterone increases glomerular filtration rate, urinary kallikrein excretion and uric acid clearance in normal women. Braz J Med Biol Res Revista brasileira de pesquisas medicas e biologicas. 1988;21(1):71–4.
Nicholls A, Snaith ML, Scott JT. Effect of oestrogen therapy on plasma and urinary levels of uric acid. BMJ. 1973;1(5851):449–51.
Hak AE, Curhan GC, Grodstein F, Choi HK. Menopause, postmenopausal hormone use and risk of incident gout. Ann Rheum Dis. 2010;69(7):1305–9. https://doi.org/10.1136/ard.2009.109884.
Rothenbacher D, Primatesta P, Ferreira A, Cea-Soriano L, Rodriguez LA. Frequency and risk factors of gout flares in a large population-based cohort of incident gout. Rheumatology (Oxf Engl). 2011;50(5):973–81. https://doi.org/10.1093/rheumatology/keq363.
Richette P, Clerson P, Périssin L, Flipo R-M, Bardin T. Revisiting comorbidities in gout: a cluster analysis. Ann Rheum Dis. 2015;74(1):142–7. https://doi.org/10.1136/annrheumdis-2013-203779.
ter Borg EJ, Rasker JJ. Gout in the elderly, a separate entity? Ann Rheum Dis. 1987;46(1):72–6.
Hadler NM, Franck WA, Bress NM, Robinson DR. Acute polyarticular gout. Am J Med. 1974;56(5):715–9.
Getta B, O’Mahony PG. Atypical presentation of acute gout in an elderly patient. J Am Geriatr Soc. 2008;56(4):764–5. https://doi.org/10.1111/j.1532-5415.2008.01619.x.
Wilson ME, Wan SH, Beyder A, Osborn TG, Beckman TJ. Acute polyarticular gout presenting as delirium. J Clin Rheumatol Pract Rep Rheum Musculoskelet Dis. 2013;19(4):221–2. https://doi.org/10.1097/RHU.0b013e318293794f.
Abhishek A, Valdes AM, Jenkins W, Zhang W, Doherty M. Triggers of acute attacks of gout, does age of gout onset matter? A primary care based cross-sectional study. PLoS One. 2017;12(10):e0186096. https://doi.org/10.1371/journal.pone.0186096.
Singh JA, Yu S. Gout-related inpatient utilization: a study of predictors of outcomes and time trends. Arthritis Res Ther. 2016;18:57. https://doi.org/10.1186/s13075-016-0936-y.
He W, Phipps-Green A, Stamp LK, Merriman TR, Dalbeth N. Population-specific association between ABCG2 variants and tophaceous disease in people with gout. Arthritis Res Ther. 2017. https://doi.org/10.1186/s13075-017-1254-8.
Macfarlane DG, Dieppe PA. Diuretic-induced gout in elderly women. Br J Rheumatol. 1985;24(2):155–7.
Mitnala S, Phipps-Green A, Franklin C, Horne A, Stamp LK, Merriman TR, et al. Clinical and genetic features of diuretic-associated gout: a case-control study. Rheumatology. 2016;55(7):1172–6. https://doi.org/10.1093/rheumatology/kew018.
Abhishek A, Roddy E, Doherty M. Gout - a guide for the general and acute physicians. Clin Med (Lond). 2017;17(1):54–9. https://doi.org/10.7861/clinmedicine.17-1-54.
Jordan KM, Cameron JS, Snaith M, Zhang W, Doherty M, Seckl J, et al. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of gout. Rheumatology (Oxf Engl). 2007;46(8):1372–4. https://doi.org/10.1093/rheumatology/kem056a.
Logan JA, Morrison E, McGILL PE. Serum uric acid in acute gout. Ann Rheum Dis. 1997;56(11):696–7. https://doi.org/10.1136/ard.56.11.696a.
Dalbeth N, Doyle AJ. Imaging of gout: an overview. Best Pract Res Clin Rheumatol. 2012;26(6):823–38. https://doi.org/10.1016/j.berh.2012.09.003.
Newberry SJ, FitzGerald JD, Motala A, Booth M, Maglione MA, Han D, et al. Diagnosis of gout: a systematic review in support of an american college of physicians clinical practice guideline. Ann Intern Med. 2017;166(1):27–36. https://doi.org/10.7326/m16-0462.
Loffler C, Sattler H, Peters L, Loffler U, Uppenkamp M, Bergner R. Distinguishing gouty arthritis from calcium pyrophosphate disease and other arthritides. J Rheumatol. 2015;42(3):513–20. https://doi.org/10.3899/jrheum.140634.
Abhishek A, Doherty M. Update on calcium pyrophosphate deposition. Clin Exp Rheumatol. 2016;34(4 Suppl 98):32–8.
Ea HK, Liote F. Diagnosis and clinical manifestations of calcium pyrophosphate and basic calcium phosphate crystal deposition diseases. Rheum Dis Clin North Am. 2014;40(2):207–29. https://doi.org/10.1016/j.rdc.2014.01.011.
Kohn NN, Hughes RE, Mc CD Jr, Faires JS. The significance of calcium phosphate crystals in the synovial fluid of arthritic patients: the “pseudogout syndrome”. II. Identification of crystals. Ann Intern Med. 1962;56:738–45.
Dieppe PA, Doherty M, Macfarlane DG, Hutton CW, Bradfield JW, Watt I. Apatite associated destructive arthritis. Br J Rheumatol. 1984;23(2):84–91.
Khanna D, Khanna PP, Fitzgerald JD, Singh MK, Bae S, Neogi T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res. 2012;64(10):1447–61. https://doi.org/10.1002/acr.21773.
Richette P, Doherty M, Pascual E, Barskova V, Becce F, Castañeda-Sanabria J, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017;76(1):29–42. https://doi.org/10.1136/annrheumdis-2016-209707.
Terkeltaub RA, Furst DE, DiGiacinto JL, Kook KA, Davis MW. Novel evidence-based colchicine dose-reduction algorithm to predict and prevent colchicine toxicity in the presence of cytochrome P450 3A4/P-glycoprotein inhibitors. Arthritis Rheum. 2011;63(8):2226–37. https://doi.org/10.1002/art.30389.
Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW. High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010;62(4):1060–8. https://doi.org/10.1002/art.27327.
Wason S, Mount D, Faulkner R. Single-dose, open-label study of the differences in pharmacokinetics of colchicine in subjects with renal impairment, including end-stage renal disease. Clin Drug Investig. 2014;34(12):845–55. https://doi.org/10.1007/s40261-014-0238-6.
Wechalekar MD, Vinik O, Schlesinger N, Buchbinder R. Intra-articular glucocorticoids for acute gout. Cochrane Database Syst Rev. 2013. https://doi.org/10.1002/14651858.CD009920.pub2.
Man CY, Cheung IT, Cameron PA, Rainer TH. Comparison of oral prednisolone/paracetamol and oral indomethacin/paracetamol combination therapy in the treatment of acute goutlike arthritis: a double-blind, randomized, controlled trial. Ann Emerg Med. 2007;49(5):670–7. https://doi.org/10.1016/j.annemergmed.2006.11.014.
Janssens HJ, Janssen M, van de Lisdonk EH, van Riel PL, van Weel C. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial. Lancet (Lond Engl). 2008;371(9627):1854–60. https://doi.org/10.1016/s0140-6736(08)60799-0.
Jackson RL, Hunt B, MacDonald PA. The efficacy and safety of febuxostat for urate lowering in gout patients ≥65 years of age. BMC Geriatr. 2012;12:11. https://doi.org/10.1186/1471-2318-12-11.
Stamp LK, Chapman PT, Barclay ML, Horne A, Frampton C, Tan P, et al. A randomised controlled trial of the efficacy and safety of allopurinol dose escalation to achieve target serum urate in people with gout. Ann Rheum Dis. 2017;76(9):1522–8. https://doi.org/10.1136/annrheumdis-2016-210872.
Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984;76(1):47–56.
Lavan BE, McWherter C, Choi YJ. Arhalofenate, a novel uricosuric agent, is an inhibitor of human uric acid transporters [abstract]. Ann Rheum Dis. 2013;71(Suppl 3):450–1.
Choi YJ, Larroca V, Lucman A, Vicena V, Abarca N, Rantz T. Arhalofenate is a novel dual-acting agent with uricosuric and anti-inflammatory properties [abstract]. Arthritis Rheum. 2012;64(Suppl):S697.
Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–41. https://doi.org/10.1038/nature04516.
Miner J, Tan PK, Hyndman D, Liu S, Iverson C, Nanavati P, et al. Lesinurad, a novel, oral compound for gout, acts to decrease serum uric acid through inhibition of urate transporters in the kidney. Arthritis Res Ther. 2016;18(1):214. https://doi.org/10.1186/s13075-016-1107-x.
Segal R, Lubart E, Leibovitz A, Berkovitch M, Habot B, Yaron M, et al. Early and late effects of low-dose aspirin on renal function in elderly patients. Am J Med. 2003;115(6):462–6.
Caspi D, Lubart E, Graff E, Habot B, Yaron M, Segal R. The effect of mini-dose aspirin on renal function and uric acid handling in elderly patients. Arthritis Rheum. 2000;43(1):103–8. https://doi.org/10.1002/1529-0131(200001)43:1<103:aid-anr13>3.0.co;2-c.
Choi HK, Soriano LC, Zhang Y, Rodríguez LAG. Antihypertensive drugs and risk of incident gout among patients with hypertension: population based case-control study. BMJ. 2012;344:d8190. https://doi.org/10.1136/bmj.d8190.
McAdams DeMarco MA, Maynard JW, Baer AN, Gelber AC, Young JH, Alonso A, et al. Diuretic use, increased serum urate levels, and risk of incident gout in a population-based study of adults with hypertension: the Atherosclerosis Risk in Communities cohort study. Arthritis Rheum. 2012;64(1):121–9. https://doi.org/10.1002/art.33315.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
Dr. A. Abhishek has received departmental research grants from AstraZeneca and OxfordImmunotech.
Rights and permissions
About this article
Cite this article
Abhishek, A. Managing Gout Flares in the Elderly: Practical Considerations. Drugs Aging 34, 873–880 (2017). https://doi.org/10.1007/s40266-017-0512-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-017-0512-4