Abstract
Gout flares, caused by monosodium urate crystals in joints, are debilitating and linked to poor health outcomes. Gout prevalence increases with age, but effective treatment is available even in those with associated renal, cardiovascular and metabolic comorbidities. Treatment includes immediate pain relief with low-dose colchicine, non-steroidal anti-inflammatory drugs, or oral/parenteral corticosteroids; with parenteral corticosteroids useful in older patients. Lifelong urate-lowering therapy and patient education, are typically recommended to reduce the risk of recurring gout flares.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Treat gout to avoid unnecessary pain and sequelae
Gout is a manageable form of inflammatory arthritis [1, 2], but it is also the most common form affecting 1–6% of people across the world [2, 3]. Its prevalence and the proportion of women affected both increase significantly with age, with 20% of men aged ≥ 70 years (vs 4.7% of working-age people) self-reporting gout in Australian studies [1] and 4% of female US veterans aged ≥ 80 years affected [3].
Gout flares occur when monosodium urate (MSU) crystals accumulate in joints and periarticular tissues [3], especially the feet and ankles, with the classical presentation being gout in the big toe (podagra) [3]. Gout causes severe pain, reduced mobility, poor sleep, depression, anxiety, and consequent reductions in quality of life [1, 3]. Chronic gout with recurrent flares can damage joints and is linked to poor renal, gastrointestinal (GI), cardiovascular (CV) and infection-related outcomes; all resulting in a 17% increase in overall mortality [4]. The mechanisms of these associations are unclear, including any potential effects from gout treatment [3]. The financial costs of gout in elderly patients are also high [3].
In most patients, gout flares can be completely eliminated and long-term outcomes improved by standard treatment [1, 2]. Despite the availability of guidelines from the American College of Rheumatology (ACR) [5], the European League Against Rheumatism (EULAR) [2] and others, good treatment is usually not provided [1], leaving most patients to experience flares at ever-increasing intervals [6]. This article outlines the recommended management of gout flares in elderly patients, as reviewed by Kumar et al. [3], and relevant recommendations from ACR [5] and EULAR [2] treatment guidelines.
Ask about, don’t assume, gout risk factors
Risk factors for gout include hyperuricaemia [elevated serum urate (SU)], older age, male gender, post-menopause, metabolic, CV and renal comorbidities, lifestyle factors (Table 1) and iatrogenic causes [1, 3]. Hyperuricaemia is generally regarded as the first stage of gout [2]. Genetic factors can decrease the excretion of urate from the gut and kidneys, which subsequently results in hyperuricaemia [7]. However, the link between genetics and clinical gout is less clear [7].
Diabetes, obesity, coronary artery disease, hypertension, hyperlipidaemia and nephrolithiasis often coexist with gout [3, 8]. Many of these comorbidities reflect lifestyle and most increase in prevalence as people age [1]. While food and drink may catalyse flares and provide up to one-third of a patient’s urate load [3], stigmatising patients is unhelpful [1, 5].
Surgery (including bariatric surgery), severe illness, minor trauma and the early stages of urate-lowering therapy (ULT) can all precipitate flares [3]. Long-term use of diuretics [5], aspirin, cyclosporin and tacrolimus [9], which are all commonly used in older people, increase SU [5]. Age-related decline in renal and hepatic function may decrease renal urate clearance and exacerbate these effects [1].
Treatment for asymptomatic hyperuricaemia is not recommended
The relationship between asymptomatic hyperuricaemia (AH) and gout flares is unpredictable [3]. AH is common and linked to increased mortality [2], but even very high SU levels may never cause a gout flare [3]. In one 5-year study, only 20% of people with SU > 9 mg/dL developed gout [5]. Despite poor non-gout outcomes, for cost-benefit reasons, the pharmacological management of AH (defined as SU > 6.8 mg/dL or 400 mmol/L) is not recommended, including in patients with chronic kidney disease (CKD) or other comorbidities [5].
Both gout and hyperuricaemia are also strongly linked to CKD [2], with 86% of people with SU >10 mg/dL (595 μmol/L) having stage 2 or worse disease; 53% of gout patients in the same study had CKD [2]
Flare, crystals and serum urate all add up to gout
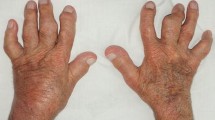
Patients with gout may have a history of risk factors, previous flares and/or elevated SU [3]. Those with a classic flare report a sudden onset of pain in one peripheral joint or bursa, which may appear warm, swollen and red. Use of the joint, pressure or even touch may be unbearable. Tophi, which are subcutaneous nodules, may be present in joints and suggest advanced disease. Patients may also present with fever and leucocytosis; aseptic cellulitis and other forms of arthritis, including septic arthritis, must be excluded [3].
Chronic, advanced disease also increases in frequency with age [10]. In approximately half of patients aged > 65 years, subacute flares in several joints occur concurrently [10]. Taking a careful history and utilizing gout scoring systems can help confirm a diagnosis where presentation is atypical [2, 3, 5].
In patients with probable gout, diagnosis may be confirmed in 95% of patients by measuring SU and assessing the synovial fluid or tophus aspirate for needle-shaped MSU crystals whilst excluding infection [11]. Repeating the analysis for SU some weeks later is suggested, as levels can drop during a gout flare, often to normal [12].
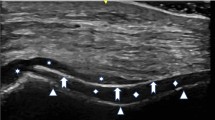
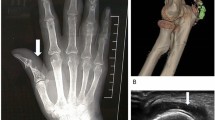
Imaging may be appropriate [3], particularly where the diagnosis is unclear [5]. Dual-energy CT is sensitive and specific for gout, particularly for the atypical presentations common in older patients [3], and ultrasound is recommended by the ACR and EULAR [2, 5]. Imaging facilitates fluid aspiration procedures, and may also reveal tophi or the double-contour sign; this sign is highly specific for gout as it shows the deposition of MSU crystals across hyaline cartridge [12].
In older patients, kidney and hepatic function tests, including an estimate of creatinine clearance (CLCR), plus other investigations relevant to known or suspected comorbidities (e.g. glycosylated haemoglobin for diabetes) are recommended [3]. EULAR guidelines recommend screening for CKD, heart failure (HF), hypertension and peripheral artery disease, smoking, diabetes and lifestyle [2]. Such screening systematically elucidates polypharmacy, and resultant potential drug interactions, that are common in older patients (Table 2) [1].
Approach flare treatment from all angles
The aspects of gout flare treatment should be discussed with patients [3]. The immediate need is the relief of acute pain and inflammation (Table 2); however, over the longer-term, lifelong ULT, along with lifestyle changes, are usually recommended to prevent further flares [2, 5]. Prophylaxis against repeat flares early in ULT is also recommended as they are paradoxically more likely as MSU crystals disperse from joints [2].
Reviewing non-gout medications is also recommended [2, 5]. When gout flares occur in hypertensive patients receiving thiazide or loop diuretics [2], these should be replaced where possible with losartan (an angiotensin-converting enzyme inhibitor) or calcium channel blockers [2]. Patients with hyperlipidaemia may benefit from a statin (subject to colchicine interactions, Table 2) or fenofibrate [2], although the latter is not a recommended switch in the USA [5]. Low-dose aspirin, where clearly indicated, should be continued in the absence of alternatives [5].
Choose from three helpful first-line flare choices
While gout flare management in older and/or renally impaired patients may be more complex than in younger people, treatment options that may preserve kidney and CV function are available (Table 2) [2, 3, 5]. Selecting an appropriate first-line acute flare therapy in an older patient depends on their profile (Table 2), including comorbidities and the potential for adverse drug events (ADEs) [3]. Recommendations in severe CKD vary, especially with colchicine (Table 2) [2, 13].
Using colchicine only at low doses (≤ 1.2 mg and ≤ 0.6 mg 1 h later) is consistently suggested (Table 2) [2, 5] as it results in GI ADEs similar to placebo (e.g. nausea and diarrhoea), with no drop in efficacy versus a higher dose (4.8 mg over 6 h) [14]. A 24 h drug-free interval on day 2 (Table 2) may also reduce colchicine ADEs [1]. In older people, where colchicine is contraindicated, selecting shorter-acting non-steroidal anti-inflammatory drugs (NSAIDs) may likewise minimise ADEs (Table 2) [1].
Parenteral corticosteroids may be preferred for patients who have comorbidities that are contraindications to colchicine or NSAIDs and/or have significant drug interactions, as is often the case in older patients (Table 2). If oral corticosteroids are used, systemic ADEs should be discussed with patients (Table 2) [5].
Conversely, for severe flares such as tophaceous gout or if multiple joints are affected [2], combination therapy such as colchicine in combination with NSAIDs or glucocorticoids may be tolerated, although high quality efficacy data are lacking [3]. Concomitant NSAID and glucocorticoid use is not recommended due to potential GI toxicity [3].
Adjuvant therapies such as topical ice [5] and limited use of non-NSAID analgesics may be helpful [3]. Opioids are widely dispensed to gout patients, despite their routine use not being recommended in guidelines [2, 5]. Opioids are especially inappropriate in elderly patients with CKD and other comorbidities in whom ADEs are likely [3].
Interleukin-1 antagonists are second-line options
Limited data support the use of subcutaneous injections with interleukin (IL)-1 blockers, anakinra and canakinumab, as second-line options for recurrent flares [2, 5]. IL-1β influences MSU-induced inflammation and IL-1 blockers may have a future role in gout prophylaxis [2]. Anakinra 100 mg/day for 3–4 days [16] and canakinumab 150 mg as a single dose were each at least as effective as intramuscular (IM) triamcinolone [2]; the shorter half-life of anakinra may be helpful in older patients [3]. Occult sepsis must be excluded before IL-1 inhibitors are started, and ULT adjusted after their administration [2]. Despite their efficacy, access to IL-1 inhibitors may be limited due to cost in the USA [5].
While IM adrenocorticotropic hormone 1 mg acts quickly with similar efficacy to triamcinolone [6], it is a third-line option in the US and not recommended in Europe [2, 5] due to cost, inaccessibility and limited tolerability data [3].
Long-term treatment and patient education are important
All patients with recurrent flares should receive lifelong ULT [2, 5]. Preventing repeat flares with ULT is a key therapy goal [2, 5] and they may be eliminated if patients adhere to treatment [2]. ULT aims to reduce SU to concentrations below where it crystallises (< 6 mg/dL or 360 μmol/L) [8], as a treat-to-target approach [2, 5]; the risk of gout increases above this target [2, 5, 9]. In severe gout, the target is 5 mg/dL (300 μmol/L) to speed MSU crystal dissolution [2]. The benefits of lowering SU to < 3 mg/dL are not clear and this is not currently recommended [2].
At present, many patients are not given the option of ULT, and in patients who are offered long-term ULT, approximately half discontinue treatment [1]. Adherence significantly improves with patient education, with 92% of better informed patients effectively treated after 1 year in an observational study [2]. Furthermore, patients should be included in the decision making process to improve adherence [2].
Potential ADEs and the possibility of flares early in the course of ULT should be discussed, so that patients understand that either medications or dosages can be changed if needed, rather than discontinuing ULT [2]. Early flares caused by the dissolution of MSU crystals can be minimised by a gradual start to ULT and the use of prophylactic treatments (Table 2), with colchicine preferred in most older patients [1]. Patients may alternatively be prescribed on-demand medication to take as soon as flare symptoms occur [3]; the immediacy of on-demand treatment should be emphasised (Table 2) [2].
Patient education and encouraging lifestyle changes should always be a part of gout management (Table 1) [2]. Certain foods and drinks, such as beer or meat, are recommended to be consumed in moderation [2]. Weight loss and regular exercise are recommended for all patients (Table 1) [2, 5].
Long-term use of low-dose colchicine may also be effective in the prophylaxis of gout flares (Table 2) [15]. Safety concerns relating to cancer or infection risk with long-term colchicine was reported in one trial; however, other trials did not corroborate this result and its safety is supported by 50 years of clinical experience [15]. While many patients decline prophylaxis without experiencing numerous flares, low-dose colchicine may have other benefits. For instance, colchicine reduced major CV events in two trials in patients with coronary heart disease [2].
Eliminate flares with urate-lowering therapy
ULT should be started during or after a first flare [2], where:
-
patients have urolithiasis, moderate to severe renal [5] or CV comorbidities, such as HF, hypertension or ischaemic heart disease [2], all of which affect many older patients [1]; and/or
-
SU is very high (> 8 or 9 mg/dL or > 480 or 535 μmol/L [2, 3, 5]; and
-
patients aged < 40 years (EULAR recommendation) [2].
In the US, starting ULT during a flare is recommended for practical reasons as patients are with their clinicians and motivated to consider therapy [5]. Regular SU testing is an essential part of ULT due to its treat-to-target goals, and nurse- or pharmacist-led programmes are helpful [5].
Use allopurinol, subject to genetic risks
The xanthine oxidase inhibitor (XOI) allopurinol is the first-line option for ULT [2]. Starting ULT with low doses and titrating to target is an effective and well tolerated approach, including in older patients [5]. In patients of Asian and African origin, genetic testing is recommended prior to starting allopurinol due to the higher risk for severe hypersensitivity [1]. There are also several provisos relevant to older patients with CKD:
-
In mild-moderate disease (CLCR ≥ 30 mL/min) allopurinol may be replaced with febuxostat starting at ≤ 40 mg/day [5], or added to/replaced with the uricosurics benzbromarone or probenecid [2], although hepatotoxicity is a problem with the latter [1].
-
In moderate-to-severe disease, a dose reduction of ≥ 50% based on CLCR is recommended [2, 5].
-
The preferred second-line option in this group is an XOI [1] (e.g. febuxostat starting at ≤ 40 mg/day) despite its CV [1] and mortality [17] risks, rather than probenecid starting at 500 mg once or twice daily [5].
-
In severe, chronic, tophaceous gout with poor life quality, pegloticase is a second-line option due to cost and tolerability issues [2, 5].
Take home messages
-
Gout flares, caused by MSU crystal accumulation in and around joints, are painful and debilitating; both immediate management of pain and inflammation in addition to long-term preventative strategies are required.
-
Colchicine, NSAIDs and oral or parenteral corticosteroids (parenteral corticosteroids are well suited to elderly patients) are all first-line options for treating gout flares.
-
The presence of comorbidities (e.g. CKD or CV) and the potential for pharmacokinetic drug interactions may guide treatment selection.
-
Comorbidities and polypharmacy in older people, who are most prone to gout, do not preclude the effective treatment and eventual elimination of gout flares.
-
Effective patient education and long-term ULT (typically with allopurinol) can prevent the recurrence of gout flares, improving quality of life and long-term renal and CV outcomes.
References
Day RO, Lau W, Stocker SL, et al. Management of gout in older people. J Pharm Pract Res. 2019;49(1):90–7.
Richette P, Doherty M, Pascual E, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017;76(1):29–42.
Kumar M, Manley N, Mikuls TR. Gout flare burden, diagnosis, and management: navigating care in older patients with comorbidity. Drugs Aging. 2021;38(7):545–57.
Vargas-Santos A, Neogi T, da Rocha C-PG, et al. Cause-specific mortality in gout: novel findings of elevated risk of non-cardiovascular-related deaths. Arthritis Rheumatol (Hoboken, NJ). 2019;71(11):1935–42.
FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). 2020;72(6):744–60.
Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and anti-inflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447–61.
Major TJ, Dalbeth N, Stahl EA, et al. An update on the genetics of hyperuricaemia and gout. Nat Rev Rheumatol. 2018;14(6):341–53.
Mouradjian MT, Plazak ME, Gale SE, et al. Pharmacologic management of gout in patients with cardiovascular disease and heart failure. Am J Cardiovasc Drugs. 2020;20(5):431–45.
Richette P, Doherty M, Pascual E, et al. 2018 updated European League Against Rheumatism evidence-based recommendations for the diagnosis of gout. Ann Rheum Dis. 2020;79(1):31–8.
Stamp LK, Jordan S. The challenges of gout management in the elderly. Drugs Aging. 2011;28(8):591–603.
Lawry GV, Fan PT, Bluestone R. Polyarticular versus monoarticular gout: a prospective, comparative analysis of clinical features. Medicine (Baltimore). 1988;67(5):335–43.
Schlesinger N. Diagnosis of gout: clinical, laboratory, and radiologic findings. Am J Manag Care. 2005;11(Suppl 15):S443–8.
Camber Pharmaceuticals Inc. Colchicine: US prescribing information. 2021. https://dailymed.nlm.nih.gov/dailymed. Accessed 29 June 2022.
Terkeltaub RA, Furst DE, Bennett K, et al. High versus low dosing of oral colchicine for early acute gout flare: twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010;62(4):1060–8.
Robinson PC, Terkeltaub R, Pillinger MH, et al. Consensus statement regarding the efficacy and safety of long-term low-dose colchicine in gout and cardiovascular disease. Am J Med. 2022;135(1):32–8.
Saag KG, Khanna PP, Keenan RT, et al. A randomized, phase 2 study evaluating the efficacy and safety of anakinra in the treatment of gout flares. Arthritis Rheumatol. 2021;73(8):1533–42.
White WB, Saag KG, Becker MA, et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med. 2018;378(13):1200–10.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
C. Fenton, a contracted employee of Adis International Ltd/Springer Nature, and A. Lee, a salaried employee of Adis International Ltd/Springer Nature, declare no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent for publication, Availability of data and material, Code availability
Not applicable.
Rights and permissions
About this article
Cite this article
Fenton, C., Lee, A. Educate and treat to eliminate gout flares in elderly patients. Drugs Ther Perspect 38, 349–354 (2022). https://doi.org/10.1007/s40267-022-00934-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-022-00934-6