Abstract
Givosiran (Givlaari®) is an δ-aminolevulinic acid synthase 1 (ALAS1)-directed small interfering RNA (siRNA) approved for the treatment of acute hepatic porphyria (AHP). In the phase 3 ENVISION trial, givosiran significantly reduced the annualized rate of composite porphyria attacks (i.e. attacks requiring hospitalization, urgent healthcare visit or intravenous hemin administration at home) compared with placebo in patients with recurrent acute intermittent porphyria (the most common type of AHP) attacks. Givosiran also improved several other outcomes, including hemin use and pain (the cardinal symptom of AHP). While generally well tolerated with an acceptable safety profile, the drug may increase the risk of hepatic and kidney adverse events. Givosiran offers the convenience of once-monthly subcutaneous administration. Available evidence indicates that givosiran is an important newer therapeutic option for patients with AHP and severe recurrent attacks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.14226518 |
ALAS1-directed siRNA |
Convenient once-monthly subcutaneous administration |
Significantly reduces the annualized rate of composite porphyria attacks versus placebo |
Generally well tolerated and acceptable safety profile |
1 Introduction
Acute hepatic porphyria (AHP) is a family of four rare inherited disorders caused by mutations in genes encoding enzymes involved in the haem biosynthesis pathway [1,2,3]. The four types are acute intermittent porphyria (AIP), hereditary coproporphyria, variegate porphyria and δ-aminolevulinic acid dehydratase (ALAD)-deficient porphyria, caused by mutations in the HMBS, CPOX, PPOX and ALAD genes, respectively [3]. The estimated prevalence is 5–10, 0.5 and 0.5 per 100,000 individuals for AIP, hereditary coproporphyria and variegate porphyria, respectively; ALAD-deficient porphyria is very rare, with only 12 reported cases worldwide [3]. The estimated clinical penetrance of AIP is ≈ 1% [4]. AHP attacks occur mostly in women of reproductive age [1, 5].
AHP is characterized by upregulation of hepatic δ-aminolevulinic acid synthase 1 (ALAS1), leading to accumulation of neurotoxic haem intermediates, δ-aminolevulinic acid (δ-ALA) and porphobilinogen (PBG) [1, 2, 6,7,8]. These compounds cause injury mainly to the nervous system, resulting in debilitating and potentially life-threatening acute porphyria attacks as well as chronic symptoms. The acute attacks manifest as neurovascular symptoms such as diffuse abdominal pain (primary symptom), nausea, vomiting, weakness, constipation and psychiatric symptoms [1, 2, 6]. AHP can also contribute to long-term comorbidities, including chronic kidney disease, systemic arterial hypertension, chronic neuropathy and liver disease [1, 2, 5, 9]. Acute porphyria attacks typically last 3–5 days [10], although they can last 1–2 weeks or longer in some patients [11].
AHP presents considerable clinical heterogeneity, with at least four major subgroups identified: symptomatic patients with sporadic attacks; symptomatic patients with recurrent acute attacks; asymptomatic patients with elevated urinary δ-ALA and PBG levels (chronic high excretors); and, asymptomatic patients without elevated urinary δ-ALA and PBG levels [7]. Most symptomatic patients will experience a few attacks in their lifetime and up to 8% of patients will have severe disease and recurrent attacks [12], typically defined as ≥ 4 attacks requiring admission to hospital for treatment in one or more years [13]. There is a need for treatment to durably decrease the frequency of recurrent AHP attacks and reduce the long-term complications of these attacks.
A novel approach to preventing recurrent AHP attacks is to reduce hepatic ALAS1 activity with synthetic small interfering RNAs (siRNAs) [14,15,16]. Subcutaneous givosiran (Givlaari®) is an ALAS1-directed siRNA approved for the treatment of AHP. This review focuses on the efficacy and tolerability of givosiran in patients with AHP and briefly summarizes its pharmacological properties.
2 Pharmacodynamic Properties of Givosiran
Givosiran is a synthetic double-stranded siRNA that inhibits the production of ALAS1 in hepatocytes and thereby reduces circulating levels of δ-ALA and PBG [2, 17, 18]. It is designed to be selectively delivered to hepatocytes through asialoglycoprotein receptor-mediated endocytosis. Givosiran is composed of a metabolically stable siRNA covalently conjugated to a synthetic trivalent N-acetylgalactosamine ligand. The conjugate binds to the asialoglycoprotein receptors on hepatocytes with high specificity and affinity, triggering endocytosis. Once internalized, the siRNA is incorporated into the RNA-induced silencing complex (RISC), followed by separation of the two strands. When the RISC with the functional (antisense) strand binds to ALAS1 mRNA, a catalytic process ensues where a single RISC cleaves and degrades a large number of target mRNAs, preventing ALAS1 protein synthesis [2, 17, 18].
A preclinical proof-of-concept study demonstrated the effectiveness of a siRNA in silencing hepatic ALAS1 in a mouse model of AIP [19]. In a subsequent study, prophylactic administration of givosiran prevented phenobarbital-induced upregulation of hepatic ALAS1 expression in a rat model of AIP, with corresponding reductions in urinary δ-ALA and PBG levels [20]. In a mouse model of AIP, givosiran was significantly (p < 0.05) more effective than hemin in reducing liver and serum ALAS1 mRNA at 24 h and 48 h post treatment. Furthermore, a single subcutaneous administration of givosiran dose-dependently reduced liver, serum and urinary ALAS1 mRNA levels in cynomolgus monkeys; with multiple weekly administrations, the reduction in serum ALAS1 mRNA levels was rapid, robust and sustained throughout the dosing phase, with the levels returning to baseline 42 days after the last dose [20].
Givosiran showed robust pharmacodynamic activity in patients with AIP in a multicentre, randomized, placebo-controlled, 3-part phase 1 trial [21]. A single 0.035–2.5 mg/kg dose (part 1) or two 0.35 or 1.0 mg/kg doses 28 days apart (part 2) produced rapid, dose-dependent reductions from baseline in serum ALAS1 mRNA and urinary levels of δ-ALA and PBG in chronic high excretors. In part 3, patients with recurrent porphyria attacks were randomized to four once-monthly 2.5 or 5.0 mg/kg doses, two once-quarterly 2.5 or 5.0 mg/kg doses or placebo in a double-blind manner. The maximum reductions in serum ALAS1 mRNA from baseline were 67% and 74% with monthly 2.5 and 5.0 mg/kg doses, and 49% and 53% with quarterly 2.5 and 5.0 mg/kg doses. These reductions were sustained through the end of the trial (day 168) and were accompanied by rapid and sustained reductions in urinary δ-ALA and PBG levels (> 90% reduction with monthly doses). The once-monthly regimen produced greater reductions in urinary δ-ALA and PBG levels and lower peak-to-trough fluctuations than the once-quarterly regimen. Givosiran recipients who achieved the greatest reduction in δ-ALA level had the lowest annualized attack rate (AAR) [21]. The effect of the approved givosiran regimen on δ-ALA and PBG levels in patients with AIP in the phase 3 ENVISION trial is discussed in Sect. 4.
3 Pharmacokinetic Properties of Givosiran
Subcutaneous givosiran and its active metabolite AS(N-1)3′ givosiran (equipotent to givosiran) exhibit linear plasma pharmacokinetics over a dose range of 0.35–2.5 mg/kg [22, 23]. At the recommended 2.5 mg/kg once monthly regimen, givosiran exhibits time-independent pharmacokinetics [22, 23].
The key pharmacokinetic properties of givosiran and its active metabolite are summarised in Table 1 [23, 24]. Following subcutaneous administration, givosiran is rapidly absorbed and is > 90% plasma protein-bound at the 2.5 mg/kg once monthly dosage [22, 23]. Since givosiran is a liver-targeted therapy, plasma concentrations are not reflective of the extent or duration of its pharmacodynamic activity. Givosiran is rapidly taken up by, and distributes primarily to, the liver where it exhibits a long half-life resulting in sustained pharmacodynamic activity over the monthly dosing interval. Givosiran is metabolised by nucleases to oligonucleotides of shorter lengths and is not metabolized by CYP450 enzymes. AS(N-1)3′ givosiran is a major metabolite in plasma, with 45% exposure (area under the plasma concentration-time curve from time zero to 24 h) relative to givosiran at the 2.5 mg/kg once monthly dosage. Givosiran and AS(N-1)3′ givosiran are eliminated primarily by kidneys, with up to 14% of the administered dose recovered in the urine [22, 23]. The pharmacokinetics of givosiran and its active metabolite after multiple doses were generally similar to that after single dose, with no accumulation [24].
No clinically relevant differences in givosiran pharmacokinetics were observed based on age, gender, race, kidney impairment [mild, moderate or severe; estimated glomerular filtration rate (eGFR) ≥ 60 to < 90, ≥ 30 to < 60 and ≥ 15 to < 30 mL/min/1.73 m2, respectively) or mild hepatic impairment [bilirubin ≤ 1 × upper limit of normal (ULN) and aspartate transaminase (AST) > 1 × ULN, or bilirubin > 1 to 1.5 × ULN] [22, 23]. The effects of kidney failure and moderate to severe hepatic impairment on the pharmacokinetics of givosiran have not been studied [22, 23].
Downregulation of ALAS1 mRNA expression in the liver by givosiran, combined with defective haem synthesis in AHP, could lower hepatic haem content, which in turn could reduce the activity of haem-dependent hepatic proteins such as CYP450 enzymes. In a drug–drug interaction study in patients with AIP, givosiran moderately inhibited CYP1A2 and CYP2D6, weakly inhibited CYP3A4 and CYP2C19, and had no effect on CYP2C9 [25]. Caution is recommended when substrates of CYP1A2 (e.g. caffeine) and CYP2D6 (e.g. dextromethorphan) are concomitantly used with givosiran, as givosiran may increase plasma concentrations of these substrates and alter their adverse event (AE) profiles [22, 23]. If concomitant use is unavoidable, reducing the CYP1A2 and CYP2D6 substrate dosage in accordance with approved product labelling should be considered [22, 23].
4 Therapeutic Efficacy of Givosiran
Givosiran reduced the AAR and hemin use in patients with AIP who had recurrent attacks in a phase 1 study (Sect. 2) [21] and its open-label extension (OLE) [26]. This section focuses on the efficacy of givosiran in patients with recurrent AHP attacks in the randomized, double-blind, multinational phase 3 ENVISION trial [27] and its OLE period [28].
The key eligibility criteria in ENVISION included age ≥ 12 years, documented diagnosis of AHP, urinary δ-ALA or PBG levels ≥ 4 × ULN, a confirmed AHP mutation or biochemical and clinical presentation of AHP (if a AHP mutation was not identified), and at least two composite porphyria attacks (i.e. those requiring hospitalization, urgent healthcare or intravenous hemin at home) within the 6 months prior to screening [27]. The key exclusion criteria were alanine aminotransferase (ALT) > 2 × ULN, total bilirubin > 1.5 × ULN, eGFR < 30 mL/min/1.73 m2, international normalized ratio > 1.5 (> 3.5 if on anticoagulants), active HIV, hepatitis B or C virus infection, and anticipated liver transplantation [27].
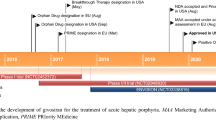
Randomization was stratified by AHP types, previous use of hemin prophylaxis and AAR in the previous 12 months [27]. Patients received givosiran 2.5 mg/kg or placebo once monthly for 6 months (Fig. 1). Prophylactic hemin was not permitted during the trial and was discontinued ≥ 4 days prior to screening; acute attacks were treated as per local standard of care, which could include intravenous hemin. The primary end point was the AAR of composite porphyria attacks in patients with AIP during the 6-month treatment period in the full analysis set [27].
Trial design of the randomized, double-blind, multinational phase 3 ENVISION trial in patients with AHP with ongoing attacks [27]. Primary endpoint results are reported in the animated figure (available online). AAR annualized rate of composite porphyria attacks, AHP acute hepatic porphyria, AIP acute intermittent porphyria, qM once monthly
Supplementary file1 (MP4 11692 kb)
Of the 94 randomized patients, 89 (95%) had AIP with an identified mutation in the HMBS gene, one had hereditary coproporphyria, two had variegate porphyria and two had AHP without an identified mutation [27]. Baseline demographic and clinical characteristics were generally similar between the treatment groups. At baseline, patients had a mean age of 38.8 years, 89% of patients were females, 78% were White, 40% had received hemin prophylaxis, the median historical ARR was 8 and the mean time since AHP diagnosis was 9.7 years [27].
Givosiran started to decrease the frequency of porphyria attacks within the first month and the effect was sustained through 6 months’ treatment [27]. Givosiran reduced the AAR of composite porphyria attacks by 74% relative to placebo in patients with AIP (Table 2); reductions were seen for all three component treatment settings (i.e. attacks requiring hospitalization, urgent healthcare and intravenous hemin use). Consistent AAR reductions were seen in all analysed prespecified subgroups of AIP, based on age at screening (< 38 vs ≥ 38 years), race (White vs non-White), geographic region (North America vs other; Europe vs other), baseline body mass index (< 25 vs ≥ 25 kg/m2), prior hemin prophylaxis (yes vs no), historic AAR (< 7 vs ≥ 7 with hemin prophylaxis; < 12 vs ≥ 12 without hemin prophylaxis), prior chronic opioid use when not having attacks (yes vs no) and prior chronic symptoms when not having attacks (yes vs no). AAR rate ratios for givosiran to placebo ranged from 0.18 to 0.43 for these subgroups (all significant based on 95% CI, except for patients who had used opioids chronically when not having attacks). During the 6-month treatment period, 50.0% of givosiran recipients (vs 17.4% of placebo recipients) had no porphyria attacks [27].
In the overall AHP population (i.e. all types included), the AAR was 73% lower in the givosiran than in the placebo group (Table 2) [27]. In a post hoc analysis of the AHP population, givosiran provided similar benefit in patients with or without prior hemin prophylaxis (median AAR reductions > 90% and 100% vs placebo) [29].
In patients with AIP, givosiran significantly reduced urinary δ-ALA and PBG levels and hemin use, compared with placebo (Table 2) [27]. In the givosiran group, δ-ALA and PBG levels decreased by a median 86% and 91% from baseline at 6 months. Hemin use decreased by 77% relative to placebo; 54% of givosiran recipients (vs 23% of placebo recipients) had zero days of hemin use [27].
Givosiran treatment was also associated with improvements in some patient-reported outcomes [27]. Daily worst pain, fatigue and nausea were measured on a 10-point numerical rating scale (10 = severe) and health-related quality of life was assessed using the 12-Item Short-Form Health Survey (SF-12) as secondary endpoints. Givosiran significantly decreased pain versus placebo (Table 2), but not fatigue. Due to prespecified hierarchical testing rules, statistical significance was not tested for nausea and SF-12 scores [27]. Nevertheless, SF-12 physical component summary scores improved in givosiran recipients (nominal p < 0.05 vs placebo) [23]. In exploratory analyses, givosiran improved the overall health status, activities of daily living and treatment satisfaction in patients with AHP, as assessed by the Patient Global Impression of Change scale and the Porphyria Patient Experience Questionnaire (PPEQ) [27].
In post hoc analyses, givosiran reduced attack severity versus placebo, as assessed by attacks with median pain scores ≥ 7 (21.1% vs 32.0% of total number of attacks) and the proportion of patients with ≥ 1 attack with median pain scores ≥ 7 (41.7% vs 63.2%) [30]. Givosiran also reduced pain during attack-free periods versus placebo, as assessed by the proportion of days with daily worst pain scores above baseline (19.1% vs 28.1%) and pain score ≥ 7 (6.8% vs 12.2%). Treatment-related pain reduction was not due to increased analgesics use, as fewer givosiran than placebo recipients had used opioids during attacks (73.3% vs 85.0%) and attack-free periods (56.3% vs 69.6%). Givosiran recipients reported greater improvement in the SF-12 bodily pain domain suggesting the reduction in daily worst pain was clinically relevant, as the pain reduction was accompanied by reduced interference with normal activities [30].
4.1 Open-Label Extension
All eligible patients completing the ENVISION double-blind period entered a 30-month OLE period during which givosiran recipients continued givosiran while placebo recipients were crossed over to givosiran [28]. In the OLE, some patients were assigned to givosiran 1.25 mg/kg once monthly initially, with subsequent increase to 2.5 mg/kg once monthly following a protocol amendment [28].
The efficacy of givosiran was maintained in 12-month [28] and 18-month [31] interim analyses of the OLE, with placebo crossover patients attaining similar benefits to givosiran recipients during the double-blind period. With continued treatment, reductions in the AAR, urinary δ-ALA/PBG levels and hemin use were sustained at 12 months, while the crossover patients experienced 83%, > 75% and 100% reductions in these parameters, respectively [28]. At 18 months, the proportion of patients with no attacks increased to 60.9% and 40.0% in the continued givosiran and crossover groups during OLE [31].
The continued givosiran group had further improvements in daily worst pain (through 12-month interim analysis), most SF-12 categories and all PPEQ categories during the OLE [28, 31]. The crossover patients had improvements in these measures, consistent with givosiran recipients during the double-blind treatment period. In both continued givosiran and crossover groups, givosiran provided greatest improvements from baseline for role physical, bodily pain, general health and social functioning among the SF-12 categories, and for overall satisfaction with treatment, convenience of treatment and travelling > 1 day for work or pleasure among the PPEQ categories. Overall, > 74% of patients responded as ‘much better’ or ‘always’ for convenience of treatment and overall satisfaction with treatment [28, 31].
5 Tolerability of Givosiran
Givosiran had an acceptable safety profile and was generally well tolerated in patients with AHP in clinical studies [21, 27]. Although AEs (90% vs 80%), severe AEs (17% vs 11%) and serious AEs (21% vs 9%) were more common with givosiran than with placebo in the ENVISION trial, only one givosiran recipient discontinued the treatment because of an AE in the double-blind period [27]. AEs with 5% greater incidence in the givosiran than in the placebo group included injection-site reactions (25% vs 0%), nausea (27% vs 11%), chronic kidney disease (10% vs 0%), decreased eGFR (6% vs 0%), rash (6% vs 0%), increased ALT (8% vs 2%) and fatigue (10% vs 4%) [27]. The safety profile of givosiran remained acceptable in the ENVISION OLE with no new safety signals [28, 31]; one givosiran recipient discontinued treatment because of hypersensitivity to the drug at 18-month data cut-off [31].
Givosiran has low immunogenic potential [22, 23]. In clinical studies, 1 of 111 patients with AHP (0.9%) receiving givosiran developed treatment-emergent anti-drug antibodies. These antibodies had no clinically relevant effect on the efficacy, safety, pharmacokinetics or pharmacodynamics of givosiran [22, 23].
5.1 Adverse Events of Special Interest
Injection-site reactions with givosiran included erythema, pain, pruritus, rash, discoloration and swelling around the injection site [22, 23]. The majority of injection-site reactions were mild or moderate in severity and did not result in treatment discontinuation [27]. In clinical studies, anaphylactic reaction was reported in one givosiran recipient who had a history of allergic asthma and atopy [22, 23].
Serum ALT levels > 3 × ULN were more common with givosiran than with placebo (15% vs 2%), occurring mainly 3–5 months after initiation of study treatment [27]. One givosiran recipient had a serious AE of ALT 9.9 × ULN, requiring treatment discontinuation [27]. Liver function should be monitored before and during (at monthly intervals) givosiran treatment and the treatment should be interrupted or discontinued if severe or clinically significant ALT elevation occurs [22, 23].
Kidney-related AEs, manifesting mostly as an increase in serum creatinine level or a decrease in eGFR, occurred in 15% of givosiran recipients and 7% of placebo recipients [27]. In the givosiran group, serum creatinine increased by a median 0.07 mg/dL at 3 months accompanied by a reduction in eGFR, both of which resolved over time without dose modifications. Five givosiran recipients had onset or worsening of chronic kidney disease. Two of the worsening cases were serious (the only serious AE reported in at least two givosiran recipients); these patients had porphyria-related coexisting conditions (hypertension and nephropathy). No givosiran or placebo recipients discontinued treatment because of kidney-related AEs [27]. Kidney function should be monitored during givosiran treatment [22, 23].
6 Dosage and Administration of Givosiran
Givosiran is indicated for the treatment of AHP in several countries, including those in the EU (in adults and adolescents aged ≥ 12 years) [23] and the USA (in adults) [22]. The recommended dosage is 2.5 mg/kg once monthly administered as a subcutaneous injection [22, 23]. In patients who have dose interruption because of severe [22] or clinically significant [22, 23] transaminase elevations and subsequent improvement, givosiran can be resumed at 1.25 mg/kg once monthly. If these patients do not have recurrence of severe or clinically significant transaminase elevations, the dose may be increased to 2.5 mg/kg once monthly [22]. Local prescribing information should be consulted for detailed information, including contraindications, precautions, drug interactions and use in special patient populations.
7 Place of Givosiran in the Management of Acute Hepatic Porphyria
Clinical features of AHP resemble other common medical conditions, often leading to missed or delayed diagnosis [7, 8]. Therefore, optimal AHP management should start with a comprehensive initial assessment, including porphyria‐specific biochemical testing, standard blood tests, genetic testing, review of complete medical history and physical examination. AHP treatment and care should be individualised, depending on the clinical subgroup (see Sect. 1) [7, 8].
Specific treatment options for AHP are limited, and current treatment guidelines predate the approval of givosiran [7, 8]. Conventionally, AHP is managed by the elimination of precipitating factors, symptomatic supportive treatment, carbohydrate loading and hemin therapy. The most common precipitating factors for AHP are drugs (many commonly prescribed drugs are unsafe in AHP), alcohol, reduced calorie intake, fluctuating sex hormone levels, stress, infection, smoking and recreational drugs. Supportive measures include medications to treat pain, nausea, vomiting, hypertension, tachycardia, convulsions and fluid imbalance. As ALAS1 is upregulated during fasting, carbohydrate loading may be used in mild attacks, along with supportive measures, for up to 48 h. In case of severe attacks, hemin treatment should be started immediately. In women with recurrent premenstrual porphyria attacks, hormonal suppression therapy may be used. Liver transplantation is indicated as the last resort for patients with severe, disabling, intractable attacks that are refractory to hemin therapy [7, 8]. Intravenous hemin remains the standard of care for sporadic acute AHP attacks and it is not approved for prophylactic use in patients with recurrent attacks [7, 8, 32,33,34]. On the other hand, givosiran has not been studied as a treatment for sporadic acute attacks, but was evaluated for preventing severe recurrent AHP attacks in the phase 3 ENVISION trial [27].
In the EXPLORE natural history study of AHP, patients with recurrent attacks often required hospitalization, had impaired health-related quality of life and had increased healthcare utilization [9]. In ENVISION, givosiran significantly reduced the AAR of AIP attacks that required hospitalization, urgent healthcare or intravenous hemin at home (Sect. 4). This effect was consistently seen in all prespecified subgroups, including patients with a high number of attacks at baseline and those who had used hemin prophylaxis previously. A number of secondary outcomes also favoured givosiran over placebo, including urinary δ-ALA and PBG levels and hemin use. Of note, givosiran reduced patient-reported pain (the cardinal symptom in AHP) and analgesic use during attack and attack-free periods. With continued treatment, the efficacy of givosiran was maintained through to 18 months (Sect. 4.1). Givosiran treatment was associated with a high level of patient-reported convenience and overall satisfaction in the ENVISION trial (Sect. 4).
Givosiran was generally well tolerated and had an acceptable safety profile in patients with AHP in the ENVISION trial (Sect. 5). The main safety concerns with givosiran were hepatic and kidney AEs, which were transient and resolved in most patients (Sect. 5.1). Interpretation of hepatic and kidney safety data is also confounded by the fact that elevated liver enzymes and chronic kidney disease are comorbid and long-term complications of AHP. Nevertheless, liver and kidney function should be monitored during givosiran treatment.
Based on the ENVISION results, givosiran was recently approved in the EU, USA and several other countries for the treatment of AHP (Sect. 6). The American Porphyria Foundation [35], European Porphyria Network [13] and the National Organization for Rare Disorders [36] recognize givosiran as a new treatment option for AHP. Expert opinion is that givosiran is an effective treatment option for the subset of patients with AHP and recurrent acute attacks that were severe enough to require hospital admission and/or hemin treatment, a population similar to that enrolled in the ENVISION trial [32, 33, 37].
While the ENVISION trial demonstrated the efficacy and safety of givosiran in AHP, this clinical experience is limited in terms of number of patients and treatment duration. In this regard, the OLE data are promising, although additional long-term efficacy and safety studies, as well as real-world clinical experience, are required to firmly establish the relative role of givosiran in the management of AHP. Research into mechanisms and potential biomarkers of hepatic and kidney AEs with givosiran would also be valuable. Finally, since AHP poses an enormous economic and healthcare utilization burden, cost implications of AHP therapy is of paramount importance. Robust cost-effectiveness analyses of givosiran are required to guide healthcare resource allocation decisions. Patient-reported outcomes point to increased health utility values for givosiran (Sect. 4) [27, 28].
In conclusion, givosiran reduces the AAR of acute composite porphyria attacks in patients with AHP and is generally well tolerated, with an acceptable safety profile. The efficacy and tolerability of givosiran is sustained through to 18 months, although additional longer-term data required. With its convenient once-monthly subcutaneous administration, available data indicate that givosiran is an important newer therapeutic option for patients with AHP and severe recurrent attacks.
Data Selection Givosiran: 141 records identified
Duplicates removed | 48 |
Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 37 |
Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 19 |
Cited efficacy/tolerability articles | 6 |
Cited articles not efficacy/tolerability | 31 |
Search Strategy: EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data. Key words were givosiran, Givlaari, ALN-AS1, porphyria. Records were limited to those in English language. Searches last updated 23 March 2021. | |
References
Wang B, Rudnick S, Cengia B, et al. Acute hepatic porphyrias: review and recent progress. Hepatol Commun. 2019;3(2):193–206.
Bissell DM, Anderson KE, Bonkovsky HL. Porphyria. N Engl J Med. 2017;377(9):862–72.
Kazamel M, Desnick RJ, Quigley JG. Porphyric neuropathy: pathophysiology, diagnosis, and updated management. Curr Neurol Neurosci Rep. 2020;20(12):56.
Chen B, Solis-Villa C, Hakenberg J, et al. Acute intermittent porphyria: predicted pathogenicity of HMBS variants indicates extremely low penetrance of the autosomal dominant disease. Hum Mutat. 2016;37(11):1215–22.
Bonkovsky HL, Maddukuri VC, Yazici C, et al. Acute porphyrias in the USA: features of 108 subjects from Porphyrias Consortium. Am J Med. 2014;127(12):1233–41.
Bissell DM, Lai JC, Meister RK, et al. Role of delta-aminolevulinic acid in the symptoms of acute porphyria. Am J Med. 2015;128(3):313–7.
Balwani M, Wang B, Anderson KE, et al. Acute hepatic porphyrias: recommendations for evaluation and long-term management. Hepatology. 2017;66(4):1314–22.
Stein P, Badminton M, Barth J, et al. Best practice guidelines on clinical management of acute attacks of porphyria and their complications. Ann Clin Biochem. 2013;50(Pt 3):217–23.
Gouya L, Ventura P, Balwani M, et al. EXPLORE: a prospective, multinational, natural history study of patients with acute hepatic porphyria with recurrent attacks. Hepatology. 2020;71(5):1546–58.
Simon A, Pompilus F, Querbes W, et al. Patient perspective on acute intermittent porphyria with frequent attacks: a disease with intermittent and chronic manifestations. Patient. 2018;11(5):527–37.
Puy H, Gouya L, Deybach JC. Porphyrias. Lancet. 2010;375(9718):924–37.
Schmitt C, Lenglet H, Yu A, et al. Recurrent attacks of acute hepatic porphyria: major role of the chronic inflammatory response in the liver. J Intern Med. 2018;284(1):78–91.
European Porphyria Network. Acute porphyria. 2020. https://porphyria.eu. Accessed 16 Mar 2021.
de Paula Brandao PR, Titze-de-Almeida SS, Titze-de-Almeida R. Leading RNA interference therapeutics part 2: silencing delta-aminolevulinic acid synthase 1, with a focus on givosiran. Mol Diagn Ther. 2020;24(1):61–8.
Debacker AJ, Voutila J, Catley M, et al. Delivery of oligonucleotides to the liver with GalNAc: from research to registered therapeutic drug. Mol Ther. 2020;28(8):1759–71.
Gonzalez-Aseguinolaza G. Givosiran—running RNA interference to fight porphyria attacks. N Engl J Med. 2020;382(24):2366–7.
European Medicines Agency. Givlaari: public assessment report. 2020. https://www.ema.europa.eu. Accessed 16 Mar 2021.
Alnylam Pharmaceuticals. How GIVLAARI® (givosiran) works? 2020. https://www.givlaarihcp.com. Accessed 16 Mar 2021.
Yasuda M, Gan L, Chen B, et al. RNAi-mediated silencing of hepatic Alas1 effectively prevents and treats the induced acute attacks in acute intermittent porphyria mice. Proc Natl Acad Sci USA. 2014;111(21):7777–82.
Chan A, Liebow A, Yasuda M, et al. Preclinical development of a subcutaneous ALAS1 RNAi therapeutic for treatment of hepatic porphyrias using circulating RNA quantification. Mol Ther Nucleic Acids. 2015;4:e263.
Sardh E, Harper P, Balwani M, et al. Phase 1 trial of an RNA interference therapy for acute intermittent porphyria. N Engl J Med. 2019;380(6):549–58.
Alnylam Pharmaceuticals. Givlaari (givosiran) injection, for subcutaneous use: US prescribing information. 2019. https://www.fda.gov. Accessed 16 Mar 2021.
European Medicines Agency. Givlaari 189 mg/mL solution for injection: summary of product characteristics. 2020. https://www.ema.europa.eu. Accessed 16 Mar 2021.
Agarwal S, Simon AR, Goel V, et al. Pharmacokinetics and pharmacodynamics of the small interfering ribonucleic acid, givosiran, in patients with acute hepatic porphyria. Clin Pharmacol Ther. 2020;108(1):63–72.
Agarwal S, Sardh E, Harper P, et al. A drug-drug interaction study investigating the effect of givosiran on activity of five drug metabolizing CYP450 enzymes in acute hepatic porphyria (AHP) patients [abstract no. PII-088]. Clin Pharm Ther. 2020;107(Suppl 1):S59.
Stein P, Rees D, Anderson K, et al. A phase 1/2 open label extension study of givosiran, an investigational RNAi therapeutic, in patients with acute intermittent porphyria [abstract no. FRI310 plus poster]. J Hepatol. 2020;73(Suppl 1):S553–S4.
Balwani M, Sardh E, Ventura P, et al. Phase 3 trial of RNAi therapeutic givosiran for acute intermittent porphyria. N Engl J Med. 2020;382(24):2289–301.
Sardh E, Balwani M, Rees D, et al. Twelve-month interim analysis of efficacy and safety of givosiran, and investigational RNAi therapeutic for acute hepatic porphyria, in the envision open label extension. 2020. https://www.alnylam.com. Accessed 23 Dec 2020.
Bonkovsky HL, et al. Clinical outcomes in patients with acute hepatic porphyria treated with givosiran who stopped hemin prophylaxis at study entry: a post hoc analysis of data from the phase 3 ENVISION study through month 12 [abstract no. P2094 (S1154)]. In: American College of Gastroenterology 2020 Annual Scientific Meeting. 2020.
Kauppinen R, Kuo HC, Oh J, et al. Reduction in pain during and between attacks in patients with acute hepatic porphyria treated with givosiran: a posthoc analysis of the phase 3 ENVISION study [abstract no. O4015]. In: 6th Congress of the European Academy of Neurology. 2020.
Kuter D, Keel S, Parker C, et al. Eighteen-month interim analysis of efficacy and safety of givosiran, an RNAi therapeutic for acute hepatic porphyria, in the envision open label extension [abstract no. 222]. In: 62nd American Society of Hematology Annual Meeting and Exposition. 2020.
Thapar M, Rudnick S, Bonkovsky HL. Givosiran, a novel treatment for acute hepatic porphyrias. Expert Rev Precis Med Drug Dev. 2021;6(1):9–19.
Bustad HJ, Kallio JP, Vorland M, et al. Acute intermittent porphyria: an overview of therapy developments and future perspectives focusing on stabilisation of HMBS and proteostasis regulators. Int J Mol Sci. 2021;22(2).
Ventura P, Corradini E, Di Pierro E, et al. Hyperhomocysteinemia in patients with acute porphyrias: a potentially dangerous metabolic crossroad? Eur J Intern Med. 2020;79:101–7.
American Porphyria Foundation. Treatment options. 2020. https://porphyriafoundation.org. Accessed 16 Mar 2021.
National Organization for Rare Disorders. Acute intermittent porphyria. 2019. https://rarediseases.org. Accessed 16 Mar 2021.
Honor A, Rudnick SR, Bonkovsky HL. Givosiran to treat acute porphyria. Drugs Today. 2021;57(1):47–59.
Acknowledgements
During the peer review process the manufacturer of givosiran was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
Yahiya Y. Syed is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
The manuscript was reviewed by: P. Berraondo, Program of Immunology and Immunotherapy, CIMA Universidad de Navarra, Pamplona, Spain; H. L. Bonkovsky, Wake Forest University School of Medicine/NC Baptist Hospital, Winston-Salem, NC, USA; E. I. Minder, Municipal Hospital Triemli, Institute of Laboratory Medicine, Zurich, Switzerland; E. Sardh, Department of Endocrinology and Centre for Inherited Metabolic Disease, Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden; A. K. Singal, Avera McKennan University Hospital Transplant Institute, University of South Dakota Sanford School of Medicine, Sioux Falls, SD, USA.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 8696 kb)
Rights and permissions
About this article
Cite this article
Syed, Y.Y. Givosiran: A Review in Acute Hepatic Porphyria. Drugs 81, 841–848 (2021). https://doi.org/10.1007/s40265-021-01511-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-021-01511-3