Abstract
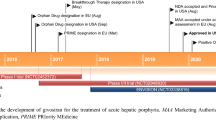
In November 2019 givosiran became the second small interfering RNA (siRNA)-based drug to receive US Food and Drug Administration (FDA) approval, it has been developed for the treatment of acute intermittent porphyria (AIP), a disorder characterized by life-threatening acute neurovisceral attacks. The porphyrias are a group of disorders in which enzymatic deficiencies in heme production lead to toxic accumulation of delta-aminolevulinic acid (ALA) and porphobilinogen (PBG), which are involved in the neurovisceral attacks. Givosiran acts as a conventional siRNA to trigger RNA interference (RNAi)-mediated gene silencing on delta-ALA synthase 1 (ALAS1), thus returning ALA and PBG metabolites to the physiological level to attenuate further neurotoxicity. Givosiran makes use of a new hepatic-delivery system that conjugates three GalNac (N-acetylgalactosamine) molecules to the siRNA passenger strand. GalNac binds to the liver asialoglycoprotein receptor, favoring the internalization of these GalNac-conjugated siRNAs into the hepatic cells. In a phase I study, subcutaneous monthly administration of givosiran 2.5 mg/kg reduced > 90% of ALA and PBG content. This siRNA is being analyzed in ENVISION (NCT03338816), a phase III, multicenter, placebo-controlled randomized controlled trial. In preliminary results, givosiran achieved clinical endpoints for AIP, reducing urinary ALA levels, and presented a safety profile that enabled further drug development. The clinical performance of givosiran revealed that suppression of ALAS1 by GalNac-decorated siRNAs represents an additional approach for the treatment of patients with AIP that manifests recurrent acute neurovisceral attacks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Givosiran is a small interfering RNA (siRNA) that targets delta-aminolevulinic acid synthase 1 when administered subcutaneously in patients with acute intermittent porphyrias (AIP). |
siRNAs are conjugated with three N-acetylgalactosamine molecules for drug delivery into hepatic cells. |
Givosiran has reduced the annualized attack rate and the frequency of hemin use in patients with AIP. |
1 Introduction
Givosiran is a small interfering RNA (siRNA)-based drug developed to treat a genetic disease called acute intermittent porphyria (AIP) [1,2,3]. The term porphyria refers to the ancient Greek “porphura,” which means purple, referencing the pigmented metabolites that accumulate in the patient’s body [4]. At normal levels in healthy individuals, porphyrins are natural chemicals involved in physiological functions. They play an essential role in oxygen carriage as part of the heme component in hemoglobin, and they stain our erythrocytes and blood a vivid red color [5, 6].
Porphyrias are a group of disorders related to increased levels of the metabolites that form porphyrin [2, 7]. The root of this metabolic disease lies in a genetic defect in the enzymes involved in the heme biosynthetic pathway [8]. Enzymatic deficiency in heme production leads to a toxic accumulation of metabolic precursors, such as delta-aminolevulinic acid (ALA) and porphobilinogen (PBG), which are thought to be the main mechanisms precipitating neurovisceral attacks and other clinical signs of porphyria [9]. This rare, life-threatening genetic group of pathologies can be autosomal dominant or recessive or have X-linked inheritance [8, 10]. Clinical management across the natural history of the disease may require a multidisciplinary effort from professionals with expertise in neurology, hematology, and dermatology.
For simplicity, we classify porphyrias into photocutaneous porphyrias and acute hepatic porphyrias. The photocutaneous presentation may arise if photosensitizing porphyrins accumulate in the liver or bone marrow [5] and leads to increased skin fragility, blistering, and scarring [11]. Conversely, acute hepatic porphyrias develop when the heme precursors ALA and PBG accumulate in hepatic cells. These neurotoxic metabolites reach the nervous system and trigger a neuropathy manifesting as acute neurovisceral pain [2]. Finally, acute hepatic porphyrias are classified as AIP (the target disease of givosiran), variegate porphyria, ALA dehydratase deficiency porphyria, and hereditary coproporphyria [7]. AIP is the most prevalent of these four disorders worldwide and the most commonly symptomatic.
The present review focuses on givosiran, a new drug developed by Alnylam Pharmaceuticals recently approved by the FDA for the treatment of AIP. Givosiran is an siRNA-based drug that silences the enzyme delta-ALA synthase 1 (ALAS1), which further reduces ALA and PBG metabolites involved in most neurological manifestations of AIP.
2 How Dysregulated Heme Biosynthesis Shapes the Clinical Presentation of Porphyrias
The heme molecule is a cofactor for proteins with essential functions in cell biology, including hemoglobin, myoglobin, cytochromes P450 (CYP450), mitochondrial cytochromes, and others. While heme biosynthesis occurs in most human body tissues, that in the bone marrow and liver accounts for 75% and 15%, respectively, of the total amount [8]. Eight enzymes catalyze the formation of heme in the mitochondria or cell cytoplasm (Fig. 1). The first and rate-limiting step is the synthesis of delta-aminolevulinate by ALA synthase from glycine and succinyl-coenzyme A (CoA) [2, 8, 12]. ALA synthase is strictly regulated by negative feedback exerted by intracellular iron levels and heme concentration. The second step requires the enzyme ALA dehydratase, which catalyzes the conversion of delta-aminolevulinate to PBG and water. Four PBG molecules can then be converted to hydroxymethylbilane by the uroporphyrinogen I synthase/porphobilinogen deaminase, currently named hydroxymethylbilane synthase (HMBS). Uroporphyrinogen III synthase can then form a ring of uroporphyrinogen III from the linear tetrapyrrole hydroxymethylbilane. The acetyl groups of uroporphyrinogen III are converted to methyl groups via decarboxylation, generating coproporphyrinogen III. Mitochondrial coproporphyrinogen III oxidase may, in turn, form protoporphyrinogen, later oxidized to protoporphyrins. Finally, iron incorporation forms the heme molecule (Fig. 1).
Heme biosynthesis pathway and porphyrias. The metabolic steps leading to heme formation from eight molecules of glycine and succinyl-coenzyme A (CoA) (derived from the Krebs cycle). Enzymes are represented by rounded blue rectangles and porphyria subtypes by rounded yellow rectangles. The synthesis of delta-ALA by ALAS is the rate-limiting step of this metabolic pathway. By knocking-down ALAS messenger RNA, givosiran prevents ALA from accumulating, thus counteracting acute hepatic porphyrias. Acute hepatic porphyrias are highlighted with bold formatting. ALA aminolevulinic acid, ALAD delta-aminolevulinic acid dehydratase, ALAS ALA synthase, CoA coenzyme A, PBG porphobilinogen
Genetic dysfunction in each enzyme of heme biosynthesis plays a role in a specific type of porphyria: (1) X-linked erythropoietic protoporphyria (ALA synthase), (2) ALA dehydratase deficiency porphyria (ALA dehydratase), (3) AIP (porphobilinogen deaminase, now called hydroxymethylbilane synthase), (4) congenital erythropoietic porphyria (uroporphyrinogen III cosynthase), (5) porphyria cutanea tarda (uroporphyrinogen decarboxylase), (6) hereditary coproporphyria (coproporphyrinogen oxidase), (7) variegate porphyria (protoporphyrinogen oxidase), and (8) erythropoietic protoporphyria (ferrochelatase).
3 Acute Intermittent Porphyria (AIP)
First described in 1889, AIP is the most common type of acute hepatic porphyria. In northern Europe, the disease has a relatively high prevalence of 5.4 per 1 million; in Sweden it reaches even higher values and has been named “Swedish porphyria” [13, 14]. Patients with AIP manifest attacks characterized by insidious fatigue and difficulty concentrating; severe poorly localized abdominal pain, nausea, vomiting, neurological (motor neuropathy) and autonomic dysfunction; and hepatic, renal, and neurological involvement (seizures). Hyponatremia is a recurrent accompanying sign [7]. Inducers of CYP450 (e.g., oral or subdermal contraceptives, antiepileptic drugs, anesthetic agents, and some antibiotics) and reduction in carbohydrate intake (due to fasting, dieting, gastrointestinal distress) are factors that are well-known to worsen the symptoms of AIP. The typical patient is an adult woman in the second to fourth decade of life [15]. Diagnosis may be challenging and requires a high index of suspicion, with further testing of urine ALA and PBG. AIP may be difficult to discriminate from acute abdomen, Guillain-Barré syndrome, acute psychosis, epilepsy, and lead poisoning [7, 15].
Most mutation carriers of the HMBS gene remain asymptomatic, and data have suggested a clinical penetrance of about 1% in the general population [16, 17]. Most symptomatic patients have one or few attacks during their lifetime and experience full recovery. A minority of patients (3–5%) may have recurrent attacks, defined as four or more attacks per year. This population mainly comprises young women with hormonal triggers, who suffer substantial disability [18, 19].
The current treatment is based on heme replacement using hematin or heme arginate, which improves the negative feedback on the ALAS1 enzyme. However, heme reposition therapy also involves short- and long-term issues. The drug is administered intravenously, requires up to four daily infusions, and must be freshly prepared. In acute porphyria attacks, a beneficial clinical effect of hematin 2–4 mg/kg once daily is expected within 1–2 days after treatment initiation. Although the infusion protocol typically lasts up to 4 days, the therapy may be extended for 2 weeks in severe cases. Chronic use of hematin, for example, in the prophylaxis of premenstrual exacerbations of AIP, can lead to iron deposition in the liver. Oral carbohydrate loading is an alternative therapy for mild attacks. Finally, liver transplantation is reserved for patients with severe recurrent attacks of AIP refractory to standard therapy [2, 3, 20, 21].
4 Givosiran
The biopharmaceutical company Alnylam has developed a novel treatment for recurrent AIP: givosiran, a GalNac (N-acetylgalactosamine)-conjugated siRNA for knockdown of ALAS1 [22]. Cellular mechanisms of RNA interference (RNAi)-mediated gene silencing were recently reviewed in another article in this journal [44]. Givosiran’s siRNA was designed based on nucleotide sequence NM_020559.2 and first tested in an AIP mouse model [23]. The authors used intravenous injection to administer the anti-ALAS1 siRNA, which at that time was formulated in lipid nanoparticles, to obtain a dose-dependent knockdown of ALAS1. To improve drug biodistribution to the liver, Alnylam developed a delivery system based on the conjugation of three GalNac molecules to the siRNA passenger strand. GalNac binds to the liver asialoglycoprotein receptor, increasing siRNA internalization and gene silencing without the need for a nanoparticulated formulation (Fig. 2) [24, 25]. Within the hepatocyte, givosiran duplexes interact with the dicer/HIV-1 trans-activation response RNA-binding protein (TRBP) and further with the RNA-induced silencing complex (RISC) to finally trigger the RNAi-mediated gene silencing of ALAS1 messenger RNA (mRNA). The reduced content of the ALAS1 enzyme consequently decreases the production of toxic metabolites ALA and PBG, thus counteracting a crucial underlying mechanism of porphyria (Fig. 2). Another noteworthy advance with givosiran is that it circumvents the intravenous route that requires healthcare professionals to administer the drug. GalNac-conjugated ALAS1-targeted siRNAs of givosiran are infused subcutaneously [25].
Givosiran therapy for AIP. a Patients with AIP receive givosiran by subcutaneous injection. b Anti-ALAS1 siRNA contains three GalNac molecules conjugated at the 3′-end of the passenger strand. c GalNac moiety binds to ASGPR on hepatocytes. d An endocytic vesicle is formed and internalize the siRNA molecule into the cell cytoplasm. e siRNA–GalNac conjugate is delivered in the cytoplasm. f The siRNA molecule forms a complex with dicer and TRBP proteins that will eliminate the passenger strand. g The remaining siRNA guide strand loaded in RISC binds to complementary sequences in ALAS1 mRNA, allowing AGO2 enzyme to execute the cleavage of ALAS1 mRNA. h Knockdown of ALAS1 prevents further formation of toxic intermediates (ALA and PBG), reducing clinical signs of porphyria. AGO2 argonaute 2, AIP acute intermittent porphyria, ALA aminolevulinic acid, ALAS aminolevulinic acid synthase, ASGPR asialoglycoprotein receptor, GalNac N-acetyl galactosamine, mRNA messenger RNA, PBG porphobilinogen, RISC RNA-induced silencing complex, siRNA small interfering RNA, TRBP HIV-1 trans-activation response RNA-binding protein
4.1 Biotechnological Inputs on Small Interfering RNA (siRNA)-GalNac Conjugates: Vectorizing to Hepatic Cells with Increased Drug Stability
Delivering small RNAs to target organs has brought remarkable progress to RNAi biotechnology, offering advantageous dose regimens that might contribute to mitigating the costs of therapy, a concern with RNAi-based drugs. GalNac conjugation represents a valuable platform for hepatic delivery of small RNA therapeutics [26], as this moiety has affinity for the asialoglycoprotein receptor of hepatocytes [27]. Alnylam developed a GalNac ligand that binds to this transmembrane receptor with high affinity and specificity [28,29,30]. siRNA duplexes decorated with three GalNac moieties are thus internalized into hepatocytes and trigger RNAi-mediated gene silencing on ALAS1 (see Fig. 2). Along with gains in siRNA stability, as discussed in the following paragraphs of this section, GalNac technology undoubtedly represents a fundamental pillar in the effectiveness of givosiran [25].
siRNA duplexes are composed of chains of ribonucleotides and are thus vulnerable to enzymatic attack executed mainly by ribonucleases. Chemical changes in the siRNA backbone have provided molecules with increased stability, for example, the typical substitution of a phosphodiester for a phosphorothioate linkage between nucleotides [31]. From a therapeutic perspective, a more stable siRNA would allow fewer drug injections and a reduced therapeutic dose. Stability favors potency and durability of effects, which consequently improves cost effectiveness of treatment, an issue discussed in the accompanying article about patisiran [44].
In givosiran, the siRNA molecules are naked, i.e., short RNAs completely exposed to enzymatic attack in the organism. They are not encapsulated into lipid carriers, and the GalNac-conjugated molecules have no protective role. Nucleases would digest givosiran duplexes in subcutaneous tissue where the drug is injected and absorbed, then during transport in the circulatory system to the liver, and, finally, in hepatocyte cytoplasm. However, some strategies were adopted to protect givosiran. First, the ribosugar moieties were modified with either 2′-deoxy-2′-fluoro or 2′-O-methyl, a feature also incorporated in other siRNAs from this company [32,33,34,35,36].
Additional phosphorothioate linkages at the 5′-end of the guide and passenger siRNA strands also protect against nuclease attack, thereby increasing the potency and duration of GalNac–siRNA conjugates injected subcutaneously in rodents and nonhuman primates [37, 38]. The changes were collectively named enhanced stabilization chemistry and provided a mean effective dose ≤ 1 mg/kg after a single subcutaneous injection. When chronically injected, weekly subcutaneous doses resulted in a dose-dependent knockdown in the liver without adverse reactions in rodents. Effects remained for 9 months, a long-term duration valuable in therapeutics for lifelong diseases [37]. These findings, together with ongoing studies about siRNA designs, may bring further improvements in siRNA-based therapeutics for AIP [25, 39].
4.2 Givosiran Phase I Study (NCT02452372)
The phase I study examined the safety and tolerability of givosiran in patients with AIP as the primary outcomes and addressed some pharmacokinetic and pharmacodynamic parameters. Patients with a diagnosis of AIP and a urinary PBG level > 4 mmol/mol of creatinine were recruited for this phase I clinical trial [1]. The following clinical outcomes were assessed: safety (side effects profile), pharmacokinetics (serum and urine drug concentration), and pharmacodynamics (monitoring of ALA and PBG levels in urine, and ALAS1 mRNA in the circulation). The study also evaluated the capacity of givosiran to reduce the frequency of attacks and the decline of heme administration in patients with recurrent relapses.
The trial comprised three parts: parts A and B included patients without any porphyria attacks in the previous 6 months (n = 23). Part C involved patients who had experienced porphyria attacks in the previous 6 months or were undergoing a heme injection regimen (n = 13 givosiran; n = 4 placebo); these participants had to discontinue hemin treatment during the hospitalization and intervention periods. The level of urinary ALAS1 mRNA was measured. Participants in part C reported a baseline frequency of approximately nine to ten porphyria attacks in the previous 12 months.
Patients in part A were randomized 3:1 to receive a single subcutaneous injection of givosiran or placebo. The ascending doses examined were 0.035, 0.10, 0.35, 1.0, and 2.5 mg/kg. Patients in part B were also randomized in a 3:1 ratio to receive monthly subcutaneous injections of either 0.35 or 1.0 mg/kg. Patients with AIP who manifested recurrent attacks (part C) were also randomized and received subcutaneous administrations of givosiran at higher doses of 2.5 or 5.0 mg/kg either monthly (total of four injections) or at 3-monthly intervals (total of two injections) over a period of 12 weeks. In part A, patients showed a dose-dependent knockdown of ALAS1 mRNA, ALA, and PBG in urine samples. In part B, givosiran caused a decrease in mRNA of ALAS1, ALA, and PBG similar to those found in part A regardless of the dose used (0.35 or 1.0 mg/kg).
In part C, the monthly injections of givosiran reduced > 90% of ALA and PBG content, with no effect differences between doses of 2.5 or 5.0 mg/kg. Monthly administration produced better results than quarterly administration. Moreover, the mean number of annual attacks decreased in treated patients: 7.2 versus 16.7 attacks per year with givosiran and placebo, respectively. The annualized mean number of AIP attacks correlated with different categories of ALA reduction, i.e., the lower the ALA levels achieved, the lower the frequency of attacks. In terms of safety, the authors found no difference in the rate of patients reporting adverse events from treatment with givosiran or placebo. For example, adverse effects occurred in 91% (30/33) of patients receiving givosiran versus 100% (10/10) in the placebo group. These data must be interpreted carefully, as both groups included patients with AIP, and some of the adverse events that were reported are also symptoms commonly seen in the natural course of the disease. While considering this inherent limitation, the most common adverse events found in the trial were nasopharyngitis, abdominal pain, nausea, and diarrhea. Six patients experienced serious events [1].
4.3 Phase III Trial (NCT03338816)
The ongoing phase III study (ENVISION) aimed to evaluate the therapeutic efficacy of givosiran in patients with recurrent attacks of acute hepatic porphyria, to measure decreases in urinary ALA and PBG levels, and to monitor drug safety. This clinical trial was a randomized (1:1), double-blind, multicenter (18 countries, 36 sites), placebo-controlled study. Givosiran 2.5 mg/kg or placebo was administered subcutaneously monthly to patients diagnosed with acute hepatic porphyria (N = 94) who had experienced at least two attacks within the previous 6 months and were willing to not use hemin prophylaxis. Patients had a median of three attacks during the 6 months that antedated the study: 40% were receiving hemin prophylaxis, whereas 50% had chronic symptoms between attacks. The recruited sample had a high comorbidity burden (e.g., liver or chronic kidney disease, peripheral neuropathy, and iron overload) [40]. The selected primary endpoint was a composite annualized rate of attacks requiring hospitalization, an urgent healthcare visit, or hemin administration at home at 6 months. The secondary endpoints were pharmacodynamic effect on urine ALA and PBG levels, annualized rate of hemin administration, composite annualized attacks over 6 months, pain (measured with the Brief Pain Inventory-Short Form), fatigue (measured with the Brief Fatigue Inventory-Short Form), nausea (measured with a numeric rating scale), and the physical component summaries (PCS) of the 12-item Short Form Survey (SF-12).
Partial results from the randomized first 6-month period of this in-progress phase III trial were recently reported at the annual meeting of the European Association for the Study of the Liver and are available on the manufacturer’s website [41]. In summary, givosiran resulted in a 74% mean reduction in the annualized composite rate of AIP attacks relative to placebo, with 50% of patients in the givosiran group and 16.3% of those in the placebo group being attack free. Givosiran resulted in a mean reduction of 77% in days of hemin use and a sustained lowering of ALA (86%) and PBG (91%) urinary levels in relation to baseline.
When patient-centered outcomes were analyzed, patients in the givosiran group had a greater reduction in daily worst pain as recorded on a numeric rating scale. Likewise, givosiran had an effect on SF-12 PCS scores because of changes in bodily pain, social functioning, and role-physical. Using a patient global impression of change scale, 59% of those receiving givosiran reported “very much improved” or “much improved,” whereas only 18.4% of those in the placebo group reported “much improved.” Givosiran also had a significant impact on activities of daily life and functioning, as measured by the Porphyria Patient Experience Questionnaire (exploratory endpoint), a Likert-built scale that contains eight items about impacts and treatment experience [41].
Two important secondary outcomes related to chronic symptoms between AIP attacks were not affected by givosiran therapy: fatigue and nausea. It is well-known that chronic symptoms might relate to underlying chronic axonal autonomic neuropathy [19] and encephalopathy [21], and 6 months of givosiran therapy, in a putative hypothesis, could have been insufficient to promote neuronal regeneration in the autonomic and central nervous system. Results of the open-label extension (OLE) study (NCT02949830), with repeated assessment at 1 year of follow-up, will help to clarify this.
Based on interim study data, the authors concluded that givosiran achieved the endpoint regarding reductions in urinary ALA—a critical disease biomarker—and presented a safety profile that enables further drug development [42]. Givosiran received FDA approval on November 20 2019, with the trade name Givlaari.
5 Adverse Effects of Givosiran
The phase I trial of givosiran (NCT02452372) revealed serious adverse events in 6 of 33 (18%) patients treated with givosiran versus 0 (0%) patients treated with placebo. Severe adverse effects (grade 3 or higher) occurred in 4 of 33 patients (12%) treated with givosiran versus 2 of 10 patients treated with placebo (20%). In total, serious or severe adverse effects occurred in 30% of those treated with givosiran versus 20% receiving placebo [1].
The preliminary results from the phase III ENVISION trial (NCT03338816) reported serious adverse events in 10 of 48 (20.8%) patients receiving givosiran compared with 4 of 46 (8.7%) receiving placebo [43]. A total of 8 of 48 (16.7%) patients receiving givosiran and 5 of 46 (10.9%) receiving placebo experienced severe adverse events. In summary, a higher percentage of patients treated with givosiran showed serious or severe side effects (37.5%) in comparison with the placebo group (19.6%).
The company reported that givosiran caused changes in hepatic and renal function that had resolved or stabilized by month 6. As such, Alnylam considered that givosiran had an overall acceptable safety and tolerability profile. An ongoing OLE study (NCT02949830) addressing the safety of givosiran will provide new information regarding tolerability and effectiveness.
6 Impact of this Novel Treatment for Acute Hepatic Porphyrias
Givosiran prevents the accumulation of toxic metabolites that presumably cause porphyria attacks, as a prophylactic treatment addressing the underlying mechanisms of the disease. Recurrent attacks of AIP decreased by 74%, which represents progress in clinical management and a positive impact on patient quality of life. Moreover, the drug averted porphyria attacks for 6 months in nearly half of the treated patients. The current performance of givosiran in ENVISION and its ongoing 30-month open-label phase means it is likely to present an additional therapeutic option for this disabling disorder.
References
Sardh E, Harper P, Balwani M, Stein P, Rees D, Bissell DM, et al. Phase 1 Trial of an RNA Interference Therapy for Acute Intermittent Porphyria. N Engl J Med. 2019;380:549–58. https://doi.org/10.1056/NEJMoa1807838.
Bissell DM, Anderson KE, Bonkovsky HL. Porphyria. N Engl J Med [Internet]. 2017;377:2101. https://www.ncbi.nlm.nih.gov/pubmed/29166231. Accessed 23 Nov 2017
Balwani M, Desnick RJ. The porphyrias: advances in diagnosis and treatment. Hematol. Am Soc Hematol Educ Progr. 2012;2012:19–27. https://www.ncbi.nlm.nih.gov/pubmed/23233556. Accessed 13 Dec 2017
Nick L. Born to the purple: the story of porphyria—Scientific American. Sci Am. 2012. https://www.scientificamerican.com/article/born-to-the-purple-the-st/. Accessed 16 Dec 2002
Dayan FE, Dayan EA. Porphyrins: one ring in the colors of life. Am Sci. 2011;99:236–44.
Bren KL, Eisenberg R, Gray HB. Discovery of the magnetic behavior of hemoglobin: a beginning of bioinorganic chemistry. Proc Natl Acad Sci. 2015;112:13123–7.
Ramanujam VM, Anderson KE. Porphyria diagnostics-Part 1: a brief overview of the porphyrias. Curr Protoc Hum Genet. 2015;86:17.20.1–26. https://doi.org/10.1002/0471142905.hg1720s86.
Wang B, Rudnick S, Cengia B, Bonkovsky HL. Acute hepatic porphyrias: review and recent progress. Hepatol Commun [Internet]. 2019;3:193–206. https://www.ncbi.nlm.nih.gov/pubmed/30766957. Accessed 16 Feb 2019
Pischik E, Kauppinen R. Neurological manifestations of acute intermittent porphyria. Cell Mol Biol. (Noisy-le-grand). 2009;55:72–83.
Singal AK, Parker C, Bowden C, Thapar M, Liu L, McGuire BM. Liver transplantation in the management of porphyria. Hepatology [Internet]. 2014;60:1082–9. https://www.ncbi.nlm.nih.gov/pubmed/24700519. Accessed 05 Apr 2014
Maranda EL, Heifetz R, Estes WA, Cortizo J, Shareef S, Jimenez JJ. Porphyria and vampirism—a myth, sensationalized. JAMA Dermatol. 2016;152:975.
Bissell DM, Wang B. Acute Hepatic Porphyria. J Clin Transl Hepatol [Internet]. 2015;3:17–26. https://www.ncbi.nlm.nih.gov/pubmed/26357631. Accessed 12 Sept 2015
Elder G, Harper P, Badminton M, Sandberg S, Deybach JC. The incidence of inherited porphyrias in Europe. J Inherit Metab Dis [Internet]. 2013;36:849–57. Available from: https://www.ncbi.nlm.nih.gov/pubmed/23114748. Accessed 02 Nov 2012
Floderus Y, Shoolingin-Jordan PM, Harper P. Acute intermittent porphyria in Sweden. Molecular, functional and clinical consequences of some new mutations found in the porphobilinogen deaminase gene. Clin Genet [Internet]. 2002;62:288–97. https://www.ncbi.nlm.nih.gov/pubmed/12372055. Accessed 10 Oct 2002
Besur S, Hou W, Schmeltzer P, Bonkovsky H. Clinically important features of porphyrin and heme metabolism and the porphyrias. Metabolites. 2014;4:977–1006.
Lenglet H, Schmitt C, Grange T, Manceau H, Karboul N, Bouchet-Crivat F, et al. From a dominant to an oligogenic model of inheritance with environmental modifiers in acute intermittent porphyria. Hum Mol Genet [Internet]. 2018;27:1164–73. https://www.ncbi.nlm.nih.gov/pubmed/29360981. Accessed 24 Jan 2018
Yasuda M, Chen B, Desnick RJ. Recent advances on porphyria genetics: inheritance, penetrance and molecular heterogeneity, including new modifying/causative genes. Mol Genet Metab [Internet]. 2018. https://www.ncbi.nlm.nih.gov/pubmed/30594473. Accessed 31 Dec 2018
Manceau H, Gouya L, Puy H. Acute hepatic and erythropoietic porphyrias. Curr Opin Hematol. 2017;24:198–207.
Stein PE, Badminton MN, Rees DC. Update review of the acute porphyrias. Br J Haematol. 2017;176:527–38.
Mustajoki P, Nordmann Y. Early administration of heme arginate for acute porphyric attacks. Arch Intern Med [Internet]. 1993;153:2004–8. https://www.ncbi.nlm.nih.gov/pubmed/8357285. Accessed 13 Sept 1993
Pischik E, Kauppinen R. An update of clinical management of acute intermittent porphyria. Appl Clin Genet [Internet]. 2015;8:201–14. https://www.ncbi.nlm.nih.gov/pubmed/26366103. Accessed 15 Sept 2015
Nikam RR, Gore KR. Journey of siRNA: clinical developments and targeted delivery. Nucleic Acid Ther. 2018;28:209–24.
Yasuda M, Gan L, Chen B, Kadirvel S, Yu C, Phillips JD, et al. RNAi-mediated silencing of hepatic Alas1 effectively prevents and treats the induced acute attacks in acute intermittent porphyria mice. Proc Natl Acad Sci USA [Internet]. 2014;111:7777–82. https://www.ncbi.nlm.nih.gov/pubmed/24821812. Accessed 14 May 2014
Shen X, Corey DR. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res [Internet]. 2018;46:1584–600. https://www.ncbi.nlm.nih.gov/pubmed/29240946. Accessed 15 Dec 2017
Chan A, Liebow A, Yasuda M, Gan L, Racie T, Maier M, et al. Preclinical development of a subcutaneous ALAS1 RNAi therapeutic for treatment of hepatic porphyrias using circulating RNA quantification. Mol Ther Nucleic Acids. 2015;4:e263.
Huang Y. Preclinical and clinical advances of GalNAc-decorated nucleic acid therapeutics. Mol Ther Nucleic Acids. 2017;6:116–32. https://doi.org/10.1016/j.omtn.2016.12.003.
Matsuda S, Keiser K, Nair JK, Charisse K, Manoharan RM, Kretschmer P, et al. siRNA conjugates carrying sequentially assembled trivalent N-acetylgalactosamine linked through nucleosides elicit robust gene silencing in vivo in hepatocytes. ACS Chem Biol. 2015;10:1181–7.
Khorev O, Stokmaier D, Schwardt O, Cutting B, Ernst B. Trivalent, Gal/GalNAc-containing ligands designed for the asialoglycoprotein receptor. Bioorg Med Chem. 2008;16:5216–31.
Pricer WE, Hudgin RL, Ashwell G, Stockert RJ, Morell AG. [87] A membrane receptor protein for asialoglycoproteins. Methods Enzymol. 1974;34:688–91.
Rensen PCN, Van Leeuwen SH, Sliedregt LAJM, Van Berkel TJC, Biessen EAL. Design and synthesis of novel N-acetylgalactosamine-terminated glycolipids for targeting of lipoproteins to the hepatic asialoglycoprotein receptor. J Med Chem. 2004;47:5798–808.
Titze-de-Almeida R, David C, Titze-de-Almeida SS. The race of ten synthetic RNAi-based drugs to the pharmaceutical market. Pharm Res [Internet]. 2017;34:1339–63. https://www.ncbi.nlm.nih.gov/pubmed/28389707. Accessed 09 Apr 2017
Cummins LL, Owens SR, Risen LM, Lesnik EA, Freier SM, Mc Gee D, et al. Characterization of fully 2′-modified oligoribonucleotide hetero-and homoduplex hybridization andnuclease sensitivity. Nucleic Acids Res. 1995;23:2019–24.
Layzer JM, McCaffrey AP, Tanner AK, Huang Z, Kay MA, Sullenger BA. In vivo activity of nuclease-resistant siRNAs. RNA. 2004;10:766–71.
Takahashi M, Minakawa N, Matsuda A. Synthesis and characterization of 2′-modified-4′-thioRNA: a comprehensive comparison of nuclease stability. Nucleic Acids Res. 2009;37:1353–62.
Allerson CR, Sioufi N, Jarres R, Prakash TP, Naik N, Berdeja A, et al. Fully 2′-modified oligonucleotide duplexes with improved in vitro potency and stability compared to unmodified small interfering RNA. J Med Chem. 2005;48(4):901–4. https://doi.org/10.1021/jm049167j.
Prakash TP, Kinberger GA, Murray HM, Chappell A, Riney S, Graham MJ, et al. Synergistic effect of phosphorothioate, 5′-vinylphosphonate and GalNAc modifications for enhancing activity of synthetic siRNA. Lett: Bioorg Med Chem; 2016.
Nair JK, Willoughby JL, Chan A, Charisse K, Alam MR, Wang Q, et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J Am Chem Soc. 2014;136(49):16958–61. https://doi.org/10.1021/ja505986a.
Nair JK, Attarwala H, Sehgal A, Wang Q, Aluri K, Zhang X, et al. Impact of enhanced metabolic stability on pharmacokinetics and pharmacodynamics of GalNAc-siRNA conjugates. Nucleic Acids Res. 2017;45:10969–77.
Foster DJ, Brown CR, Shaikh S, Trapp C, Schlegel MK, Qian K, et al. Advanced siRNA designs further improve in vivo performance of GalNAc-siRNA conjugates. Mol Ther. 2018;26:708–17.
Balwani M, Sardh E, Gouya L, Rees DC, Stein P, Stölzel U et al. Disease characteristics of patients with acute hepatic porphyria patients: ENVISION, a Phase 3 global, multicenter, randomized, double-blind, placebo-controlled trial. https://www.alnylam.com/wp-content/uploads/2019/09/ICPP_Balwani_ENVISION-Disease-Characteristics.pdf. Accessed 10 Oct 2019
Alnylam_Pharmaceuticals. ENVISION, a Phase 3 study to evaluate the efficacy and safety of givosiran, an investigational RNAi therapeutic targeting aminolevulinic acid synthase 1, in acute hepatic porphyria patients. ICPP|Milan, Italy [Internet]. 2019. https://www.alnylam.com/wp-content/uploads/2019/09/ICPP_Gouya_ENVISION.pdf. Accessed 10 Sept 2019
Abstracts of The International Liver CongressTM 2019—54th annual meeting of the European Association for the Study of the Liver April 10–14 Vienna, Austria. J Hepatol. 2019;70:e1–e952. 2019. No Title [Internet]. https://www.journal-of-hepatology.eu/article/S0168-8278(19)30196-5/pdf. Accessed 10 Jul 2019
Balwani M, Gouya L, Rees DC, Stein P, Stölzel U, Aguilera Peiro P, et al. ENVISION, a Phase 3 study to evaluate the efficacy andsafety of givosiran, an investigational RNAi therapeutictargeting aminolevulinic acid synthase 1, in acute hepatic porphyria patients. 2019. https://www.alnylam.com/wp-content/uploads/2019/04/Balwani_ENVISION_EASL_FINAL2-2.pdf. Accessed 08 Oct 2019.
Titze-de-Almeida SS, Brandão PRP, Faber I, Titze-de-Almeida R. Leading RNA interference therapeutics part 1: silencing hereditary transthyretin amyloidosis, with a focus on patisiran. Mol Diagn Ther. 2019. https://doi.org/10.1007/s40291-019-00434-w(Epub ahead of print).
Acknowledgements
Pedro Renato de Paula Brandão, Simoneide S. Titze-de-Almeida, and Ricardo Titze-de-Almeida are members of the Network for Translational Neuroscience-International Consortium for Academic Cooperation in Experimental and Clinical Studies Regarding Neurodegenerative Diseases (http://dgp.cnpq.br/dgp/espelhogrupo/5933421119277338).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to conduct this study or prepare this manuscript.
Conflicts of interest
Pedro Renato de Paula Brandão, Simoneide S. Titze-de-Almeida, and Ricardo Titze-de-Almeida have no conflicts of interest that are directly relevant to the content of this study.
Rights and permissions
About this article
Cite this article
de Paula Brandão, P.R., Titze-de-Almeida, S.S. & Titze-de-Almeida, R. Leading RNA Interference Therapeutics Part 2: Silencing Delta-Aminolevulinic Acid Synthase 1, with a Focus on Givosiran. Mol Diagn Ther 24, 61–68 (2020). https://doi.org/10.1007/s40291-019-00438-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-019-00438-6