Abstract
Introduction
Data suggest that the beneficial cardiovascular effects of statins are maximized after the first year of statin use; yet, the timeline of statin-associated adverse events is not well delineated.
Objective
To examine the associations of short-term statin use (≤1 year) with short- and long-term adverse events and beneficial cardiovascular outcomes in a ‘healthy’ cohort.
Participants and Methods
A cohort study of a healthy Tricare population (fiscal year [FY] 2002 through FY 2011) who have no cardiovascular disease, major comorbidities requiring medications, or functional limitations. Statin users used statins for 90–365 days during FY 2005 as their only prescription medication. Nonusers had medical encounters but did not receive prescription medications during FY 2005, and did not receive any statins throughout the study period from FY 2002 to FY 2011. Outcomes were the occurrence of major acute cardiovascular events, diabetes mellitus and its complications, kidney diseases, musculoskeletal diseases, obesity, cataracts, malignancy, and death.
Results
We matched 1525 statin users to 1525 nonusers. During the follow-up period (FY 2006 to FY 2011), statin users had significantly higher odds of developing diabetes and diabetic complications that persisted throughout follow-up (odds ratio [OR] 1.93, 95 % confidence interval [CI] 1.55–2.41 and OR 2.15, 95 % CI 1.20–3.86, respectively). Short-term statin use was not associated with decreased odds of major acute cardiovascular events (OR 1.17, 95 % CI 0.72–1.92). There were no differences in risks of kidney diseases, musculoskeletal diseases, or malignancy.
Conclusions
Short-term statin use for primary prevention in this healthy cohort was associated with an increased risk of long-term diabetes and diabetic complications without cardiovascular benefits. Further study using pragmatic studies and prospective observational studies appropriately equipped to eliminate unidentified confounders are urgently needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Short-term statin use, in comparison to no use, was associated with a higher risk of developing diabetes mellitus and diabetic complications without any decreased risk of major acute cardiovascular events. |
Risk of kidney diseases, musculoskeletal diseases, or malignancy did not differ between short-term statin users and nonusers. |
The increased risk of diabetes and diabetic complications persisted throughout the follow-up period (5 years) after discontinuing statins. |
Further studies appropriately equipped to eliminate unidentified confounders and confounding by indication are urgently needed. |
1 Introduction
Despite their effectiveness in lowering cardiovascular morbidity and mortality, statins have been associated with an increased risk of diabetes mellitus [1], diabetic complications [2], obesity [3], and musculoskeletal diseases [4, 5]. The overall benefit/risk of statins in primary prevention is mainly related to a patient’s baseline risk of cardiovascular diseases [6, 7]. However, various cardiovascular risk calculators yield different estimates with variables accuracies and can result in overestimating the risk in lower risk populations [6, 7].
Statin studies suggest that 6–12 months of treatment is needed to demonstrate their beneficial cardiovascular effects [8, 9]. However, the timeline of the risks of adverse events is not well known and some studies suggested that the risk of adverse events may remain increased after discontinuing statin therapy [4]. Hence, it is possible that short-term statin use may increase the risk of adverse events without achieving desired cardiovascular benefits especially in low-cardiovascular-risk populations. The long-term effects of short-term statin use in healthy adults have not been examined in any randomized controlled trial (RCT). In a statin primary prevention RCT, the outcomes of those who withdrew from trials after enrollment (arguably short-term users) are not known and are analyzed using an intention-to-treat analysis, indiscriminant from those who continued statins [10].
The objective of this study was to examine the associations of short-term statin use (≤1 year) with short- and long-term known beneficial cardiovascular outcomes, such as acute myocardial infraction and acute cerebrovascular accidents, known statin-associated adverse events such as diabetes and musculoskeletal diseases, and suspected/debated statin-associated adverse events such as kidney diseases and cataract, in a ‘healthy’ cohort of statin users and nonusers.
2 Methods
2.1 Study Design
This cohort study was approved by the Institutional Review Board at Brooke Army Medical Center and the VA North Texas Health System. After obtaining permission from the Tricare Management Activity office, we extracted national Tricare archival data from fiscal year (FY) 2002 to FY 2011 (10/1/2001 to 9/30/2011) from the Military Health System (MHS) Data Repository (MDR). Tricare is the healthcare program for the US uniformed service members (active duty service members, National Guard/Reserve, and retired) and their families. To comply with the Tricare Data Sharing Agreement, all data were de-identified, to include rounding dates of medical encounters to the nearest quarter of the year.
The MDR contains patient information, outpatient and inpatient medical encounters within MHS (direct care) and outside of MHS (purchased care), direct care laboratory data, and Pharmacy Data Transaction Service. The Pharmacy Data Transaction Service tracks drug use and receives weekly feeds that include medications and prescription details regardless of pharmacy location or affiliation in the USA. Each patient is uniquely identified by an identification code that is consistent throughout the MDR. MDR data are managed according to a published and approved protocol to ensure raw data integrity and quality [11, 12]. MDR data are routinely used in administrative and leadership decision making, as well as in research [11–13].
2.2 Study Interval
The study was divided into three periods: baseline period (from FY 2002 to 2004 [10/1/2001 to 9/30/2004]), which was used to describe baseline characteristics; exposure period (FY 2005), which was used to identify patients as statin users or nonusers; and follow-up period (FY 2006 to 2011), which was used to capture outcomes.
2.3 Healthy Cohort Formation
We included all adults older than 35 years of age, who had at least one medical encounter in the exposure period (FY 2005) and had at least one medical encounter during FY 2011 (the last year of the follow-up period). To form a healthy cohort, we used two criteria: (1) we included patients who were not prescribed any prescription medication during the exposure period (FY 2005) except for potentially a statin; and (2) we excluded patients with conditions at baseline that would severely shorten life expectancy or interfere with physical activity (Electronic Supplementary Material Appendix 1).
We identified two treatment groups: short-term statin users and statin nonusers. Short-term statin users were patients who: (a) newly received and filled at least two statin prescriptions with a cumulative duration of at least 90 days during FY 2005; (b) continued to use statins in FY 2005 only; and (c) did not receive other prescription medications in FY 2005. Statin users were considered new users if they did not receive statins in FYs 2002–2004. We also required that statin users did not continue statins beyond FY 2005 to achieve the objective of our study for examining the effect of short-term statin use. This exclusion also helps to minimize confounding by indication; short-term statin users are more likely to be free from diseases that compel long-term statin prescription [14, 15]. We also ascertained that statin users did not receive other prescription medications in FY 2005 to ensure that we included healthy adults with no interacting drugs. Unexpected drug interactions may contribute to the incidence of adverse events; for example, in a large observational study, the concomitant use of clarithromycin and a statin was associated with increased risk of acute kidney injury and death [16].
Statin nonusers were defined as those patients who: (a) never received a statin throughout the study period (FY 2002 to FY 2011); and (b) never received any prescription medication during FY 2005. Because the number of these patients was very large, we limited nonusers to those who had medical encounters in the first 6 months of FY 2005.
2.4 Outcome Measures
Our outcomes were selected a priori to constitute known beneficial cardiovascular effects of statins such as acute myocardial infraction and acute cerebrovascular accidents, known statin-associated adverse events such as diabetes and musculoskeletal diseases, and suspected/debated statin-associated adverse events such as kidney diseases and cataract. An event was defined as the occurrence of International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM] codes during the follow-up period in an inpatient or outpatient setting. Our outcomes were pre-specified diagnosis groups defined by the Agency for Health Research and Quality-Clinical Classifications Software (AHRQ-CCS) as the following: [17] (1) major acute cardiovascular events (MACE), which comprised acute myocardial infarction, cardiac arrest and ventricular fibrillation, acute cerebrovascular disease, and peripheral and visceral atherosclerosis; (2) diabetes; (3) diabetes with complications; (4) nephritis and nephrosis; (5) acute and unspecified renal failure; (6) chronic kidney disease; (7) osteoarthritis and arthropathies; (8) spondylosis, intervertebral disc, back disorders; (9) dislocation, strain, sprain; (10) obesity; (11) cataracts; (12) malignancy; [18], and (13) death. Death was identified if the disposition code of an inpatient or outpatient encounter, in accordance with the MDR data dictionary, indicated death and there was no further medical encounters after the date of death. We could not ascertain death from social security administrative data because the investigators were working with de-identified data according to the Tricare Data Sharing Agreement. Details of our outcomes codes are delineated in Electronic Supplementary Material Appendix 2.
To increase the specificity of our chronic diseases diagnosis groups as previously published [19, 20], we required that two separate encounters carried ICD-9-CM codes consistent with the diagnosis group of interest. However, we accepted one encounter only in defining acute diseases such as cardiac arrest or in conditions known to have low sensitivity in identification using ICD-9-CM codes such as obesity [21, 22]. A complete list of diagnoses defined using two separate encounters is in Electronic Supplementary Material Appendix 3.
2.5 Statistical Analyses
Patients’ comorbidities were identified using ICD-9-CM codes, and their Charlson comorbidity total score was calculated using Deyo’s method [18]. Baseline characteristics of groups were compared using Chi square for categorical variables and the Student’s t test for continuous variables. We used a propensity score approach to match statin users to similar nonusers using 51 variables including: demographics, personal history, comorbidities, occurrence of outcomes of interest at baseline, undergoing musculoskeletal procedures, duration of baseline and follow-up period, and healthcare use (Table 1) [17]. We included in our propensity score variables what we believed would either be potentially associated with the use of statins or the outcomes of interest. Statin prescription may be a surrogate marker for social status confounders, or access to healthcare; [10, 23] therefore, we included in the propensity score marital status, active duty status, pay grade status, and number of inpatient and outpatient encounters. Additionally, some studies have shown that healthy user bias may contribute to favorable outcomes among statin users [24], therefore, we included variables indicative of healthcare use, visits for immunizations, and undergoing procedures as a surrogate marker for health-conscious behavior. We used logistic regression to create the propensity score and performed nearest number matching with a caliper of 0.01 using previously described routines [25–29]. After matching, we assessed the balance of covariates based on standardized differences [30, 31]. We also examined the proportions of patients in both treatment arms that underwent cardiovascular procedures (Electronic Supplementary Material Appendix 3) to ensure their comparability.
2.5.1 Primary Analysis
We used conditional logistic regression to calculate odds ratios (ORs) and 95 % confidence intervals (95 % CI) in the propensity score-matched cohort in each year of follow-up (FY 2006 to FY 2011).
2.5.2 Secondary Analysis
We performed two analyses:
-
1.
We stratified outcomes by active duty status. Each outcome was examined in a separate logistic regression model, where outcome was the dependent variable and statin use and propensity score as covariates in the model.
-
2.
We restricted analysis to individuals who did not have diabetes or obesity at baseline; thereafter, we examined the risk of each outcome in a separate logistic regression model, where outcome was the dependent variable and statin use and propensity score as covariates in the model.
2.5.3 Sensitivity Analyses
To further examine if our findings from the primary analysis may be owing to unrecognized confounders, we performed several sensitivity analyses, in which we adjusted for variables that were not incorporated into the propensity score and that may serve as markers of health risk. These variables (measured at the end of the follow-up period [FY 2011]) included systolic blood pressure, diastolic blood pressure, body mass index, high-density-cholesterol, and low-density-cholesterol. Because at FY 2011, both statin users and nonusers had not taken statins for 6 years, we adjusted for these variables in multivariable logistic regression analyses to account for any potential residual confounding. We also adjusted the OR of MACE for undergoing the following cardiovascular procedures during baseline or follow-up: electrocardiogram, echocardiography, stress test, and cardiac catheterization. Finally, we performed Cox-proportional hazard regression to assess the impact of statin use on time to development of each outcome of interest. In these analyses, time to first event of each outcome was the dependent variable, statin use was a predictor variable.
Statistical significance was defined as two-tailed p values ≤0.05. Statistical analyses were performed using SPSS version 19 (IBM Corp., Armonk, NY, USA).
3 Results
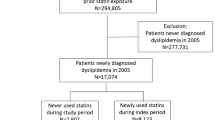
The details of the cohort assembly are shown in Fig. 1. We identified 185,005 nonusers and 1554 statin users that met study inclusion criteria. The distribution of race/ethnicity was as follows: 64.1 % unknown, 23.8 % Caucasians, 6.3 % African Americans, 0.2 % Hispanics, 0.1 % Native Americans, and 5.6 % others including southwest Asians, Asians, Filipino, and others. Because multiple sub-classifications weaken statistical power, and with such small proportions having a meaningful interpretation is unlikely, we limited our race/ethnicity groups to Caucasians, African Americans, and others/unknown. We successfully matched 1525 pairs of nonusers and statin users with good balance on all variables with two exceptions (Table 1); there were modestly higher rates of pregnancy and delivery (1.2 vs. 0.2 %) and syncope (10.9 vs. 8.7 %) in nonusers vs. statin users. As expected, our cohort was very healthy at baseline with low rates of chronic diseases (mean Charlson comorbidity score = 0.11). Despite being healthy subjects, they had many medical encounters during the baseline period (approximately 24 outpatient visits), which is partly owing to the mandatory requirements of MHS for periodic health assessments for active duty soldiers. To ensure that we were able to successfully match cardiovascular risk in both groups, we examined rates of cardiovascular procedures during the baseline period. Use of electrocardiography and echocardiography were similar in both groups, but the proportion of those who had a cardiac stress test was lower in nonusers vs. statin users (3.7 vs. 5.9 %); however, only one patient underwent cardiac catheterization and none underwent a revascularization procedure.
Among statin users, the mean duration of statin use was 254 days (standard deviation 89 days); 76 % of the prescriptions were for simvastatin, 19 % for atorvastatin, 3 % for pravastatin, and 1 % for rosuvastatin. At some point during FY 2005, 311 (20.4 %) used low-intensity statins, 1211 (79.4 %) used moderate-intensity statins, and 145 (9.5 %) used high-intensity statins (not additive because a single patient may be given different strengths at different times). Statin intensity was defined according to the 2013 American College of Cardiology/American Heart Association cholesterol guidelines with modification to include simvastatin 80 mg in the high-intensity group [32], which was in use at the study time but not of the guideline writings.
3.1 Outcomes
Tables 2 and 3 depict our outcomes; 0.9 % of nonusers and 1.8 % of statin users were diagnosed with diabetes during the first year of follow-up (FY 2006), OR 2.18 (95 % CI 1.12–4.22). The increased risk of diabetes consistently remained high throughout the follow-up years; in FY 2011, 9.0 % of nonusers and 16.0 % of statin users were diagnosed with diabetes (OR 1.93, 95 % CI 1.55–2.41). The risk for diabetes with complications also was higher among statin users beginning at FY 2008 with OR 3.52 (95 % CI 1.16–10.73) and consistently remained high throughout the follow-up years; in FY 2011, 1.1 % of nonusers and 2.4 % of statin users were diagnosed with diabetes with complications (OR 2.15, 95 % CI 1.2–3.86). Similarly, the risk of obesity was higher among statin users from FY 2006 (OR 1.7, 95 % CI 1.11–2.62) to FY 2011 (OR 1.21, 95 % CI 1.01–1.45), except for FY 2007 (OR 1.25, 95 % CI 0.94–1.66). There was no difference in the risk of musculoskeletal diseases, renal diseases, or malignancy between statin users and nonusers.
Only 3 (0.2 %) nonusers and 2 (0.1 %) statin users were diagnosed with MACE in FY 2006. During FY 2006 to FY 2011, only 2 % of nonusers and 2.3 % of statin users were diagnosed with MACE and the risk of MACE was not significantly different between treatment groups (OR 1.17, 95 % CI 0.72–1.92). During FY 2006 to FY 2011, only 1 % of nonusers and 1 % of statin users underwent cardiac catheterization, 0.3 % of nonusers and 0.4 % of statin users underwent percutaneous coronary intervention, and 0.2 % of nonusers and 0.5 % of statin users underwent coronary artery bypass graft surgery.
Secondary analyses were generally in line with primary analysis showing that statin users in comparison to nonusers in active duty and non-active duty subgroups had no statistically significant OR for MACE, but had higher OR for diabetes (Table 4). Restricting analysis to individuals without obesity or diabetes at baseline showed consistent results similar to the primary analysis. Our sensitivity analysis confirmed the findings of our primary analysis (Tables 3, 5); the risk of diabetes, diabetes complications, and obesity persisted throughout the follow-up period (FY 2006 to FY 2011), despite various adjustments. Assuming that baseline confounders may have contributed to the higher risk of diabetes among statin users (for example, clinicians may have prescribed statins because of their perception of a patient’s unwillingness to diet and exercise), we adjusted for body mass index, systolic blood pressure, and diastolic blood pressure at FY 2011, and propensity score. Adjusting for these covariates did not change our findings; OR 2.43 (95 % CI 1.82–3.24) for diabetes and OR 2.34 (95 % CI 1.10–5.05) for diabetes with complications. Table 6 delineates types of diabetic complications in statin users and nonusers. Similarly, adjusting for these variables and lipid values, or undergoing cardiologic procedures during the follow-up did not change our findings regarding MACE (OR 2.12, 95 % CI 0.81–5.60). Of note, five patients of nonusers died during the follow-up period, but none of the statin users; however, it did not reach statistical significance. The cause of deaths could not be ascertained but they were not associated with trauma/injury according to MDR dictionary coding.
Additionally, we performed a post hoc analysis examining the relation of statin use and the presence of diabetes and obesity at baseline to further determine if confounding by indication might have played a role. In these analyses, each of diabetes and obesity at baseline were entered as an outcome in a separate logistic regression model, and statin use as a covariate. Neither diabetes nor obesity at baseline was significantly related to statin therapy (OR 1.16, 95 % CI 0.84–1.56 for obesity at baseline and OR 1.37, 95 % CI 0.63–2.99 for diabetes at baseline).
Time-to-event analyses showed a similar pattern (Table 7) of increased hazards of diabetes and diabetes complications in statin users with no difference in MACE (Fig. 2).
4 Discussion
This study of healthy adults demonstrated that short-term statin use (averaging about 8 months) for primary prevention was associated with approximately double the odds of developing diabetes and diabetic complications. Of more concern, this increased risk persisted for at least 5 years after statin use stopped. While short-term statin exposure was associated with increased adverse outcomes, we found no evidence of countervailing cardiovascular benefits in this healthy low-cardiovascular-risk population. Statin users had the same low rates of MACE as non-users, as well as a similarly low incidence of invasive cardiac and revascularization procedures.
Our study did not find a difference in MACE between statin users and nonusers, which may be owing to the short period of statin administration (mean 254 days), and the low overall incidence of MACE in our healthy cohort. This study’s population is uniquely different from populations examined in major statin, primary prevention RCTs or observational studies. First, >50 % of subjects were active duty soldiers who can be considered very active physically. The mandatory biannual military physical testing, which include measured push-ups, sit-ups, and a timed two-mile run, is a good marker of physical fitness [33]. This can explain the very low rates of MACE in our cohort because excellent functional capacity status (>10 Metabolic Equivalents) has been shown to be associated with lower cardiovascular morbidity and mortality regardless of the anatomical extent of coronary artery disease [34, 35]. Such a healthy physically active population was never intentionally included in any statin primary prevention RCT. Second, no statin primary prevention RCT limited inclusion to participants who received no other prescription medications other than statins or ensured inclusion of healthy adults only. In contrast, a meta-analysis of statins primary prevention trials included patients with several comorbidities including diabetes, peripheral vascular diseases, heart failure, or elevated C-reactive protein [36].
It may seem non-intuitive that the risk of diabetes and diabetic complications persisted after statin use stopped. However, several points need to be considered. First, statins beneficial effects/adverse events may linger for prolonged periods after discontinuing therapy. Several studies noted that statins cardiovascular benefits persisted for at least 5 years after their discontinuation [37–39], an effect referred to as “a legacy effect” [40]. However, a large observational study (more than 2 million subjects) noted that prior statin use was associated with higher risks of moderate or serious myopathy and acute renal failure, which persisted 3 years after stopping statins [4]. Second, there is a lag time between acquiring type 2 diabetes as a disease and the formal diagnosis, typically 4–6 years [41]. Therefore, the diabetes that was diagnosed in 2008 might have occurred at the time of statin exposure but was diagnosed later. Indeed, Table 3 shows that the OR of incident diabetes and diabetic complications peaked at FY 2008 (fourth year after prescribing statin). Third, statins effects on the immune system and transcription factors in cells offer a plausible mechanism that may explain why their effect may last after the discontinuation of therapy, as detailed later. Last, the timeline of developing diabetes in relation to statin use is not known, although the increased risk of diabetes among statin users is well described in several studies [1, 2, 42–44]. In the JUPITER trial (the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin), a higher incidence of diabetes was noted among statin users despite the short median follow-up of 1.9 years [42]. Our study indicates that the risk of diabetes is higher with a much shorter duration of statin use and it may persist after the discontinuation of statins.
Although there is no well-defined mechanism that explains the relation of statins to diabetes, statins have complex pleotropic effects inclusive of several pathways other than lipid-lowering effects [23]. Statins mitochondrial effects include inhibition of ubiquinone synthesis, which consequently reduce adenosine triphosphate, resulting in inhibition of adenosine triphosphate-dependent potassium secretion and reduction in insulin secretion. Statins have been shown to inhibit glucose-induced, calcium signaling-dependent insulin secretion [45, 46]. Additionally, atorvastatin was noted to negatively affect the glucose transporter-4 expression on adipocytes, leading to impaired glucose uptake [47]. Using the available experimental evidence, some authors proposed a paradigm for statin-induced impairment of glucose metabolism including the decrease of important downstream cellular products such as coenzyme Q10, farnesyl pyrophosphate, geranylgeranyl pyrophosphate, and dichol; their depletion leads to reduced intracellular signaling [48]. Other mechanisms included inhibition of adipocyte differentiation, through inhibition of important transcription factors, resulting in increased insulin resistance; inhibition of isoprenoid synthesis, resulting in increasing adipocyte insulin resistance; inhibition of adiponectin secretion, which correlates with insulin resistance, obesity, and the metabolic syndrome; negatively regulating β-cell mass through suppression of leptin secretion from adipocytes; uncoupling protein 3, resulting in insulin resistance; expression on microRNA, resulting in impairment on insulin secretion and induction of β-cell dysfunction [48]. Statins effects on oxidation and overproduction of nitric oxide were also thought to result in apoptosis of β cells [46, 49]. Additionally, statins have proinflammatory effects (in addition to their well-known anti-inflammatory effects). Statins have been shown to increase interleukin-1β and interleukin-18 through their effect on the NOD-like receptor family pyrin domain containing 3 (NLRP 3) inflammosome [50]. NLRP 3 shows increased activity in patients with type 2 diabetes, and blocking or deleting components of the inflammosome reduces insulin resistance in animal models. Therefore, some authors proposed that statins proinflammatory effects through their effects on NLRP 3 inflammasome are a major factor in increasing the risk of diabetes in statin users [50]. Last, a recent Mendelian randomization study noted that common variants in the hydroxyl-methylglutaryl-coenzyme-A reductase (HMGR) gene (that would result in an effect similar to statins) were associated with lower low-density lipoprotein-serum levels, increased body mass index, increased insulin resistance, and a higher likelihood of being diagnosed with type 2 diabetes [51]. Such findings further support the link between statins and higher risk of diabetes and obesity and demonstrates that such an effect is an “on-target” effect [52]. Both statins effects on the immune system and transcription factors might explain their “legacy effects” after the discontinuation of therapy.
Several studies noted that statin use is associated with high blood glucose levels. In one study, effects of atorvastatin (10 mg/day) on glycemic profiles of 78 patients with diabetes were studied over 12 weeks [47]. Treatment with atorvastatin attenuated adipocyte maturation by inhibiting isoprenoid biosynthesis and significantly increased glycosylated hemoglobin; this effect was greater in the non-obese subgroup [47]. In another retrospective study including 1060 patients, statin use was associated with increased glycosylated hemoglobin in comparison to non-users [53]. In another observational study (345,417 patients, 20 % statin users) from the Veterans Affairs Health Care System, the change in fasting plasma glucose (FPG) over a mean time of 2 years was examined [54]. Among patients without diabetes, FPG increased with statin use from 98 to 105 mg/dL, and among patients with diabetes, FPG increased with statin use from 102 to 141 mg/dL. After adjustment, the change in FPG in non-diabetic statin users was still higher than nonusers [54]. Such an effect may partially explain the association of statins with a higher risk of diabetic complications. In a recent study, we have reported the association of statin use with the increased risk of diabetic complications including uncontrolled diabetes, diabetes with renal manifestations, diabetes with ophthalmic manifestations, diabetes with neurological manifestations, and diabetes with peripheral circulation manifestations, but not diabetes with hypoglycemia [2].
A large body of studies strongly associates statins with obesity. In addition to in vitro studies summarized earlier, in an experiment on 70 Zücker rats, which were treated with different statins for 6 weeks, liver and white adipose tissue were dissected and weighted. Statin-treated rats had a higher body weight than controls, increased adipose tissue size, increased de novo lipogenesis, increased fatty acid uptake from circulating triacylglycerols, and increased hepatic triacylglycerols content [55]. Additional evidence from randomized trials, observational studies from patients’ survey, retrospective cohort studies, and Mendelian randomization studies also support such relation. A cross-sectional study from the NHANES study (National Health and Nutrition Examination Survey), including 27,886 US adults, evaluated caloric and fat intake and body weight from 1999 through 2010 [3]. Among statin users, caloric intake in the 2009–2010 period was 9.6 % higher (95 % CI 1.8–18.1; p = 0.02) than that in the 1999–2000 period, in contrast of nonusers. Statin users fat intake also increased 14.4 % (95 % CI 3.8–26.1) while not changing significantly among nonusers. Additionally, body mass index increased more among statin users (+1.3) than among nonusers (+0.4) [3]. Additionally, a RCT of 1016 subjects noted that statin users reported higher levels of “decrease in energy” and “fatigue with exertion” in comparison to nonusers [56]. Several studies and a metaanalysis have reported that statins increased the risk of myopathy and reduced muscle strength and exercise tolerance. Fatigue and myopathy may be reasonably argued to result in decreased physical activity and increased obesity. Last, a recent Mendelian randomized trial (summarized earlier) gives additional insight into the link between HMGR activity and obesity [51, 52].
Our findings raise concerns about the risk–benefit ratio of statins among lower risk, physically active, healthy individuals because short-term statin exposure doubled the risk of adverse consequences without any of the desired cardiovascular benefits. In other words, “a little bit of a good thing may not be necessarily good”. Consequently, further study is urgently needed to appropriately inform patients about the risks associated with non-commitment to statin therapy.
From a provider perspective, these findings should be taken as a cautionary message that prescribing statins in low-risk populations for primary prevention might have a very different harm and benefit balance than what would be inferred from extrapolating the powerful benefits of statins in higher risk populations. Concerns have been raised that risk overestimation by the recent ACC/AHA pooled risk calculator [6, 7] may entice practitioners to accept that more healthy individuals will be treated with statins [7]. Our study offers a cautionary note against this approach.
Our study has several limitations including its retrospective observational design. Comparing risks of cardiovascular diseases, diabetes, and diabetic complications between statin users and nonusers in observational data is subject to confounding by indication. Despite our best effort to include relevant confounders in our propensity score, unidentified confounders is a major limitation of observational studies. To minimize the latter, we excluded outcomes during the exposure period as previously recommended [57–59]. We also limited our statin users to those who used statins in FY 2005 only because studies have shown that non-persistent patients commonly have lower comorbidities [14, 15]. We also excluded those with chronic diseases and those who received any prescription medication other than a statin in the statin user group to ensure homogeneity of our cohort. At the year 2005, the prevalent guidelines for prescribing statins (modified ATPIII) did not recommend statins for obese individuals; rather, the guidelines clearly and explicitly recommended lifestyle modifications and not statins [60]. We also performed post hoc regression analysis, which did not show that statin use was related to obesity or diabetes at baseline (as detailed earlier). Additionally, adjusting the risk of outcomes (including diabetes) for actual body mass index and other laboratory tests at the end of the follow-up period in our sensitivity analyses did not change or weaken our findings, rather it increased the OR of outcomes. Additionally, if our findings were mainly owing to confounding, we would expect that other outcomes have yielded positive findings, specifically, MACE or other outcomes related to obesity (such as osteoarthritis, arthropathy, kidney diseases, and malignancy). The increased OR of diabetes among statin users peaked at FY 2008 and then started to decrease. This timeline, which is in line with the known time lag between diabetes incidence and its diagnosis, suggests a true relation; if the increased OR was owing to confounders, we would expect the OR to continue to rise. We also examined the risk of undergoing cardiac procedures during baseline, which were not included in our propensity score matching, to serve as a measure for our success in minimizing confounding; only one patient underwent cardiac catheterization and none of the patients had any revascularization procedures. Cook and Ridker suggested doubling the risk of cardiovascular events to adjust for confounding by indication among statin users [61]; even if we applied such an assumption to our data, the absolute number of MACE would be still small in comparison to patients with diabetes and diabetic complications. Probably, using a larger sample size might have detected a difference in MACE. The lack of laboratory data and vital sign data at baseline to be incorporated into propensity score creation is a major limitation for our study. However, adjusting for these data at the end of follow-up, when available, did not change our findings as detailed in Table 3. Of note, the available vital signs and laboratory data at follow-up were missing from a significant proportion of patients, specifically the nonusers group, which is another limitation. Additionally, the ethnicity of our propensity score-matched cohort was unknown in more than half of the study subjects, resulting in an inability to know the actual proportions of African Americans and Hispanics in our sample. Therefore, we may not have adequately adjusted for ethnic variations in our study and the proportions of ethnic groups in our sample do not reflect their actual presence in the military. Similarly, the presence of a potential sex difference in outcomes cannot be examined because >82 % of our population were men (only 265 women nonusers and 270 women statin users). Using ICD-9-CM codes is another limitation to our study as it may not reflect disease severity. Although the use of ICD-9 codes, in general, and AHRQ-CCS codes in particular, to extract obesity diagnoses has been use in healthcare research [62–65], as well as in generating reports on use and cost of obesity [66–68], it has low sensitivity but high specificity [21, 22]. In one study, ICD-9 codes had a sensitivity of 24.6 %, a specificity of 99.3 %, a positive predictive value of 75.9 %, and a negative predictive value of 93.6 % [21]. However, we do not know of any reason for differential bias between statin users vs. nonusers (i.e., underestimation or overestimation is likely to affect both treatments equally).
Both statin users and nonusers had many outpatient visits and procedures (Table 1), which would minimize ascertainment bias in both treatment arms. Furthermore, adjusting for actual values of blood pressure, body mass index, blood glucose, glycosylated hemoglobin, and serum lipid at the end of follow-up did not significantly change our results.
The reason for short statin therapy in our study cannot be ascertained from pharmacy data and may be owing to intolerance or simply non-persistence, both of which are well-known problems that limit statin efficacy. Studies have shown that persistence with statin therapy is generally low [15, 69]. In one study, the percentage of patients who persisted with statin therapy was 46 % after 1 month, 40.3 % after 2 months, 27 % after 6 months, and 20.1 % after 12 months [69]. Even clinical trials, with their patient selectivity and meticulous follow-up, non-persistence with statin therapy has been a problem. For example, in the JUPITER trial (the largest and most recent statin primary prevention trial), 25 % of the statin arm stopped taking the study medication although the study mean duration was 1.9 years only [10]. We also could not account for the type or dose of statin used because patients occasionally received different types of statins at different doses. Therefore, ascribing a specific outcome to a specific type or dose of a statin is not possible. Owing to the relatively small sample size, subclassification of statin users based on the type or dose of statin was unfeasible.
5 Conclusion
Short-term statin use in a healthy physically active population was associated with doubled the odds of persistent obesity, diabetes, and its complications without cardiovascular benefits. This should be taken as a cautionary message that the use of statins in a low-risk population might be ‘more harm than good’ or ‘all harm and no good.’ Further studies including RCTs, pragmatic studies, and prospective observational studies appropriately equipped to eliminate unidentified confounders and confounding by indication are urgently needed.
References
Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375(9716):735–42.
Mansi I, Frei CR, Wang CP, Mortensen EM. Statins and new-onset diabetes mellitus and diabetic complications: a retrospective cohort study of US healthy adults. J Gen Intern Med. 2015;30(11):1599–610.
Sugiyama T, Tsugawa Y, Tseng CH, Kobayashi Y, Shapiro MF. Different time trends of caloric and fat intake between statin users and nonusers among US adults: gluttony in the time of statins? JAMA Intern Med. 2014;174(7):1038–45.
Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010;340:c2197.
Lee DS, Markwardt S, Goeres L, Lee CG, Eckstrom E, Williams C, et al. Statins and physical activity in older men: the osteoporotic fractures in men study. JAMA Intern Med. 2014;174(8):1263–70.
DeFilippis AP, Young R, Carrubba CJ, McEvoy JW, Budoff MJ, Blumenthal RS, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162(4):266–75.
Ridker PM, Cook NR. Comparing cardiovascular risk prediction scores. Ann Intern Med. 2015;162(4):313–4.
Simes J, Furberg CD, Braunwald E, Davis BR, Ford I, Tonkin A, et al. Effects of pravastatin on mortality in patients with and without coronary heart disease across a broad range of cholesterol levels. The Prospective Pravastatin Pooling project. Eur Heart J. 2002;23(3):207–15.
Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–78.
Mansi I. Statin adverse events in primary prevention: between randomized trials and observational studies. Am J Med Sci. 2015;350(4):330–7.
Moniz C. Outpatient workload (RVU) predictors: age, gender and beneficiary category. Graduate Management Project. Baltimore, MD: US Army Medical Department Center and School, Medicine JH; 2008 June 15, Report No.: Contract No.: 34-08.
Gantt CJ, Neely JA, Villafana IA, Chun CS, Gharabaghli SM. Analysis of weight and associated health consequences of the active duty staff at a major Naval medical center. Mil Med. 2008;173(5):434–40.
George SZ, Childs JD, Teyhen DS, Wu SS, Wright AC, Dugan JL, et al. Brief psychosocial education, not core stabilization, reduced incidence of low back pain: results from the Prevention of Low Back Pain in the Military (POLM) cluster randomized trial. BMC Med. 2011;9(1):128.
Lilly SM, Mortensen EM, Frei CR, Pugh MJ, Mansi IA. Comparison of the risk of psychological and cognitive disorders between persistent and nonpersistent statin users. Am J Cardiol. 2014;114(7):1035–9.
Mann DM, Woodward M, Muntner P, Falzon L, Kronish I. Predictors of nonadherence to statins: a systematic review and meta-analysis. Ann Pharmacother. 2010;44(9):1410–21.
Li DQ, Kim R, McArthur E, Fleet JL, Bailey DG, Juurlink D, et al. Risk of adverse events among older adults following co-prescription of clarithromycin and statins not metabolized by cytochrome P450 3A4. CMAJ Can Med Assoc J J de l’Association Med Can. 2015;187(3):174–80.
Elixhauser A, Steiner C, Palmer L. Clinical Classifications Software (CCS) for ICD-9-CM. Databases and related tools from the healthcare cost and utilization project (HCUP) [Internet]. 2012 01/01/2012: [Appendix A p.]. http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed 16 Mar 2015.
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9.
Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(Suppl 2):B10–21.
Kim SY, Servi A, Polinski JM, Mogun H, Weinblatt ME, Katz JN, et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. 2011;13(1):R32.
Quan H, Li B, Saunders LD, Parsons GA, Nilsson CI, Alibhai A, et al. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43(4):1424–41.
Goff SL, Pekow PS, Markenson G, Knee A, Chasan-Taber L, Lindenauer PK. Validity of using ICD-9-CM codes to identify selected categories of obstetric complications, procedures and co-morbidities. Paediatr Perinat Epidemiol. 2012;26(5):421–9.
Mansi I, Mortensen E. The controversy of a wider statin utilization: why? Expert Opin Drug saf. 2013;12(3):327–37.
Dormuth CR, Patrick AR, Shrank WH, Wright JM, Glynn RJ, Sutherland J, et al. Statin adherence and risk of accidents: a cautionary tale. Circulation. 2009;119(15):2051–7.
Ho D, Imai K, King G, Stuart E. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal. 2007;15:199–236.
Ho D, Imai K, King G, Stuart E. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42(8):1–28.
Hansen BB. Full matching in an observational study of coaching for the SAT. J Am Stat Assoc. 2004;99(467):609–18.
Hansen BB, Bowers J. Covariate balance in simple, stratified and clustered comparative studies. Stat Sci. 2008;23(2):219–36.
Thoemmes F. An SPSS R menu for propensity score matching. 2011. http://www.arxiv.org. Accessed 21 May 2015.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale: Lawrence Erlbaum Associates Publishers; 1988.
Yang D, Dalton JE. A unified approach to measuring the effect size between two groups using SAS, SAS Global Forum 2012—Statistics and Data Analysis; Paper 335-2012. http://support.sas.com/resources/papers/proceedings12/335-2012.pdf.
Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–45.
Army Regulation 40-501; Standards of Medical Fitness. Washington, DC: Headquarters, Medical Services, Department of the Army, 14 December 2007. 2007.
Mark DB, Hlatky MA, Harrell FE Jr, Lee KL, Califf RM, Pryor DB. Exercise treadmill score for predicting prognosis in coronary artery disease. Ann Intern Med. 1987;106(6):793–800.
Weiner DA, Ryan TJ, McCabe CH, Chaitman BR, Sheffield LT, Ferguson JC, et al. Prognostic importance of a clinical profile and exercise test in medically treated patients with coronary artery disease. J Am Coll Cardiol. 1984;3(3):772–9.
Taylor F, Huffman MD, Macedo AF, Moore TH, Burke M, Davey Smith G, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1:CD004816.
Strandberg TE, Pyorala K, Cook TJ, Wilhelmsen L, Faergeman O, Thorgeirsson G, et al. Mortality and incidence of cancer during 10-year follow-up of the Scandinavian Simvastatin Survival Study (4S). Lancet. 2004;364(9436):771–7.
Ford I, Murray H, Packard CJ, Shepherd J, Macfarlane PW, Cobbe SM, et al. Long-term follow-up of the West of Scotland Coronary Prevention Study. N Engl J Med. 2007;357(15):1477–86.
Tonkin A, Aylward P, Colquhoun D, Glasziou P, Harris P, MacMahon S, Magnus P, Newel D, Nestel P, Sharpe N, Hunt D. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349–57.
Enas EA, Kuruvila A, Khanna P, Pitchumoni CS, Mohan V. Benefits and risks of statin therapy for primary prevention of cardiovascular disease in Asian Indians—a population with the highest risk of premature coronary artery disease and diabetes. Indian J Med Res. 2013;138(4):461–91.
Porta M, Curletto G, Cipullo D, Rigault de la Longrais R, Trento M, Passera P, et al. Estimating the delay between onset and diagnosis of type 2 diabetes from the time course of retinopathy prevalence. Diabetes Care. 2014;37(6):1668–74.
Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–207.
Culver AL, Ockene IS, Balasubramanian R, Olendzki BC, Sepavich DM, Wactawski-Wende J, et al. Statin use and risk of diabetes mellitus in postmenopausal women in the Women’s Health Initiative. Arch Intern Med. 2012;172(2):144–52.
Park ZH, Juska A, Dyakov D, Patel RV. Statin-associated incident diabetes: a literature review. Consult Pharm. 2014;29(5):317–34.
Yada T, Nakata M, Shiraishi T, Kakei M. Inhibition by simvastatin, but not pravastatin, of glucose-induced cytosolic Ca2+ signalling and insulin secretion due to blockade of L-type Ca2+ channels in rat islet beta-cells. Br J Pharmacol. 1999;126(5):1205–13.
Sampson UK, Linton MF, Fazio S. Are statins diabetogenic? Curr Opin Cardiol. 2011;26(4):342–7.
Nakata M, Nagasaka S, Kusaka I, Matsuoka H, Ishibashi S, Yada T. Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): implications in glycaemic control. Diabetologia. 2006;49(8):1881–92.
Brault M, Ray J, Gomez YH, Mantzoros CS, Daskalopoulou SS. Statin treatment and new-onset diabetes: a review of proposed mechanisms. Metab Clin Exp. 2014;63(6):735–45.
Sattar N, Taskinen MR. Statins are diabetogenic–myth or reality? Atheroscler Suppl. 2012;13(1):1–10.
Henriksbo BD, Schertzer JD. Is immunity a mechanism contributing to statin-induced diabetes? Adipocyte. 2015;4(4):232–8.
Swerdlow DI, Preiss D, Kuchenbaecker KB, Holmes MV, Engmann JE, Shah T, et al. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet. 2015;385(9965):351–61.
Frayling TM. Statins and type 2 diabetes: genetic studies on target. Lancet. 2015;385(9965):310–2.
Liew SM, Lee PY, Hanafi NS, Ng CJ, Wong SS, Chia YC, et al. Statins use is associated with poorer glycaemic control in a cohort of hypertensive patients with diabetes and without diabetes. Diabetol Metab Syndr. 2014;6:53.
Sukhija R, Prayaga S, Marashdeh M, Bursac Z, Kakar P, Bansal D, et al. Effect of statins on fasting plasma glucose in diabetic and nondiabetic patients. J Investig Med Off Publ Am Fed Clin Res. 2009;57(3):495–9.
Aguirre L, Hijona E, Macarulla MT, Gracia A, Larrechi I, Bujanda L, et al. Several statins increase body and liver fat accumulation in a model of metabolic syndrome. J Physiol Pharmacol Off J Pol Physiol Soc. 2013;64(3):281–8.
Golomb BA, Evans MA, Dimsdale JE, White HL. Effects of statins on energy and fatigue with exertion: results from a randomized controlled trial. Arch Intern Med. 2012;172(15):1180–2.
Danaei G, Rodriguez LA, Cantero OF, Logan R, Hernan MA. Observational data for comparative effectiveness research: an emulation of randomised trials of statins and primary prevention of coronary heart disease. Stat Methods Med Res. 2013;22(1):70–96.
Jee SH, Sull JW, Park J, Lee SY, Ohrr H, Guallar E, et al. Body-mass index and mortality in Korean men and women. N Engl J Med. 2006;355(8):779–87.
Allison DB, Faith MS, Heo M, Townsend-Butterworth D, Williamson DF. Meta-analysis of the effect of excluding early deaths on the estimated relationship between body mass index and mortality. Obes Res. 1999;7(4):342–54.
Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–39.
Cook NR, Ridker PM. Further insight into the cardiovascular risk calculator: the roles of statins, revascularizations, and underascertainment in the Women’s Health Study. JAMA Intern Med. 2014;174(12):1964–71.
Memtsoudis SG, Bombardieri AM, Ma Y, Walz JM, Chiu YL, Mazumdar M. Mortality of patients with respiratory insufficiency and adult respiratory distress syndrome after surgery: the obesity paradox. J Intensive Care Med. 2012;27(5):306–11.
Scott SK, Rabito FA, Price PD, Butler NN, Schwartzbaum JA, Jackson BM, et al. Comorbidity among the morbidly obese: a comparative study of 2002 US hospital patient discharges. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2006;2(2):105–11.
Vila-Corcoles A, Ochoa-Gondar O, Llor C, Hospital I, Rodriguez T, Gomez A. Protective effect of pneumococcal vaccine against death by pneumonia in elderly subjects. Eur Respir J. 2005;26(6):1086–91.
Allareddy V, Rampa S, Anamali S, Lee MK, Allareddy V, Nalliah RP. Obesity and its association with comorbidities and hospital charges among patients hospitalized for dental conditions. Journal of investigative and clinical dentistry. 2015. doi:10.1111/jicd.12146] (Epub ahead of print).
Wier L, Encinosa W. Obesity in children: Hospitalizations from 2000 to 2009. HCUP Statistical Brief #138. July 2012. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb138.pdf. Accessed 16 Mar 2015.
Weiss A, Elixhauser A. Obesity-related hospitalizations, 2004 versus 2009. HCUP Statistical Brief #137. July 2012. Agency for Healthcare Research and Quality. Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb137.pdf. Accessed 16 Mar 2015.
Elixhauser A, Steiner C. Obese patients in US Hospitals, 2004. HCUP Statistical Brief #20. December 2006. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb20.pdf. Accessed 16 Mar 2015.
Kiss Z, Nagy L, Reiber I, Paragh G, Molnar MP, Rokszin G, et al. Persistence with statin therapy in Hungary. Arch Med Sci. 2013;9(3):409–17.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Drs. Halm and Mortensen were supported in part by a grant from the Agency for Healthcare Research and Quality (R24 HS022418) for the University of Texas Southwestern Center for Patient-Centered Outcomes Research.
No funding was provided for the conduct of this study. This material is the result of work supported with resources and the use of facilities at the VA North Texas Health Care System and the University of Texas Southwestern.
Conflicts of interest
Ishak Mansi, Jenny English, Song Zhang, Eric Mortensen, and Ethan Halm declare that they have no conflicts of interest that are directly relevant to the content of this study.
Disclaimer
The views expressed herein are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, VA Healthcare administration, or the US Government. The authors are employees of the US Government. This work was prepared as part of their official duties and, as such, there is no copyright to be transferred.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mansi, I.A., English, J., Zhang, S. et al. Long-Term Outcomes of Short-Term Statin Use in Healthy Adults: A Retrospective Cohort Study. Drug Saf 39, 543–559 (2016). https://doi.org/10.1007/s40264-016-0412-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-016-0412-2