Abstract
Introduction
Even though several landmark statin trials have demonstrated the beneficial effects of statin therapy in both primary and secondary prevention of cardiovascular disease, several studies have suggested that statins are associated with a moderate increase in risk of new-onset diabetes. These observations prompted the US FDA to revise statin labels to include a warning of an increased risk of incident diabetes mellitus as a result of increases in glycosylated hemoglobin (HbA1c) and fasting plasma glucose. However, few studies have used US-based data to investigate this statin-associated increased risk of diabetes.
Objective
The primary objective of our study was to examine whether the use of statins increases the risk of incident diabetes mellitus using data from the Thomson Reuters MarketScan ® Commercial Claims and Encounters Database.
Method
This study was a retrospective cohort analysis utilizing data for the period 2003–2004. The study population included new statin users aged 20–63 years at index who did not have a history of diabetes.
Results
The proportion (3.4 %) of statin users (N = 53,212) who had incident diabetes was higher than the proportion (1.2 %) of non-statin users (N = 53,212) who had incident diabetes. Compared with no statin use and controlling for demographic and clinical covariates, statin use was significantly associated with increased risk of incident diabetes (hazard ratio 2.01; 99 % confidence interval 1.74–2.33; p < 0.0001). In addition, risk of diabetes was highest among users of lovastatin, atorvastatin, simvastatin, and fluvastatin. Diabetes risk was lowest among pravastatin and rosuvastatin users.
Discussion
Because the potential for diabetogenicity differs among different statin types, healthcare professionals should individualize statin therapy by identifying patients who would benefit more from less diabetogenic statin types.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statin therapy was significantly associated with increased risk of new-onset diabetes mellitus. |
Risk of diabetes was highest among lovastatin and atorvastatin users and lowest among pravastatin and rosuvastatin users. |
Statin therapy needs to be individualized by identifying patients who would benefit more from less diabetogenic statin types. |
1 Introduction
In February 2012, the US FDA approved important safety labeling changes for statins, including an increased risk of incident diabetes mellitus as a result of increases in glycosylated hemoglobin (HbA1c) and fasting plasma glucose (FPG) found to be associated with statin therapy [1]. The FDA based its decision on a combination of results from randomized controlled trials (RCTs) [2–5], meta-analyses of RCTs [6–8], a systematic review [9], and a few observational studies [10, 11], which indicated an increased risk of incident diabetes due to statin therapy.
The potential increased risk in diabetes associated with statin use is significant because patients with dyslipidemia being treated with statins already have a baseline increased risk of diabetes due to abnormal lipid levels, combined with comorbidities such as high blood pressure, increased weight and body mass index (BMI), metabolic syndrome, and cardiovascular diseases. Statin-induced diabetes risk is not a desirable outcome because the already increasing incidence and prevalence of prediabetes and diagnosed diabetes contributes significantly to increasing rates of morbidity and mortality among Americans [12]. Diabetes is the seventh leading cause of death in the USA, with attendant complications such as kidney failure, heart attack, stroke, and amputation [13]. Diabetes also imposes a substantial economic burden on the US population in the form of increased direct and indirect medical costs [14].
Several RCTs, meta-analyses of RCTs, and observational studies have examined the association between statin use and incidence of diabetes. The JUPITER (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) trial was one of the RCTs the FDA used in reaching a decision to change the labeling for all statins [5]. A secondary outcome of the JUPITER study results showed that rosuvastatin was significantly associated with a 26 % increased risk of diabetes [5].
Similar to the JUPITER study, the PROSPER (Pravastatin in Elderly Individuals at Risk of Vascular Disease) study found a 32 % increased risk of diabetes associated with pravastatin [15]. The results from the JUPITER and PROSPER studies were consistent with results from other RCTs [2–4] that showed statins to be associated with increased risk of diabetes. However, results from other RCTs showed that statins may be associated with a reduced risk (or protective effect) of diabetes rather than an increased risk [16, 17].
Evidence from meta-analyses of RCTs also indicates that statins may be associated with a moderate increase in risk of diabetes of about 9 % as found in the 2010 meta-analysis of 19 RCTs by Sattar et al. [8], the 2011 meta-analysis of 17 RCTs by Mills et al. [6], and the 2013 meta-analysis of 55 RCTs by Naci et al. [18].
Evidence from observational studies appears to follow that observed from RCTs and meta-analyses of RCTs with respect to the direction and strength of association between statin therapy and incidence of diabetes. The 2012 prospective cohort study by Culver et al. [11] showed that statin use was associated with a 47 % increased risk of new-onset diabetes mellitus among postmenopausal women participating in the longitudinal WHI (Women’s Health Initiative) study. The retrospective cohort studies by Danaei et al. [19], Wang et al. [20], and Zaharan et al. [21] reported a 14, 15, and 20 % increased risk in diabetes, respectively. In a more recent retrospective cohort study by Mansi et al. [22], an 87 % higher odds of incident diabetes was recorded among statin users compared with non-statin users.
The main purpose of the present study was to examine whether the use of statins increases the risk of incident diabetes mellitus using data from the Thomson Reuters MarketScan® Commercial Claims and Encounters Database. This is warranted because the findings linking statin therapy to the development of incident diabetes are inconsistent. While some studies indicate a moderate, statistically significant increase in risk of diabetes with statin therapy [2–4, 6–8, 10, 11, 18–21, 23, 24], others indicate that statins are protective or reduce the risk of diabetes [16, 17], while still others suggest the increase in risk is not statistically significant [25–27].
In addition, a majority of the observational studies that examined the association between statin use and the development of diabetes were conducted using non-US population data. Thus, further examination of this topic among the US population was required. The US population may differ from other populations in terms of the prevalence of risk factors such as overweight, obesity, and cardiovascular diseases that may put people at an increased risk of diabetes, independent of the effects of statin therapy [28]. For example, the USA has the highest rate of obesity (defined as BMI of ≥30, an independent risk factor for diabetes and cardiovascular disease) among all high-income populations such as those found in North America, Canada, and Europe [29]. This is in contrast to people from East Asia (e.g., Taiwan, China, and Japan), the BMIs for whom are known to be among the lowest in the world [29].
2 Methods
The study protocol submission was granted an institution review board waiver by the Office of Research Support of The University of Texas at Austin’s Institution Review Board because a secondary analysis of de-identified data does not meet the criteria for human subjects research.
2.1 Study Design
The study utilized a retrospective cohort design using an administrative claims database containing patients’ pharmacy and medical claims (i.e., the MarketScan ® Commercial Claims and Encounters Database) for the period of 1 January 2003 to 31 December 2004. The study population consisted of patients in the MarketScan database who (1) were aged 20–63 years at the index date (defined as date of the first prescription claim for a statin or a non-statin drug during the index period 1 July 2003 to 1 January 2004); (2) did not have a diagnosis of diabetes (International Classification of Diseases, Ninth revision, Clinical Modification [ICD-9-CM] codes 250.xx) in the pre-index period (defined as a period of at least 6 months before the first index medication was received) to ensure measurement of incident rather than prevalent diabetes cases; and (3) were continuously enrolled in their health plan during the pre-index period and for at least 1 year after the index date. To further strengthen the association between exposure and outcome, subjects were required to have a minimum drug exposure period of at least 6 months, after which the outcome (i.e., diabetes occurrence) can be considered valid. Thus, we excluded diabetes cases that occurred within 6 months of initial drug exposure.
2.2 Cohort Formation
In the retrospective cohort design, we formed two cohorts of patients from the retrospective data. The first cohort, called statin users (or the exposed group) were (1) patients who received at least one statin prescription for atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, or simvastatin during the index period ranging from 1 July 2003 to 1 January 2004; (2) new statin users (i.e., new statin use was established if a subject had no prescription for a statin medication in the 6 months before their first statin use; to establish a history of drug use of at least 6 months, the earliest first use of any statin drug was set at 1 July 2003); and (3) those who did not fill a statin drug that was in a combined dosage form with any other drug or agent.
The second cohort, called non-statin users (or the unexposed group) were patients who did not receive statin medication. These two cohorts were then “followed-up” until (1) manifestation of diabetes; or (2) end of the follow-up period (31 December 2004) without manifestation of diabetes. We based drug exposure on the intention-to-treat principle (i.e., subjects were assumed to be treated with the medication to which they were exposed at the index date).
2.3 Outcome Measures and Statistical Analysis
We analyzed the retrospective cohort design using two different statistical methods. This was to assess whether the magnitude and significance of the risk ratios associating statin use with diabetes would differ depending on the nature of the dependent variable. We used the Cox proportional hazards regression when survival time (defined as the time from receipt of index medication to manifestation of diabetes or end of the study period if no diabetes occurred) was the dependent variable of interest. In contrast, we used the binary logistic regression analysis when the dependent variable of interest was presence or no presence of incident diabetes.
Several demographic and clinical covariates were controlled for in each of the regression models, including age, sex, hyperlipidemia (ICD-9-CM codes 272.0, 272.1, 272.2, and 272.4), obesity (278.00 and 278.01), hypertension (401.0, 401.1, and 401.9), number of prescriptions for all diabetogenic medications used (i.e., thiazide diuretics, beta-blockers, antipsychotics, antidepressants, immunosuppressants, and glucocorticoids), and the Charlson Comorbidity Index (CCI) score. We estimated this score using the Dartmouth–Manitoba method [30]. We used SAS® for Windows® version 9.3 (SAS Institute, Cary, NC, USA) and IBM SPSS Statistics version 21 (SPSS Inc., Chicago, IL, USA) to perform data manipulation and analyses and evaluated statistical significance at p < 0.01.
2.4 Sensitivity Analysis
Sensitivity analyses were conducted to examine how the risk ratios were influenced by (1) using two types of statistical analysis (i.e., Cox and logistic regression models) to estimate the measures of risk; (2) controlling for significant time-dependent covariates (i.e., independent variables that violated the proportionality of hazards assumption) in the Cox models; and (3) using a regression-based propensity score covariate adjustment rather than traditionally controlling for all covariates in both the Cox and the logistic regression models. Though susceptible to the effects of unmeasured covariates, propensity score analysis helps reduce the effects of confounding when using observational data [31].
3 Results
3.1 Patient Selection Criteria and Sample Size
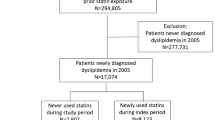
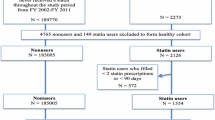
Figures 1 and 2 describe the inclusion/exclusion criteria used in selecting both the statin user and the non-statin user cohort, respectively. The study sample consisted of 106,424 subjects aged 20–63 years at index date and who had no evidence of diabetes both in the pre-index period and 6 months after the index date. The mean length of the pre-index period used to exclude prevalent diabetes cases did not significantly differ between statin users (8.86 months, standard deviation [SD] 1.74) and non-statin users (8.74 months, SD 1.76). Both the statin and non-statin user cohorts were followed from the earliest index date of 1 July 2003 until they had a diagnosis of diabetes or reached the end of the study period (31 December 2004) without a diabetes diagnosis. The study sample (N = 106,424) comprised equal proportions of statin users (N = 53,212) and non-statin users (N = 53,212).
3.2 Demographic and Clinical Characteristics
Table 1 shows the differences in demographic and clinical characteristics between statin users and non-statin users. An independent samples t test showed that mean age was significantly higher (p < 0.0001) among statin users (52.1 years) than among non-statin users (40.5 years). Similarly, the mean CCI score was significantly higher among statin users (0.24) than among non-statin users (0.04). In addition, the results showed that the proportion of statin users with a diagnosis of hyperlipidemia (50.8 vs. 6.1 %), obesity (0.9 vs. 0.3 %), and hypertension (30.6 vs. 4.1 %) was significantly higher than that of non-statin users. Furthermore, the proportion of statin users who received at least one diabetogenic medication prescription (45.6 %) or who had a CCI score of ≥1 (12.4 %) was higher than the proportion of non-statin users who received at least one diabetogenic medication prescription (12.2 %) or who had a CCI score of ≥1 (2.3 %). Given these significant differences, we controlled for all demographic and clinical variables in the Cox and logistic regression models.
3.3 Incidence of Diabetes
Table 2 shows a breakdown of the weighted and unweighted incidence density and cumulative incidence of diabetes by statin use. A total of 106,424 subjects were followed for 1,608,088.77 months, and 2478 new cases of diabetes were found, resulting in an unweighted diabetes incidence density rate of 1.54 per 1000 person-months or an unweighted diabetes cumulative incidence of 23.2 per 1000 population.
In addition, statin users (N = 53,212) were followed-up for an average of 15.01 months (SD 1.86, median 15.04) while non-statin users (N = 53,212) were followed-up for an average of 15.21 months (SD 1.81, median 15.31). The unweighted diabetes incidence density rate for statin users (2.29 per 1000 person-months) was higher than that for non-statin users (0.8 per 1000 person-months). This means that, if 1000 statin users were followed-up for 1 month, 2.29 new cases of diabetes would be recorded. This rate is higher than the 0.8 new cases of diabetes that would be recorded if 1000 non-statin users were followed-up for 1 month.
Among the statin types, the unweighted diabetes incidence density rate was highest for lovastatin (2.97 per 1000 person-months) and decreased, consecutively, for fluvastatin (2.61 per 1000 person-months), rosuvastatin (2.38 per 1000 person-months), simvastatin (2.29 per 1000 person-months), and atorvastatin (2.24 per 1000 person-months). The diabetes incidence density rate was lowest for pravastatin (2.01 per 1000 person-months).
3.4 Kaplan–Meier Survival Analysis by Exposure Type
Study results showed that the estimated mean survival time (i.e., time to a diabetes diagnosis in months) for statin users (17.8, standard error [SE] 0.006) was shorter than that for non-statin users (18.0, SE 0.003).
In addition, Fig. 3 shows two Kaplan–Meier survival curves comparing the survival probabilities against time for statin users and non-statin users. The log-rank test comparing the distribution of the two survival curves showed that statin users had a significantly shorter survival probability over time than non-statin users (χ 2 = 604; df = 1; p < 0.0001). From Fig. 3, the 12-month survival probability for statin users (98.6 %) was lower than that for non-statin users (99.3 %). At 18 months, the survival probability for statin users fell to 0.963 (i.e., 96.3 % of statin users survived past 18 months without having diabetes) compared with 98.2 % of non-statin users who survived past 18 months without having diabetes. Thus, statin users had a shorter survival time (and shorter survival probabilities over time) than non-statin users.
3.5 Kaplan–Meier Survival Analysis by Statin Type
Study results showed that the estimated mean survival time (in months) was shortest among rosuvastatin users (16.15, SE 0.03) and increased, consecutively, among lovastatin (17.74, SE 0.02), fluvastatin (17.77, SE 0.03), simvastatin (17.80, SE 0.01), and atorvastatin (17.81, SE 0.01) users. Pravastatin (17.83, SE 0.02) users had the longest estimated mean survival time.
In addition, Fig. 4 shows six Kaplan–Meier survival curves comparing the survival probabilities against time among users of different statin types. The log-rank test comparing the distributions of the six survival curves showed that at least one of the survival curves significantly differed from another (χ 2 = 20.5; df = 5; p = 0.001).
3.6 Cox Proportional Hazards Regression Models
Table 3 shows the results of the Cox proportional hazards regression model comparing the hazard of diabetes between statin users and non-statin users and between users of each statin type and non-statin users while controlling for demographic and clinical covariates. Sensitivity analysis of the hazard ratios (HRs) adjusting for significant time-dependent covariates and utilizing propensity score adjustment is also presented.
Compared with no statin use, and controlling for covariates, statin use (as a class) was significantly associated with increased risk of incident diabetes mellitus (HR 2.01; 99 % confidence interval [CI] 1.74–2.33; p < 0.0001). In other words, the hazard of incident diabetes for statin users was two times that for non-statin users.
Except for pravastatin use, which was associated with a non-significant increase in diabetes risk, the use of atorvastatin, fluvastatin, lovastatin, rosuvastatin, or simvastatin was significantly associated with an increased risk of incident diabetes. The risk of diabetes was highest among lovastatin users (HR 2.07) and lowest among pravastatin users (HR 1.29).
3.7 Logistic Regression Models
Table 4 shows the results of the logistic regression model comparing the odds of incident diabetes between statin users and non-statin users and between users of each statin type and non-statin users while controlling for demographic and clinical covariates. Sensitivity analysis of the odds ratios (ORs) utilizing propensity score adjustment is also presented. Except for the non-significance of the OR for the rosuvastatin group, the results of the logistic regression did not differ significantly from the results obtained for the Cox regression in terms of the magnitude and significance of the HRs and ORs.
The results obtained for the logistic regression model also suggest that statin use was significantly associated with increased risk of diabetes. Except for pravastatin and rosuvastatin users, who had non-significant increases in risk, diabetes risk was significantly associated with the use of atorvastatin, fluvastatin, lovastatin, or simvastatin. The risk of diabetes was highest among lovastatin users (OR 2.05) and lowest among rosuvastatin users (OR 1.21).
3.8 Sensitivity Analysis
As presented above, the results obtained for the Cox and logistic regression analyses were similar in terms of the magnitude and significance of the HRs and ORs. In addition, compared with the original Cox regression models, where only demographic and clinical covariates were adjusted for, further adjusting for significant time-dependent covariates did not change the magnitude and significance of the HRs (Table 3). However, the use of propensity score adjustment in lieu of including all covariates in the original Cox model was associated with (1) an increase in the magnitude of the HRs for all statin users and users of each statin type, and (2) the HR for pravastatin becoming significant (p = 0.019 vs. p = 0.003).
Similar to above, using a propensity score adjustment in lieu of including all covariates in the original logistic regression model resulted in an increase in the magnitude of the OR for all statin users and users of each statin type. In addition, the HR for pravastatin became significant (p = 0.01 vs. p = 0.001). However, the association of rosuvastatin therapy and risk of diabetes remained non-statistically significant even with propensity score adjustment (p = 0.19 vs. p = 0.05) (see Table 4).
Furthermore, because of the potential problem of immortal time bias, we analyzed the data without requiring subjects to have a treatment duration of at least 6 months before a diagnosis of diabetes could be considered valid. Results of this sensitivity analyses revealed that the magnitude of the HRs increased for statin users and users of each statin type, since more cases of incident diabetes would be included. In this sensitivity analysis, the HR comparing the risk of diabetes among statin users with that of non-statin users are as follows: all statin users (HR 2.75), atorvastatin (HR 2.43), fluvastatin (HR 2.06), lovastatin (HR 3.41), pravastatin (HR 1.89), rosuvastatin (HR 1.62), and simvastatin (HR 1.53). All associations were significant at p < 0.01.
4 Discussion
4.1 Incidence of Diabetes
The unadjusted cumulative incidence of diabetes (over 2003–2004) among this study sample aged 20–63 years was 23.2 per 1000 population. This rate is higher than the unadjusted incidence of diabetes of 7.8 per 1000 population of US adults aged ≥20 years [12]. This discrepancy may be because statin users constituted half the study population at risk in this study, and statin users may be more likely than the average population to have several risk factors for diabetes. Thus, it might be reasonable that the rate of incident diabetes for the current study population would be higher than that of the US population that does not have half its population at risk composed essentially of statin users.
However, the unadjusted diabetes incident density rate obtained among this study population (1.54 per 1000 person-months) was comparable to rates (unadjusted) obtained in similar population-based observational studies of statin use and incidence of diabetes as reported by Culver et al. [11] (0.85 per 1000 person-months), Danaei et al. [19] (0.96 per 1000 person-months), and Wang et al. [20] (1.75 per 1000 person-months).
Furthermore, only one observational study has compared diabetes incident density rates between statin users and non-statin users [19]. The unadjusted diabetes incident density rates reported among statin users (2.29 per 1000 person-months) and non-statin users (0.8 per 1000 person-months) in this study were comparable to those reported in the Danaei et al. [19] study, which examined the risk of type 2 diabetes mellitus among 285,864 men and women aged 50–84 years. In that study, the unadjusted diabetes incidence density rates for statin users (or statin “initiators”) and non-statin users (or statin “non-initiators”) were 1.30 per 1000 person-months and 0.94 per 1000 person-months, respectively [19].
4.2 Statin Use and Risk of Diabetes
The results of the Cox and logistic regression analyses showed that the risk of incident diabetes was higher among statin users than among non-statin users. Even though the strength of association (or magnitude of the risk ratios) of statin use and incidence of diabetes was higher in this study (HR 2.01 and OR 2.01), the increase in the risk of diabetes that was associated with statin therapy, compared with no statin therapy, is consistent with increases reported in several observational studies. Statin therapy was significantly associated with a 14, 15, 20, and 48 % increase in risk of incident diabetes, respectively, in the retrospective cohort studies by Danaei et al. [19] (HR 1.14, 95 % CI 1.10–1.19), Wang et al. [20] (HR 1.15, 95 % CI 1.08–1.22), and Zaharan et al. [21] (HR 1.20, 95 % CI 1.17–1.23), and the prospective cohort study by Culver et al. [11] (HR 1.48, 95 % CI 1.38–1.59). Our study results were more consistent with a 2015 retrospective cohort study of statin use among healthy US adults by Mansi et al. [22], which reported an 87 % increase in the odds of diabetes with statin use (OR 1.87, 95 % CI 1.67–2.01).
Meanwhile, the 2013 prospective cohort study by Izzo et al. [27] (HR 1.03, 95 % CI 0.79–1.35) and the 2004 case–control study by Jick and Bradbury [26] (OR 1.1, 95 % CI 0.8–1.4) found that statin therapy was not significantly associated with increased risk of diabetes mellitus.
4.3 Comparison of the Risk of Diabetes Among Users of Different Statin Types
The results of the Cox and logistic regression analyses showed that lovastatin users (HR 2.07, OR 2.05) had the highest risk of new-onset diabetes; this was followed, consecutively, by users of atorvastatin (HR 1.88, OR 1.89), simvastatin (HR 1.71, OR 1.74), and fluvastatin (HR 1.60, OR 1.64). Rosuvastatin (HR 1.58, OR 1.21) and pravastatin (HR 1.29, OR 1.33) users had the least risk of new-onset diabetes among all statin users.
The potency hypothesis [32]—which correlates higher statin potencies with more side effects—does not seem to explain why the risks of diabetes were highest among users of lovastatin, atorvastatin, simvastatin, or fluvastatin since higher potency statins such as atorvastatin, rosuvastatin, and simvastatin would be expected to have consistently higher risk ratios than lower potency statins such as lovastatin, pravastatin, and fluvastatin.
Evaluation of current observational studies of statin use and incidence of diabetes [11, 19–21, 26, 27, 33–37] appears to suggest that simvastatin and atorvastatin had the greatest potential to be significantly associated with increased risk of incident diabetes, while fluvastatin and lovastatin had the least potential to be significantly associated with an increase in risk. Pravastatin and rosuvastatin appear to have moderate potential to be significantly associated with increased risk of diabetes. This observation is partly consistent with our results, which showed that lovastatin, atorvastatin, simvastatin, and fluvastatin had the strongest association with risk of diabetes, while pravastatin and rosuvastatin had moderate associations with diabetes risk.
4.4 Study Strengths and Limitations
This study confirmed the hypothesis that statin therapy is associated with an increase in risk of diabetes and helped fill some gaps in the literature because there are few observational studies examining this phenomenon using US population data. This is significant because the US population may differ from other populations in terms of the risk factors for diabetes mellitus. The results of this study further confirm the findings of other studies conducted among US adult populations, where a higher diabetes risk was associated with statin therapy [10, 11, 22].
The current study has some limitations; however, it is worthwhile to acknowledge some of its merits. First, the study was adequately powered to detect significant associations between statin use and incidence of diabetes if they truly existed. Second, the study utilized two different statistical approaches, including the use of propensity score covariate adjustment, to estimate the risk of diabetes associated with statin use. To our knowledge, this is the first observational study of statin use and incidence of diabetes to conduct several sensitivity analyses utilizing a combination of robust statistical methodologies.
Despite these study strengths, it is important that the study results be interpreted in light of its limitations. One of the main study limitations was the possibility of disease misclassification. Because the data were limited to 2 years in length, a short pre-index period (ranging between 6 months and 1 year) was used to identify and exclude prevalent diabetes cases. This has the potential to increase diabetes incidence among the study population, as some prevalent diabetes cases may be misclassified as incident diabetes cases. However, this limitation might be attenuated by both statin users and non-statin users being equally exposed to the same sets of study inclusion and exclusion criteria and by further excluding diabetes cases that occurred within 6 months of subjects starting their medications. In addition, the length of the pre-index period used to exclude prevalent diabetes cases did not significantly differ between statin users and non-statin users. However, utilizing a longer pre-index period to exclude prevalent diabetes cases would have strengthened the argument for the associations observed.
Second, we were not able to control for some variables that may increase diabetes risk in one group compared with another, independent of statin use. These variables include race/ethnicity, family history of diabetes, cholesterol level, body mass index, and presence or absence of prediabetes. We attempted to attenuate these limitations by (1) adjusting for disease comorbidities (using the CCI score, which accounted for baseline diseases such as myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatologic disease, peptic ulcer disease, mild or severe liver disease, hemiplegia, renal disease, any malignancy, and AIDS) and accounting for the use of diabetogenic medications, (2) controlling for obesity (though under-reported in the data), hypertension, and hyperlipidemia diagnoses, and (3) utilizing a regression-based propensity score covariate adjustment. In particular, obesity was under-reported in our data and may not be adequately controlled for. Since obesity and metabolic syndrome are important risk factors for diabetes and may be more prevalent among statin users, it is possible that the increased risk of diabetes observed among statin users is partly explained by these inadequately controlled-for variables. Future observational studies should fully adjust for these important variables that could account for the increased risk of diabetes, irrespective of statin therapy.
Finally, the MarketScan Database that was used for this study used data collected in 2003–2004 and did not include people aged ≥65 years. Changes in statin prescribing and utilization patterns means there is a possibility that study results might be different if current data were applied. In addition, bias could be introduced because older people may have more comorbidities and risk factors for diabetes. Furthermore, the dataset was sourced mainly from large employers with private insurance and, therefore, may not generalize well to the entire US population or to other US populations such as Medicaid, Medicare, and uninsured populations.
4.5 Study Implications
Although the benefit of statins in primary and secondary prevention of cardiovascular disease is well-documented, this study and several other studies have suggested that statins are associated with a moderate increase in risk of new-onset diabetes. These previous observations prompted the FDA to revise statin labels to include a warning of an increased risk of incident diabetes mellitus [1]. Even though the precise pathway by which statins induce incident diabetes is still unclear, statins are thought to worsen glycemic control and increase FPG and insulin resistance, thereby possibly leading to diabetes mellitus [10].
Because types and doses of statin differ in their ability to reduce low-density lipoprotein cholesterol (i.e., the “bad” cholesterol) as well as in their diabetogenic potential, Navarese et al. [32] suggested there might be a need for physicians to individualize statin therapy, especially among people with low cardiovascular risk. This tailored therapy should be based on sound clinical judgment, the patient’s overall cardiovascular risk and metabolic profile, and the type and dose of statin used [32]. According to Navarese et al. [32], pravastatin seems to be the least diabetogenic statin currently available on the market, and it could be ideal for patients with hyperlipidemia who have a low risk of cardiovascular disease but who have a high predisposition for diabetes. The results of this study also support this argument, as the risk of diabetes was lowest among pravastatin and rosuvastatin users. However, it should be noted that even though study results indicate that statin therapy may be associated with increased diabetes risk, there is evidence that the cardiovascular benefits offered by statin therapy may outweigh the potential for increased risk in diabetes. The meta-analysis of 13 statin trials with 91,140 participants by Sattar et al. [8] indicated that statin therapy was associated with 5.4 fewer deaths from coronary heart disease and non-fatal myocardial infarction per 255 patients treated over 4 years. This is in contrast to only one additional case of new-onset diabetes recorded per 255 patients treated with statins over 4 years [8].
5 Conclusion
The results of the study lend support to the hypothesis that statin therapy is significantly associated with increased risk of new-onset diabetes. This increased risk was found not only in the use of statins as a class, but each statin type was also significantly associated with an increased risk of incident diabetes mellitus. Nevertheless, healthcare professionals can use a targeted approach to optimize the management of their patients by identifying those who would benefit more from less diabetogenic statin types.
References
US Food and Drug Administration. FDA Drug Safety Communication: Important safety label changes to cholesterol-lowering statin drugs. 2012. http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm. Accessed 12 May 2012.
Koh KK, Quon MJ, Han SH, Lee Y, Kim SJ, Shin EK. Atorvastatin causes insulin resistance and increases ambient glycemia in hypercholesterolemic patients. J Am Coll Cardiol. 2010;55(12):1209–16. doi:10.1016/j.jacc.2009.10.053.
Sabatine MS, Wiviott SD, Morrow DA, McCabe CH, Cannon CP. High-dose atorvastatin associated with worse glycemic control: a PROVE-IT TIMI 22 substudy. Circ J. 2004;110(Suppl I):S834.
Thongtang N, Ai M, Otokozawa S, Himbergen TV, Asztalos BF, Nakajima K, et al. Effects of maximal atorvastatin and rosuvastatin treatment on markers of glucose homeostasis and inflammation. Am J Cardiol. 2011;107(3):387–92. doi:10.1016/j.amjcard.2010.09.031.
Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–207. doi:10.1056/NEJMoa0807646.
Mills EJ, Wu P, Chong G, Ghement I, Singh S, Akl EA, et al. Efficacy and safety of statin treatment for cardiovascular disease: a network meta-analysis of 170,255 patients from 76 randomized trials. QJM. 2011;104(2):109–24. doi:10.1093/qjmed/hcq165.
Rajpathak SN, Kumbhani DJ, Crandall J, Barzilai N, Alderman M, Ridker PM. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care. 2009;32(10):1924–9. doi:10.2337/dc09-0738.
Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375(9716):735–42. doi:10.1016/s0140-6736(09)61965-6.
Kostapanos MS, Liamis GL, Milionis HJ, Elisaf MS. Do statins beneficially or adversely affect glucose homeostasis? Curr Vasc Pharmacol. 2010;8(5):612–31.
Sukhija R, Prayaga S, Marashdeh M, Bursac Z, Kakar P, Bansal D, et al. Effect of statins on fasting plasma glucose in diabetic and nondiabetic patients. J Investig Med. 2009;57(3):495–9. doi:10.231/JIM.0b013e318197ec8b.
Culver AL, Ockene IS, Balasubramanian R, Olendzki BC, Sepavich DM, Wactawski-Wende J, et al. Statin use and risk of diabetes mellitus in postmenopausal women in the Women’s Health Initiative. Arch Intern Med. 2012;172(2):144–52. doi:10.1001/archinternmed.2011.625.
US Centers for Disease Control and Prevention. National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. Atlanta: US Department of Health and Human Services; 2014.
Centers for Disease Control and Prevention. Leading causes of death. 2015. http://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm. Accessed 28 Nov 2015.
American Diabetes Association. Economic costs of diabetes in the US in 2012. Diabetes Care. 2013;36(4):1033–46. doi:10.2337/dc12-2625.
Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623–30. doi:10.1016/S0140-6736(02)11600-X.
Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615–22. doi:10.1001/jama.279.20.1615.
Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333(20):1301–7. doi:10.1056/NEJM199511163332001.
Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins: a study-level network meta-analysis of 246 955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes. 2013;6(4):390–9. doi:10.1161/CIRCOUTCOMES.111.000071.
Danaei G, García Rodríguez LA, Fernandez Cantero O, Hernán MA. Statins and risk of diabetes: an analysis of electronic medical records to evaluate possible bias due to differential survival. Diabetes Care. 2013;36(5):1236.
Wang KL, Liu CJ, Chao TF, Huang CM, Wu CH, Chen SJ, et al. Statins, risk of diabetes, and implications on outcomes in the general population. J Am Coll Cardiol. 2012;60(14):1231–8. doi:10.1016/j.jacc.2012.05.019.
Zaharan NL, Williams D, Bennett K. Statins and risk of treated incident diabetes in a primary care population. Br J Clin Pharmacol. 2013;75(4):1118–24. doi:10.1111/j.1365-2125.2012.04403.x.
Mansi I, Frei CR, Wang CP, Mortensen EM. Statins and new-onset diabetes mellitus and diabetic complications: a retrospective cohort study of US healthy adults. J Gen Intern Med. 2015;30(11):1599–610. doi:10.1007/s11606-015-3335-1.
Coleman CI, Reinhart K, Kluger J, White CM. The effect of statins on the development of new-onset type 2 diabetes: a meta-analysis of randomized controlled trials. Curr Med Res Opin. 2008;24(5):1359–62. doi:10.1185/030079908x292029.
Navarese EP, Buffon A, Andreotti F, Kozinski M, Welton N, Fabiszak T, et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol. 2013;111(8):1123–30. doi:10.1016/j.amjcard.2012.12.037.
Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368(9542):1155–63. doi:10.1016/s0140-6736(06)69472-5.
Jick SS, Bradbury BD. Statins and newly diagnosed diabetes. Br J Clin Pharmacol. 2004;58(3):303–9. doi:10.1111/j.1365-2125.2004.02142.x.
Izzo R, de Simone G, Trimarco V, Giudice R, De Marco M, Di Renzo G, et al. Primary prevention with statins and incident diabetes in hypertensive patients at high cardiovascular risk. Nutr Metab Cardiovasc Dis. 2013;. doi:10.1016/j.numecd.2012.11.002.
Graham I, Cooney MT, Bradley D, Dudina A, Reiner Z. Dyslipidemias in the prevention of cardiovascular disease: risks and causality. Curr Cardiol Rep. 2012;14(6):709–20. doi:10.1007/s11886-012-0313-7.
Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377(9765):557–67. doi:10.1016/s0140-6736(10)62037-5.
Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46(10):1075–9. doi:10.1016/0895-4356(93)90103-8 (discussion 81–90).
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi:10.1080/00273171.2011.568786.
Navarese EP, Szczesniak A, Kolodziejczak M, Gorny B, Kubica J, Suryapranata H. Statins and risk of new-onset diabetes mellitus: is there a rationale for individualized statin therapy? Am J Cardiovasc Drugs. 2014;14(2):79–87. doi:10.1007/s40256-013-0053-0.
Chen CW, Chen TC, Huang KY, Chou P, Chen PF, Lee CC. Differential impact of statin on new-onset diabetes in different age groups: a population-based case–control study in women from an Asian country. PLoS One. 2013;8(8):e71817. doi:10.1371/journal.pone.0071817.
Carter AA, Gomes T, Camacho X, Juurlink DN, Shah BR, Mamdani MM. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610. doi:10.1136/bmj.f2610.
Ko DT, Wijeysundera HC, Jackevicius CA, Yousef A, Wang J, Tu JV. Diabetes mellitus and cardiovascular events in older patients with myocardial infarction prescribed intensive-dose and moderate-dose statins. Circ Cardiovasc Qual Outcomes. 2013;6(3):315–22. doi:10.1161/circoutcomes.111.000015.
Ma T, Chang MH, Tien L, Liou YS, Jong GP. The long-term effect of statins on the risk of new-onset diabetes mellitus in elderly Taiwanese patients with hypertension and dyslipidaemia: a retrospective longitudinal cohort study. Drugs Aging. 2012;29(1):45–51. doi:10.2165/11597250-000000000-00000.
Ma T, Tien L, Fang C-L, Liou Y-S, Jong G-P. Statins and new-onset diabetes: a retrospective longitudinal cohort study. Clin Ther. 2012;34(9):1977–83. doi:10.1016/j.clinthera.2012.08.004.
Acknowledgments
The authors acknowledge Thomson Reuters for providing us with the 2003–2004 MarketScan data as part of the Thomson Reuter’s MarketScan Dissertation Support Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study complied with standards required for observational study of de-identified data. The study did not involve human subjects.
Funding
No external funding was used in the preparation of this manuscript.
Conflict of interest
All authors, Busuyi Olotu, Marvin Shepherd, Suzanne Novak, Kenneth Lawson, James Wilson, Kristin Richards, and Rafia Rasu, declare they have no conflicts of interest that might be relevant to the contents of this manuscript.
Rights and permissions
About this article
Cite this article
Olotu, B.S., Shepherd, M.D., Novak, S. et al. Use of Statins and the Risk of Incident Diabetes: A Retrospective Cohort Study. Am J Cardiovasc Drugs 16, 377–390 (2016). https://doi.org/10.1007/s40256-016-0176-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-016-0176-1