Abstract
Anticoagulant-related nephropathy, a recently recognized entity, manifests as unexplained acute kidney injury in the setting of excessive anticoagulation with oral agents. Histologic findings in warfarin-related nephropathy include glomerular hemorrhage and renal tubular obstruction by red blood cells. Affected patients are at increased risk of mortality as well as irreversible kidney injury. Patients with chronic kidney disease are particularly vulnerable to this complication. Similar case reports of anticoagulant-related nephropathy have been linked to the more novel oral anticoagulant, dabigatran. Anticoagulant-related nephropathy has been successfully reproduced in rat models. These animal models shed light on the pathogenesis of the disease including the potential role of direct thrombin and protease-activated receptor-1 inhibition. Warfarin and dabigatran also cause an increase in systolic blood pressure in rats, a risk factor for developing nephropathy. This article reviews the current evidence for anticoagulant-related nephropathy and provides data for the suggested possible mechanisms underlying this adverse effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Anticoagulant-related nephropathy has been associated with warfarin and dabigatran use. |
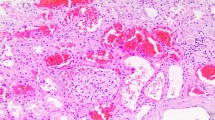
Glomerular hemorrhage is the most consistent histologic finding described. |
Chronic kidney disease is a major risk factor for over-anticoagulation and the subsequent development of anticoagulant-related nephropathy, potentially leading to irreversible kidney injury. |
Presumptive warfarin-related nephropathy is associated with increased mortality risk. |
In animal models, warfarin and dabigatran cause a dose-dependent increase in blood pressure. |
1 Introduction
Anticoagulants are a class of drugs that prevent blood clotting. Ever since its introduction, warfarin has been the only available oral anticoagulant for more than half a century. It is estimated that more than 30 million warfarin prescriptions are filled annually in the USA [1]. Warfarin produces its anticoagulant effects by interfering with the synthesis of vitamin K-dependent clotting factors through inhibition of the C1 subunit of the vitamin K epoxide reductase enzyme complex [2]. It is primarily metabolized through the cytochrome P450 pathway [3] and has a particularly narrow therapeutic range requiring close monitoring. Maintaining therapeutic concentrations is a challenge because of genetic influences affecting the drug metabolism [4], the interaction of warfarin with dietary factors [5], and commonly used medications such as antibiotics [6, 7]. This results in frequent complications, the most common of which is hemorrhage [2]. A meta-analysis showed that 44 % of bleeding complications with warfarin were associated with supratherapeutic international normalized ratios (INRs) [8]. Shortcomings attributed to frequent monitoring of INRs, difficulties in attaining optimal anticoagulation with warfarin owing to its slow onset of action, variable pharmacologic effects, and numerous food and drug interactions led to the development of new oral anticoagulants that target key coagulation factors, such as factors Xa and thrombin. Clinicians now have a broader choice of agents with newer oral anticoagulants such as dabigatran, rivaroxaban, and apixaban that have been approved by the US Food and Drug Administration (FDA) as well as the European Medicines Agency (EMA) [9]. While edoxaban has been approved for clinical use by the FDA, it is still under evaluation for human use by the EMA. The major advantage of these newer anticoagulants is that they can be administered in fixed doses without coagulation monitoring because of their predictable anticoagulant effect and that they have an overall favorable effect with respect to the risk of stroke and systemic embolism, major bleeding, total and cardiovascular mortality, and intracranial bleeding when compared with warfarin [10, 11]. Apixaban can be used in advanced chronic kidney disease (CKD) and even in end-stage renal disease [12], but the use of dabigatran, rivaroxaban, and edoxaban is not recommended in patients with advanced CKD [13–15].
The major potential renal complication associated with oral anticoagulants in the setting of over-anticoagulation is a type of acute kidney injury that has been termed anticoagulant-related nephropathy (ARN). When ARN occurs with warfarin use it is called warfarin-related nephropathy (WRN). Since the recent introduction of the other novel anticoagulants, there have been case reports of ARN occurring with dabigatran use [16, 17]. Animal studies [17–20] supplemented by observations from retrospective studies [1, 21, 22], prospective studies [23], and case reports [7, 16, 24, 25] have enriched our understanding of the pathogenesis of ARN. This article is a comprehensive review of such data to help us understand the epidemiology and potential mechanisms involved in the development of ARN.
For the purpose of this review, we conducted a published literature search through PubMed using different permutations of term combinations including warfarin, oral anticoagulants, dabigatran, rivaroxaban, apixaban, edoxaban, renal, kidney, acute renal failure, acute kidney injury (AKI), nephropathy, anticoagulant related nephropathy, and warfarin related nephropathy. The only restriction we applied to our search was the English language. No date restrictions were applied. Article types searched included case reports, clinical trials, journal articles, observational studies, randomized control trials, and review articles. We also searched the FDA Adverse Event Reporting System (FAERS) for spontaneous reports of ARN associated with newer anticoagulants using the search terms acute renal failure, acute kidney injury, renal impairment, and anticoagulant related nephropathy from October 2010 to February 2015. DailyMed, the official provider website of FDA drug label information, was used to review oral anticoagulant-related labeling information.
2 Wafarin-Related Nephropathy
WRN due to over-anticoagulation has been recently identified as a significant complication of warfarin therapy. The occurrence of AKI resulting from glomerular hemorrhage in the setting of an INR between 3 and 9 was reported as early as 2000 [26, 27]. However, WRN was first definitively described in 2009 by Brodsky et al. [25] who reviewed renal biopsies in nine CKD patients with unexplained AKI [mean serum creatinine (SCr) level 4.3 ± 0.8 mg/dL] in the setting of excessive warfarin coagulopathy (INR >3.0) [25]. The biopsies in these patients demonstrated evidence of glomerular bleeding, occlusive red blood cell (RBC) casts, and tubular injury [25]. The presence of these specific histologic lesions in the backdrop of unexplained AKI and excessive anticoagulation led to the introduction of a new subcategory of AKI called WRN [1].
Further investigation by Brodsky et al. involved the retrospective study of a cohort of 15,258 patients who initiated warfarin therapy during a 5-year period at the Ohio State University Medical Center. This study showed that WRN potentially occurred in 20.5 % of the entire cohort, 33.0 % of the CKD cohort, and 16.5 % of the no-CKD cohort [1]. Another study among Korean patients taking warfarin noted that WRN developed in 24.0 % of patients with CKD and in 17.4 % of patients without CKD [21]. Both these studies based the diagnosis of WRN on the clinical findings of AKI (increase in SCr by more than 0.3 mg/dL) within 1 week of an INR exceeding 3.0 [1, 21].
The risk of WRN is significantly increased in older patients and in those with diabetes mellitus, diabetic nephropathy, hypertension, and heart failure (Table 1). Medications that increase glomerular hydrostatic pressure (e.g., calcium channel blockers) and those that lower glomerular hydrostatic pressure (e.g., angiotensin converting enzyme inhibitors) have been associated with a statistically significant increased risk of developing WRN. Concomitant aspirin use, owing to its contribution to coagulopathy, has also been shown to increase the risk [1], as well as the presence of genetic polymorphisms that reduce warfarin metabolism predisposing to excessive anticoagulation [4].
CKD patients with excessive anticoagulation are most susceptible [28]. The interaction between CKD and over-anticoagulation with warfarin is interesting because CKD is an independent risk factor for supratherapeutic INR [29] and supratherapeutic INR itself is a risk factor for WRN [1, 21]. Furthermore, because of a decreased rate of carboxylation of clotting factors in CKD patients [30], the reversal of a supratherapeutic INR may be slower in CKD patients, exposing them to heightened risk from the deleterious effects of over-anticoagulation [30]. Data suggest that WRN also has prognostic implications as it accelerates progression of CKD and also increases mortality [22]. One-year mortality was significantly higher in WRN patients compared with those without WRN (31.1 vs. 18.9 %) representing a 65 % increased risk [1]. Similar increase in mortality rates were reported by An et al. [21] among Korean patients with WRN compared with those without WRN (42.8 vs. 26.3 %).
WRN has been reproduced in 5/6 nephrectomized (NE) rat models with morphologic changes identical to those described in humans [28]. Warfarin treatment in 5/6 NE rats leads to a dose-dependent increase in SCr and the animals with more advanced stages of CKD are more susceptible to WRN than those with earlier stages. Furthermore, treatment with vitamin K prevents a warfarin-associated increase in SCr in 5/6 NE rats [18].
The mechanisms causing WRN are multifactorial and likely involve a combination of events that occur simultaneously (Fig. 1). Glomerular hemorrhage caused by excessive anticoagulation from warfarin is an important factor [17]. Kidney biopsies from affected patients and in animal models have demonstrated glomerular hemorrhage [18, 25]. Structurally abnormal glomerular basement membranes as seen in thin and thick basement membrane disease have been described to be particularly vulnerable to glomerular hemorrhage [26–28]. The cellular and biochemical mechanisms that cause glomerular hemorrhage are still not clear [18]. Glomerular hemorrhage results in the obstruction of renal tubules by RBC casts. This obstruction leads to tubular epithelial cell injury. The RBCs within the renal tubules also cause heme toxicity and iron-associated cellular damage [31–33]. Such oxidative stress-induced damage to the renal tubules by RBCs may play a very important role in WRN [34].
Warfarin can also potentially cause direct glomerular damage by inhibiting the activation of vitamin K-dependent proteins, matrix Gla protein, and growth arrest specific gene 6 (GAS-6). These proteins and GAS-6 inhibit vascular calcification and affect vascular smooth muscle cell migration and apoptosis [35]. Their inhibition can induce vascular calcification, interfere with vascular smooth muscle cell migration, and alter glomerular hemodynamics leading to injury [19]. Warfarin also inhibits mesangial cell proliferation by interfering with the activation process of GAS-6 [36]. In rat models, excessive anticoagulation has resulted in an increased number of apoptotic cells in the kidney of endothelial and non-endothelial origin [19].
Besides WRN, warfarin has been reported to cause other rare renal side effects that include leukocytoclastic vasculitis and allergic interstitial nephritis [37–39], spontaneous atheromatous embolism [40], acute renal failure due to bilateral renal pelvis, and ureteral hematomas [41].
3 Dabigatran-Related Nephropathy
While warfarin remains the most common oral anticoagulant used to date, newer oral anticoagulants have been introduced on the market. They consist of direct thrombin inhibitors (dabigatran) and factor Xa inhibitors (apixaban, edoxaban, and rivaroxaban). These newer oral anticoagulants are being prescribed increasingly as they do not require routine coagulation monitoring and have improved clinical outcomes overall including decreased risk of major bleeding, making them particularly attractive alternatives to warfarin [10, 11]. All of the newer oral anticoagulants are renally excreted to varying degrees [15] and to date little is known about their effects on kidney function; however, two recent case reports showed that dabigatran can cause AKI [16, 42]. Dabigatran is approved for the treatment of deep venous thrombosis and pulmonary embolus as well as reducing the risk of recurrence of deep venous thrombosis and pulmonary embolus in patients previously treated. It is also approved for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation [43]. It is worth mentioning that there have been by far more case reports of AKI leading to dabigatran toxicity manifesting as major hemorrhage [44–47]. A FAERS database search did not yield any adverse reports specific to dabigatran-related nephropathy.
Morphologically, the same histologic lesions are seen with dabigatran nephropathy as WRN, namely the presence of glomerular hemorrhage and RBC casts. Studies by Ryan et al. [17] on rat models showed that dabigatran promotes AKI in a dose-dependent manner in 5/6 NE rats. Unlike warfarin, dabigatran causes dose-dependent SCr increases in 5/6 NE rats and control rats with normal kidney function. Based on these observations, the authors have suggested that not just CKD patients, but other patients as well, can be at high risk of developing AKI if the therapeutic range of anticoagulation with dabigatran is exceeded [17]. This observation is clinically relevant because detecting excessive anticoagulation with dabigatran use is difficult as coagulation parameters are neither routinely monitored nor are the tests that reflect anticoagulation with dabigatran widely available. Thus, renal function needs to be constantly monitored and this may outweigh the benefits of not monitoring coagulation parameters with newer anticoagulants [48].
Because the end result of kidney injury from warfarin and dabigatran is histologically similar despite different mechanisms of action for these two drugs, the attention shifted to downstream effects of these anticoagulants. Protease-activated receptor 1 (PAR-1) is a G protein-coupled thrombin receptor expressed on endothelial cells that participates in the regulation of the endothelial functions, vascular permeability, leukocyte migration, and adhesion [49, 50]. In vitro studies have shown that PAR-1 activation changes endothelial monolayer integrity [51]. Warfarin and dabigatran though causing anticoagulation by different mechanisms ultimately result in diminished thrombin activity (Fig. 1). Thrombin activates protein C and modulates the anticoagulation cascade by acting on thrombomodulin that is expressed on endothelial cells. Ryan et al. [17] proposed that thrombin plays an important role in the glomerular filtration barrier function, and its decreased activity owing to anticoagulation results in glomerular filtration barrier abnormalities. They also described that antagonizing PAR-1 using a selective inhibitor (SCH79797) in rat models resulted in increased SCr, hematuria, and tubular RBC casts, findings similar to those described in animals with warfarin- or dabigatran-related nephropathy. The authors hypothesized that there is a common pathway involving thrombin in the pathogenesis of ARN and that this pathway did not include vitamin K-dependent proteins. The authors concluded that the novel thrombin inhibitor dabigatran causes AKI when dosed sufficiently to cause a coagulopathy and suggested that kidney function be closely monitored while on dabigatran therapy.
In rat models, warfarin and dabigatran have been shown to increase systolic blood pressure (SBP) in a dose-dependent manner. Such increases in blood pressure can indirectly cause long-term kidney injury. Similar to the mechanism involving thrombin and PAR-1 detailed above, the authors proposed that thrombin plays an important role in the regulation of vascular tone by endothelial cells, and decreased thrombin activity, secondary to anticoagulant use, results in SBP changes [20]. This effect of warfarin on blood pressure has been observed in humans where certain subsets at higher baseline cardiovascular risk have shown significant increases in SBP or pulse pressure or both after 12 months of warfarin therapy [52]. Hypertension is an independent risk factor for WRN and progression of CKD.
4 Other Factor Xa Inhibitors
Our literature search did not yield any reports of ARN occurring with other factor Xa inhibitors besides dabigatran. Review of quarterly safety reports posted by the FAERS database on their website since approval of the newer anticoagulants did not yield any safety alerts for acute kidney injury related to newer anticoagulants use. Further, referencing of FDA label information for newer anticoagulants using the FDA’s official provider website DailyMed did not show acute kidney injury as a listed adverse effect based on data from clinical trial experience and postmarketing experience. Despite these observations it is still plausible that the other factor Xa inhibitors could cause ARN [53] based on the potential proposed role of thrombin inhibition in the pathogenesis of renal toxicity as described above. The jury is therefore still out given that these drugs are relatively new.
5 Discussion and Conclusions
In conclusion, the occurrence of ARN in the setting of over-anticoagulation is probably real but there are many questions related to the incidence, prevalence, and pathogenesis that remain unanswered. Most human studies available to date have been retrospective in nature where the cases were identified based on presumptive diagnosis defined by an elevation in creatinine that was temporally related to an abnormally elevated INR. Such a presumptive approach may not be very accurate as the diagnosis is not confirmed by kidney biopsy. Data collection by using chart reviews and diagnostic codes to identify cases may not have rigorously excluded other causes of AKI. This could lead to the overestimation of incident and prevalent cases. It is also difficult to determine the incidence of WRN as patients who have coagulopathy often do not get biopsied because of concerns of increased bleeding risk [1]. The prevalence estimates of WRN may be dampened by the increased mortality among those with WRN and CKD. In one study, approximately 30 % of such patients died within a year after the onset of WRN [1]. With specific reference to the mechanisms of WRN, it is still unclear if the toxicity of warfarin is related to consequences of blood in the glomerulus and renal tubules and heme release from RBCs or the effects of direct warfarin toxicity on the glomerular cells and renal microvasculature. The animal models used have their shortcomings. Ryan et al. [17] pointed out that ablative nephropathy in 5/6 NE rats can itself make the kidneys more sensitive to changes in coagulation disturbances owing to alterations in PAR-1. Thus, exclusive attribution of ARN changes to oral anticoagulants in treated rats would be simplistic [17]. To clearly establish mechanisms and risk factors for WRN and its consequences will require well-defined prospective studies and randomized control studies. Until then, what is absolutely clear is the fact that anticoagulation should be closely monitored, specifically in older patients who have multiple co-morbidities that put them at risk of renal injury and in those with CKD who are at increased risk of ARN, with every effort made to prevent over-anticoagulation events. Prevention is the best way to avoid ARN.
References
Brodsky SV, Nadasdy T, Rovin BH, Satoskar AA, Nadasdy GM, Wu HM, et al. Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int. 2011;80(2):181–9.
Rizk DV, Warnock DG. Warfarin-related nephropathy: another newly recognized complication of an old drug. Kidney Int. 2011;80(2):131–3.
Limdi NA, Limdi MA, Cavallari L, Anderson AM, Crowley MR, Baird MF, et al. Warfarin dosing in patients with impaired kidney function. Am J Kidney Dis. 2010;56(5):823–31.
Di Maso V, Carraro M, Bevilacqua E, Bucconi S, Artero ML, Boscutti G. Warfarin-related nephropathy: possible role for the warfarin pharmacogenetic profile. Clin Kidney J. 2014;7(6):605–8.
Franco V, Polanczyk CA, Clausell N, Rohde LE. Role of dietary vitamin K intake in chronic oral anticoagulation: prospective evidence from observational and randomized protocols. Am J Med. 2004;116(10):651–6.
Ellis RJ, Mayo MS, Bodensteiner DM. Ciprofloxacin-warfarin coagulopathy: a case series. Am J Hematol. 2000;63(1):28–31.
Nemoto C, Ikegami Y, Shimada J, Tsukada Y, Abe Y, Tase C. Acute renal failure caused by severe coagulopathy induced by the interaction between warfarin potassium and levofloxacin: a case report. J Anesth. 2012;26(6):943–4.
Oake N, Fergusson DA, Forster AJ, van Walraven C. Frequency of adverse events in patients with poor anticoagulation: a meta-analysis. CMAJ. 2007;176(11):1589–94.
Yang E. A clinician’s perspective: novel oral anticoagulants to reduce the risk of stroke in nonvalvular atrial fibrillation—full speed ahead or proceed with caution? Vasc Health Risk Manag. 2014;10:507–22.
Rui P, Erik Lerkevang G, Steen H, Sergio B, Serge B, Joao M. A meta-analysis of phase III randomized controlled trials with novel oral anticoagulants in atrial fibrillation: comparisons between direct thrombin inhibitors vs. factor Xa inhibitors and different dosing regimens. Thromb Res. 2014;134(6):1253–64.
Bacchus F, Schulman S. Clinical experience with the new oral anticoagulants for treatment of venous thromboembolism. Arterioscler Thromb Vasc Biol. 2015;35(3):513–9.
Deal EN, Pope H, Ross W. Apixaban use among patients with severe renal impairment. Ann Pharmacother. 2014;48(12):1667.
Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuunemann HJ. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):7s–47s.
Poulsen BK, Grove EL, Husted SE. New oral anticoagulants: a review of the literature with particular emphasis on patients with impaired renal function. Drugs. 2012;72(13):1739–53.
Dempfle C-E. Direct oral anticoagulants: pharmacology, drug interactions, and side effects. Semin Hematol. 2014;51(2):89–97.
Shafi ST, Negrete H, Roy P, Julius CJ, Sarac E. A case of dabigatran-associated acute renal failure. WMJ. 2014;112:173–5.
Ryan M, Ware K, Qamri Z, Satoskar A, Wu H, Nadasdy G, et al. Warfarin-related nephropathy is the tip of the iceberg: direct thrombin inhibitor dabigatran induces glomerular hemorrhage with acute kidney injury in rats. Nephrol Dial Transplant. 2014;29(12):2228–34.
Ozcan A, Ware K, Calomeni E, Nadasdy T, Forbes R, Satoskar A, et al. 5/6 nephrectomy as a validated rat model mimicking human warfarin-related nephropathy. Am J Nephrol. 2012;35(4):356–64.
Ware K, Brodsky P, Satoskar AA, Nadasdy T, Nadasdy G, Wu H, et al. Warfarin-related nephropathy modeled by nephron reduction and excessive anticoagulation. J Am Soc Nephrol. 2011;22(10):1856–62.
Ware KM, Vance JC, Muni N, Hebert LA, Satoskar AA, Nadasdy G, et al. Oral warfarin and the thrombin inhibitor dabigatran increase blood pressure in rats: hidden danger of anticoagulants? Am J Hypertens. 2015;28(2):182–9.
An JN, Ahn SY, Yoon CH, Youn TJ, Han MK, Kim S, et al. The occurrence of warfarin-related nephropathy and effects on renal and patient outcomes in korean patients. PLoS One. 2013;8(4):e57661.
Brodsky SV, Collins M, Park E, Rovin BH, Satoskar AA, Nadasdy G, et al. Warfarin therapy that results in an International Normalization Ratio above the therapeutic range is associated with accelerated progression of chronic kidney disease. Nephron Clin Pract. 2010;115(2):c142–6.
Lim AK, Campbell DA. Haematuria and acute kidney injury in elderly patients admitted to hospital with supratherapeutic warfarin anticoagulation. Int Urol Nephrol. 2013;45(2):561–70.
Santos C, Gomes AM, Ventura A, Almeida C, Seabra J. An unusual cause of glomerular hematuria and acute kidney injury in a chronic kidney disease patient during warfarin therapy. Nefrologia. 2013;33(3):400–3.
Brodsky SV, Satoskar A, Chen J, Nadasdy G, Eagen JW, Hamirani M, et al. Acute kidney injury during warfarin therapy associated with obstructive tubular red blood cell casts: a report of 9 cases. Am J Kidney Dis. 2009;54(6):1121–6.
Abt AB, Carroll LE, Mohler JH. Thin basement membrane disease and acute renal failure secondary to gross hematuria and tubular necrosis. Am J Kidney Dis. 2000;35(3):533–6.
Kabir A, Nadasdy T, Nadasdy G, Hebert LA. An unusual cause of gross hematuria and transient ARF in an SLE patient with warfarin coagulopathy. Am J Kidney Dis. 2004;43(4):757–60.
Brodsky SV. Anticoagulants and acute kidney injury: clinical and pathology considerations. Kidney Res Clin Pract. 2014;33(4):174–80.
Limdi NA, Beasley TM, Baird MF, Goldstein JA, McGwin G, Arnett DK, et al. Kidney function influences warfarin responsiveness and hemorrhagic complications. J Am Soc Nephrol. 2009;20(4):912–21.
Limdi NA, Nolin TD, Booth SL, Centi A, Marques MB, Crowley MR, et al. Influence of kidney function on risk of supratherapeutic international normalized ratio-related hemorrhage in warfarin users: a prospective cohort study. Am J Kidney Dis. 2014. (Article in press).
Homsi E, Janino P, de Faria JB. Role of caspases on cell death, inflammation, and cell cycle in glycerol-induced acute renal failure. Kidney Int. 2006;69(8):1385–92.
Patel RP, Svistunenko DA, Darley-Usmar VM, Symons MC, Wilson MT. Redox cycling of human methaemoglobin by H2O2 yields persistent ferryl iron and protein based radicals. Free Radical Res. 1996;25(2):117–23.
Tracz MJ, Alam J, Nath KA. Physiology and pathophysiology of heme: implications for kidney disease. J Am Soc Nephrol. 2007;18(2):414–20.
Ware K, Qamri Z, Ozcan A, Satoskar AA, Nadasdy G, Rovin BH, et al. N-Acetylcysteine ameliorates acute kidney injury but not glomerular hemorrhage in an animal model of warfarin-related nephropathy. Am J Physiol Renal Physiol. 2013;304(12):F1421–7.
Chang CC, Liou HH, Wu CL, Chang CB, Chang YJ, Chiu PF, et al. Warfarin slows deterioration of renal function in elderly patients with chronic kidney disease and atrial fibrillation. Clin Interv Aging. 2013;8:523–9.
Yanagita M. Gas6, warfarin, and kidney diseases. Clin Exp Nephrol. 2004;8(4):304–9.
Kapoor KG, Bekaii-Saab T. Warfarin-induced allergic interstitial nephritis and leucocytoclastic vasculitis. Intern Med J. 2008;38(4):281.
Leven C, Hudier L, Picard S, Longuet H, Lorcy N, Cam G, et al. Prospective study of drug-induced allergic nephropathy in eleven French nephrology units. Presse medicale (Paris, France: 1983). 2014;43:e369–76.
Hsu C-Y, Chen W-S, Sung S-H. Warfarin-induced leukocytoclastic vasculitis: a case report and review of literature. Intern Med. 2012;51(6):601–6.
Moll S, Huffman J. Cholesterol emboli associated with warfarin treatment. Am J Hematol. 2004;77(2):194–5.
Gultekin N, Akin F, Kucukates E. Warfarin-induced bilateral renal hematoma causing acute renal failure. Turk Kardiyoloji Dernegi arsivi: Turk Kardiyoloji Derneginin yayin organidir. 2011;39(3):228–30.
Kadiyala D, Brewster UC, Moeckel GW. Dabigatran induced acute kidney injury. In: The American Society of Nephrology annual meeting, November 1–4, 2012, San Diego, CA; 2012. p. FR-PO1122.
Perez A, Eraso LH, Merli GJ. Implications of new anticoagulants in primary practice. Int J Clin Pract. 2013;67(2):139–56.
Bachellerie B, Ruiz S, Conil JM, Crognier L, Seguin T, Georges B, et al. Patient with acute renal injury presenting dabigatran overdose: hemodialysis for surgery. Ann Fr Anesth Reanim. 2014;33(1):44–6.
Fountzilas C, George J, Levine R. Dabigatran overdose secondary to acute kidney injury and amiodarone use. NZ Med J. 2013;126(1370):110–2.
Maddry JK, Amir MK, Sessions D, Heard K. Fatal dabigatran toxicity secondary to acute renal failure. Am J Emerg Med. 2013;31(2):462.e1–2.
Wychowski MK, Kouides PA. Dabigatran-induced gastrointestinal bleeding in an elderly patient with moderate renal impairment. Ann Pharmacother. 2012;46(4):e10.
Mantha S, Cabral K, Ansell J. New avenues for anticoagulation in atrial fibrillation. Clin Pharmacol Ther. 2013;93(1):68–77.
Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3(8):1800–14.
Coughlin SR. Protease-activated receptors in vascular biology. Thromb Haemost. 2001;86(1):298–307.
Carlile-Klusacek M, Rizzo V. Endothelial cytoskeletal reorganization in response to PAR1 stimulation is mediated by membrane rafts but not caveolae. Am J Physiol Heart Circ Physiol. 2007;293(1):H366–75.
Krishnan S, Chawla N, Ezekowitz MD, Peixoto AJ. Warfarin therapy and systolic hypertension in men with atrial fibrillation. Am J Hypertens. 2005;18(12 Pt 1):1592–9.
Sergey V, Brodsky M, BH Rovin, LA Hebert. Anticoagulant-related nephropathy. In: UpToDate, Post TW, editor, UpToDate, Waltham; 2015. http://www.uptodate.com/contents/anticoagulant-related-nephropathy. Accessed 15 Feb 2015.
Funding and conflict of interest
No sources of funding were used to assist in the preparation of this article. Vinay Narasimha Krishna, David G. Warnock, Nakshatra Saxena, and Dana V. Rizk have no conflicts of interest that are directly relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Narasimha Krishna, V., Warnock, D.G., Saxena, N. et al. Oral Anticoagulants and Risk of Nephropathy. Drug Saf 38, 527–533 (2015). https://doi.org/10.1007/s40264-015-0290-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-015-0290-z