Abstract
Background
The effects of maternal use of medicines during pregnancy on tooth development has scarcely been studied; only negative effects of tetracycline on tooth germs are known (irreversible tooth discoloration and enamel hypoplasia).
Objective
The aim of this study was to investigate whether antibacterials and anti-allergic and anti-asthma medicines, being the most frequently used medicines during pregnancy, are associated with deciduous molar hypomineralisation (DMH) and, if so, which specific medicines.
Materials and Methods
To clarify this possible association, the participants of the Generation R Study, a population-based prospective cohort study from fetal life until young adulthood, were studied. Data on medicine use during pregnancy were retrieved from pharmacies. Clinical photographs of the second primary molars, which were scored for DMH, were taken with an intra-oral camera in 6,690 children (mean age 6.2 years, standard deviation [SD] ± 0.53; 49.9 % girls).
Results
During pregnancy, 20.3 % of the mothers used antibacterials, 12.3 % anti-asthma medicines and 5.4 % anti-allergic medicines. The prevalence of DMH was 9.0 % in the study group. There was no association between the use of anti-asthma medicines, anti-allergic medicines (odds ratio [OR]: 0.97 [95 % CI 0.61–1.54]; OR: 1.04 [0.54–2.03]) or antibacterials (OR: 0.73 [0.49–1.09]) during pregnancy and DMH (all p-values >0.05). The study had sufficient power (80 %) to detect significant associations.
Conclusion
Maternal use of antibacterials, anti-allergic medicines or anti-asthma medicines during pregnancy is not associated with the development of DMH in the offspring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background
Certain medicines are known to induce changes in the teeth, such as discoloration and physical damage to the tooth structure [1]. Extrinsic tooth discoloration is often seen when chlorhexidine, amoxicillin with clavulanic acid, essential oils and oral iron salts in liquid form are used [1]. Intrinsic tooth discoloration, which is permanent, occurs when the medicine has been used during tooth formation. The most common minerals and medicines causing intrinsic tooth discoloration are fluorides, tetracyclines (including minocycline) and ciprofloxacin [1]. However, in animal studies more antibacterials have been demonstrated to affect the amelogenesis [2, 3]. Antibacterials were associated with early childhood caries and probably also with tooth fluorosis [4, 5]. Amoxicillin, the most commonly prescribed antibacterial [6], has been associated with molar incisor hypomineralisation (MIH) [3, 7]. Anti-asthma medicine and severe demarcated opacities, which are part of MIH, also seemed to be associated [8]. Anti-allergic medicines, also frequently used [6], are known to give a dry mouth [9], which can negatively influence the caries progression. The influence of its maternal use on the development of the teeth of the child is not known [9].
MIH is a disturbance of the enamel of 1–4 first permanent molars, sometimes in combination with hypomineralised incisors [10]. MIH is the most prevalent dental developmental disturbance in the permanent dentition: in The Netherlands, the prevalence is 14.3 % [11].
Deciduous molar hypomineralisation (DMH) is a common hypomineralisation disturbance in the deciduous dentition in 1–4 second primary molars [12]. Its occurrence is related to the occurrence of MIH [12, 13]. For DMH and MIH, the same possible causes (perinatal problems and common childhood diseases) were suggested, although occurring somewhat earlier in life for DMH than for MIH [7, 12, 14–21]. The tooth development period of second primary molars starts around the 18th week of pregnancy and continues in the first year of life of the child, while for the first permanent molar the development starts around birth and continues until the age of 3 years [22]. Consequently, it is possible that exposure to asthma, anti-allergic and/or antibacterial treatment in utero may induce the development of DMH. However, the influence of antibacterials and asthma and anti-allergic medicines taken during pregnancy on DMH has not been studied before.
Therefore, the objective of this study was to investigate whether antibacterials and asthma and anti-allergic medicines used during pregnancy are associated with DMH in a prospective population-based study.
2 Materials and Methods
2.1 Participants
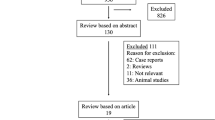
The study is embedded in the Generation R Study, a population-based prospective cohort study from early pregnancy until young adulthood. The Generation R Study, designed to identify early environmental and genetic determinants of growth, development and health, has been described in detail previously [23, 24]. The cohort included 9,897 children and their mothers living in Rotterdam, the Netherlands. Enrolment of mothers was performed at early pregnancy (gestational age <18 weeks). All children were born between April 2002 and January 2006. Of all eligible children in the study area, 61 % participated at birth in the study [24]. The study was approved by the Medical Ethics Committee of the Erasmus Medical Centre, Rotterdam; from all participants written informed consent was obtained. Approximately half of the mothers (51.0 %) and children were of Dutch origin (54.8 %) [24]. At the age of 5–6 years, the children were invited for a check-up visit at the Erasmus Medical Centre. From March 2008 to January 2012, 6,690 children (including 88 twins) visited the Erasmus Medical Centre. As part of this visit, intra-oral photographs of their teeth were taken. A flow chart of the study population is shown in Fig. 1.
2.2 Measures
Assessments included questionnaires, physical examinations and fetal ultrasound examinations and were planned in early pregnancy (gestational age <18 weeks), mid-pregnancy (gestational age 18–25 weeks) and late pregnancy (gestational age >25 weeks). Data on maternal medicine use during pregnancy were retrieved from pharmacy records. These data were available for 5,613 pregnancies of 5,654 children. The medicines studied were (1) antibacterials (e.g., broad- and small-spectrum penicillin, cephalosporin, tetracycline), (2) anti-asthma medicines (e.g., inhalation sympathomimetics, corticosteroids) and (3) anti-allergic medicines (e.g., antihistamines).
At time of birth, Apgar scores and other birth parameters such as weight and length were measured. Ethnicity [25], education level [26], household income, additional use of folic acid, and health of the mother and child were collected by questionnaires at the ages of 2, 6 and 12 months.
At ages of 5–6 years, children visited the research centre for hands-on measurements and photographs of their teeth. After brushing their teeth, photographs of clean, moist teeth were taken by trained nurses and dental students (excess saliva was removed with a cotton ball). Taking approximately ten photographs of all the teeth took 1–2 min for each child. In cases in which a few teeth could not be scored, only the teeth visible in the photographs were used in the analysis. An intra-oral camera (Poscam USB intra-oral autofocus camera (Digital Leader PointNix) or SOPRO life 717 intra-oral autofocus camera, 640 × 480 pixels) was used for the pictures of the teeth, with a minimal scene illumination of 1.4 and 30 lx. In an earlier study, the validity of this camera for clinical recognition of DMH was shown to be high [27]. From the intra-oral photographs, DMH was scored using the criteria of the European Academy of Paediatric Dentistry (EAPD) (see Table 1) [10, 27].
A second primary molar was diagnosed as having DMH when at least one of these criteria was found. The tooth was scored as ‘no judgement possible’, if the tooth or the place where the tooth should be was not shown on the photographs. The photographs were displayed on a computer in full-screen mode and scored by a single dentist (ME). To test the inter-observer agreement in this study, the data of 648 children (9.7 %) were scored independently by another dentist (JV). The two dentists were previously calibrated by scoring photographs, not belonging to this study, The Cohen’s kappa score in this study was 0.73 for DMH. In case of disagreement, the photographs were studied again, and a consensus decision was made. At least 6 weeks after the first scoring, a separate group of 649 children were scored again by the first dentist (ME). The intra-observer agreement reached the following Cohen’s kappa score for DMH of 0.82 [13].
2.3 Statistics
Statistical analyses were performed with SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). To test for any association between medicine use and DMH, a logistic regression analysis was used with adjustment for potential confounders. Selection of potential confounders was based on factors associated with MIH in the literature. First, we tested the association between the medicines and DMH with a univariate logistic regression analysis. The potential confounder was kept in the final multivariate model if it changed the β-coefficient by 10 % or more. To reduce potential bias associated with missing data, we used multiple imputation according to the Markov Chain Monte Carlo (MCMC) method (assuming no monotone missing pattern) [28]. Data were analysed in each data set separately and the results of the ten imputed analyses were pooled and reported in this paper along with the original data. A p-value of <0.05 was considered statistically significant.
Power calculation was used to establish if the size of the study population was adequate. With a power of 80 % (at an α of 0.05), our study was able to detect an odds ratio (OR) of at least 1.3 and 1.6 on DMH for exposure to antibacterials and anti-allergic medicines or anti-asthma medicines, respectively [29–31].
3 Results
Among the 6,690 participating children (mean age 6.2 years, SD ± 0.53; 49.9 % girls), photographs of good quality were made in 94.5 %, only one photograph was made in 3.2 % and no photographs were made in 2.3 % of the children. The prevalence of DMH was 9.0 % (n = 515), when calculated at the child level. Of all eligible second primary molars (n = 24,347), DMH was present in 4.1 % (n = 987) of those molars. During pregnancy, 569 mothers used antibacterials (20.3 %); anti-asthma medicines (e.g., inhalation sympathomimetics, corticosteroids) were used by 344 mothers (12.3 %), and anti-allergic medicines (antihistamines) by 151 mothers (5.4 %). Of those who used amoxicillin, 85 % did so once, 11 % twice and the remaining 4 % three to five times during pregnancy. The most frequently used daily dose of amoxicillin was 1,500 mg (84 %), with the prescribed daily doses varying between 750 and 2,500 mg. A further dose response correlation could not be assessed.
The mean age of the mothers was 30.6 years (SD ± 5.2), and 63.2 % were of Dutch-Caucasian origin. The study population is described in Table 2. First, we tested the association between antibacterials, anti-asthma medicines and anti-allergic medicines and DMH with an unadjusted logistic regression analysis. Then, the association was adjusted for different groups of confounders using a logistic regression analysis (see Table 3). In Table 3, we chose a p-value of ≤0.20 to show that most of the studied relationships were not even close to statistical significance.
The ORs for the association between DMH and antibacterials, anti-asthma medicines and anti-allergic medicines differed slightly between the different multivariate models. Overall, no statistically significant associations were found.
4 Discussion
No associations between antibacterials, anti-asthma medicines or anti-allergic medicines and DMH were found in our study. This finding is only partly in line with the literature available on this topic.
A power calculation revealed that the sample size is adequate, but only for tetracycline use as the sample size was too small.
Antibacterials are either supposed to be safe for use during pregnancy or data about the safety of separate antibacterials are lacking. Only tetracycline is contra-indicated because of known effects on tooth development [9, 32, 33]. Inhalation medicines for asthma are also supposed to be safe to use during pregnancy [34]. For anti-allergic medicines, data on their safety during pregnancy are lacking [9]. For both asthma and anti-allergic medicines, no data are available on their dental safety [9, 34].
In previous studies, several factors (including prenatal factors) were associated with hypomineralisation in the permanent dentition (MIH). These included medical problems, although sometimes controversial results were found [7, 18, 35–37]. Most of these studies were retrospective, creating potential information bias by differential recall. Pharmacies register all prescriptions in the computer before the onset of DMH and this facilitates a prospective study design in which differential misclassification of exposure is excluded.
Possible determinants of DMH have only been studied scarcely [17]. In principle, the same determinants might be expected as for MIH molars, though occurring somewhat earlier in life (pre- and perinatal instead of postnatal) [12, 14–17]. Pre- and perinatal factors did not seem to have much influence on the occurrence of MIH, but they were reported to be associated with DMH [17]. This can be explained by the period in which the teeth are developing. The development of the second primary molars and permanent molars and incisors has an overlap. The development of the second primary molar starts earlier than the development of the first permanent molars and permanent incisors [22].
In MIH, confounding cannot be excluded because it is still unknown whether it is the disease or the medicines to treat the disease that causes the hypomineralisation. The relationship of hypomineralisation with antibacterial use, especially for the commonly prescribed antibacterial amoxicillin, has been demonstrated in animal experimental research [3, 38]. In our study, the adjusted OR for tetracycline use was increased, but not significantly, possibly caused by the lack of power. Wogelius et al. [8] found a relationship between hypomineralisation in the permanent dentition and the use of anti-asthma medicines by the children themselves. We did not find an association between maternal use of anti-asthma medicine and DMH. We presume that the dose that reached the unborn child is very low because the medicines are mostly inhalational and do not pass the blood–placental barrier [9, 39].
Many different factors that could influence tooth development have been reported. In animals, specific relationships between one type of medication and its influence on amelogenesis have been investigated [2, 3]. In such studies, many medications, especially antibacterials, were found to be associated with disturbances in amelogenesis. In future research, medication use of the child and possible effects on the developing teeth have to be studied. In addition, the way the medication is taken (systemic, inhalation, topical) and the extent of the use has to be taken into account. Therefore, more research is needed to determine if the disease or the treatment influences amelogenesis, thereby being one of the factors influencing DMH.
4.1 Limitations of the Study
Our study had a power of 80 % (at an α of 0.05) to detect an OR between 1.3 and 1.6 on DMH for exposure to antibacterials, anti-allergic medicines and anti-asthma medicines, respectively. This effect size is the same or higher than that of other studies [5, 36, 37], suggesting that we had sufficient power to detect significant associations.
This study only describes the data on medication use and if the medication is prescribed or not. No conclusions can be made about the influence of the dosage of the medication.
The percentages of mothers from different ethnicities and/or with lower socioeconomic statuses were lower among the participants than expected for the overall population from Rotterdam [24]. Generalizing the results might be influenced by the selection of our study towards a more healthy and affluent population. However, the associations studied are not expected to be different in the participating population compared with the non-participating population [40]. As a result of the number of missing values, the missing data were imputed. There were no significant differences in the outcome of the analyses on the original data and on the imputed data (see Table 3).
Photographs were difficult to take in some of the young children. Unsuccessful pictures were generally seen in cases in which the child was not able to breathe nasally, e.g., because of the common cold, thus creating moisture on the lens of the camera. Despite this, the overall number of missing photographs was low (5.5 %).
5 Conclusion
The use of antibacterial, anti-asthma and anti-allergic medication taken by mothers during pregnancy was not associated with DMH in their offspring. Conclusions about tetracycline use cannot be drawn because of a lack of power. In addition, after correction for general lifestyle and health factors, no relationships with anti-asthma and anti-allergic medication and antibacterial use during pregnancy were found. These findings suggest that these medicines, used during pregnancy, do not play an important role in the aetiology of DMH in children.
References
Tredwin CJ, Scully C, Bagan-Sebastian JV. Drug-induced disorders of teeth. J Dent Res. 2005;84(7):596–602.
Lyaruu DM, van Duin MA, Bervoets TJ, et al. Effects of actinomycin D on developing hamster molar tooth germs in vitro. Eur J Oral Sci. 1997;105(1):52–8.
Laisi S, Ess A, Sahlberg C, et al. Amoxicillin may cause molar incisor hypomineralization. J Dent Res. 2009;88(2):132–6.
Alaki SM, Burt BA, Garetz SL. The association between antibiotics usage in early childhood and early childhood caries. Pediatr Dent. 2009;31(1):31–7.
Hong L, Levy SM, Warren JJ, et al. Association of amoxicillin use during early childhood with developmental tooth enamel defects. Arch Pediatr Adolesc Med. 2005;159(10):943–8.
Top 100 meest gebruikte medicijnen. http://www.medicdoc.nl/Gezondheidsnieuws/Top-100-meest-gebruikte-medicijnen.html. Accessed 15 Jul 2012.
Whatling R, Fearne JM. Molar incisor hypomineralization: a study of aetiological factors in a group of UK children. Int J Paediatr Dent. 2008;18(3):155–62.
Wogelius P, Haubek D, Nechifor A, et al. Association between use of asthma drugs and prevalence of demarcated opacities in permanent first molars in 6-to-8-year-old Danish children. Comm Dent Oral Epidemiol. 2010;38(2):145–51.
Farmacotherapeutisch kompas. http://www.fk.cvz.nl. Accessed 8 Jul 2012.
Weerheijm KL, Duggal M, Mejare I, et al. Judgement criteria for molar incisor hypomineralisation (MIH) in epidemiologic studies: a summary of the European meeting on MIH held in Athens, 2003. Eur J Paediatr Dent. 2003;4(3):110–3.
Jasulaityte L, Weerheijm KL, Veerkamp JS. Prevalence of molar-incisor-hypomineralisation among children participating in the Dutch National Epidemiological Survey (2003). Eur Arch Paediatr Dent. 2008;9(4):218–23.
Elfrink ME, Schuller AA, Weerheijm KL, et al. Hypomineralized second primary molars: prevalence data in Dutch 5-year-olds. Caries Res. 2008;42(4):282–5.
Elfrink MEC, ten Cate JM, Jaddoe VW, et al. Deciduous molar hypomineralization and molar incisor hypomineralization. J Dent Res. 2012;91(6):551–5.
Li Y, Navia JM, Bian JY. Prevalence and distribution of developmental enamel defects in primary dentition of Chinese children 3–5 years old. Comm Dent Oral Epidemiol. 1995;23(2):72–9.
Aine L, Backstrom MC, Maki R, et al. Enamel defects in primary and permanent teeth of children born prematurely. J Oral Pathol Med. 2000;29(8):403–9.
Slayton RL, Warren JJ, Kanellis MJ, et al. Prevalence of enamel hypoplasia and isolated opacities in the primary dentition. Pediatr Dent. 2001;23(1):32–6.
Ghanim AM, Morgan MV, Marino RJ, et al. Risk factors of hypomineralised second primary molars in a group of Iraqi schoolchildren. Eur Arch Paediatr Dent. 2012;13(3):111–8.
Beentjes VE, Weerheijm KL, Groen HJ. Factors involved in the aetiology of molar-incisor hypomineralisation (MIH). Eur J Paediatr Dent. 2002;3(1):9–13.
Alaluusua S, Lukinmaa PL, Vartiainen T, et al. Polychlorinated dibenzo-p-dioxins and dibenzofurans via mother’ s milk may cause developmental defects in the child’s teeth. Environ Toxicol Pharmacol. 1996;1:193–7.
Ghanim A, Manton D, Bailey D, et al. Risk factors in the occurrence of molar-incisor hypomineralization amongst a group of Iraqi children. Int J Paediatr Dent. 2013;23(3):197–206.
Jalevik B, Noren JG. Enamel hypomineralization of permanent first molars: a morphological study and survey of possible aetiological factors. Int J Paediatr Dent. 2000;10(4):278–89.
Profitt W, Fields H. Contemporary orthodontics. 3rd ed. St Louis: Mosby; 2000.
Jaddoe VW, Mackenbach JP, Moll HA, et al. The Generation R Study: design and cohort profile. Eur J Epidemiol. 2006;21(6):475–84.
Jaddoe VW, van Duijn CM, van der Heijden AJ, et al. The Generation R Study: design and cohort update 2010. Eur J Epidemiol. 2010;25(11):823–41.
Statistics Netherlands. Allochtonen in Nederland 2004. Voorburg/Heerlen; 2004.
Statistics Netherlands. Standaard onderwijs indeling 2003. Voorburg/Heerlen; 2004.
Elfrink MEC, Veerkamp JSJ, Aartman IHA, et al. Validity of scoring caries and primary molar hypomineralisation (DMH) on intraoral photographs. Eur Arch Paediatr Dent. 2009;10(Suppl. 1):5–10.
Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393.
Demidenko E. Sample size determination for logistic regression revisited. Stat Med. 2007;26(18):3385–97.
Demidenko E. Sample size and optimal design for logistic regression with binary interaction. Stat Med. 2008;27(1):36–46.
Demidenko E. Power/sample size calculation for logistic regression with binary covariate(s). http://www.dartmouth.edu/~eugened/power-samplesize.php. Accessed 8 Jul 2012.
Demers P, Fraser D, Goldbloom RB, et al. Effects of tetracyclines on skeletal growth and dentition: a report by the Nutrition Committee of the Canadian Paediatric Society. Can Med Assoc J. 1968;99(17):849–54.
Crider KS, Cleves MA, Reefhuis J, et al. Antibacterial medication use during pregnancy and risk of birth defects: National Birth Defects Prevention Study. Arch Pediatr Adolesc Med. 2009;163(11):978–85.
Lin S, Munsie JP, Herdt-Losavio ML, et al. Maternal asthma medication use and the risk of selected birth defects. Pediatrics. 2012;129(2):e317–24.
Alaluusua S. Aetiology of molar-incisor hypomineralisation: a systematic review. Eur Arch Paediatr Dent. 2010;11(2):53–8.
Jalevik B, Noren JG, Klingberg G, et al. Etiologic factors influencing the prevalence of demarcated opacities in permanent first molars in a group of Swedish children. Eur J Oral Sci. 2001;109(4):230–4.
Tapias-Ledesma MA, Jimenez R, Lamas F, et al. Factors associated with first molar dental enamel defects: a multivariate epidemiological approach. J Dent Child (Chic). 2003;70(3):215–20.
Souza JF, Santos-Pinto L, Alaluusua S, et al. Effect of amoxicillin on developing enamel of rat molars in vivo. EAPD Conference, 2012 May 24–27, Strasbourg.
Vahakangas K, Myllynen P. Drug transporters in the human blood-placental barrier. Br J Pharmacol. 2009;158(3):665–78.
Nilsen RM, Vollset SE, Gjessing HK, et al. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23(6):597–608.
Acknowledgments
The Generation R study was conducted by the Erasmus Medical Centre in close collaboration with the following: Erasmus University, Rotterdam; School of Law and Faculty of Social Sciences, Municipal Health Service Rotterdam area, Rotterdam; Rotterdam Homecare Foundation, Rotterdam; and Stichting Trombosedienst and Artsenlaboratorium Rijnmond (STAR), Rotterdam. We gratefully acknowledge the contribution of general practitioners, hospitals, midwives and pharmacies in Rotterdam. Furthermore, we acknowledge the help of Mrs. J. Wierenga-Visser for setting up the database for pharmacy data. The first phase of the Generation R Study was made possible by financial support from the Erasmus Medical Centre, Rotterdam, Erasmus University, Rotterdam and the Netherlands Organisation for Health Research and Development (ZonMw). The present study was supported by an additional and unrestricted grant from GABA, Therwil, Switzerland and financial support from the Academic Centre for Dentistry Amsterdam (ACTA), University of Amsterdam and VU University Amsterdam, Amsterdam, The Netherlands. The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elfrink, M.E.C., Moll, H.A., Kiefte-de Jong, J.C. et al. Is Maternal Use of Medicines during Pregnancy Associated with Deciduous Molar Hypomineralisation in the Offspring? A Prospective, Population-Based Study. Drug Saf 36, 627–633 (2013). https://doi.org/10.1007/s40264-013-0078-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-013-0078-y