Abstract
There is escalating interest in cell-based therapies to restore lost dopamine inputs in Parkinson’s disease. This is based upon the rationale that implanting dopamine progenitors into the striatum can potentially improve dopamine-responsive motor symptoms. A rich body of data describing clinical trials of previous cell transplantation exists. These have included multiple cell sources for transplantation including allogeneic (human embryonic mesencephalic tissue, retinal pigment epithelial cells) and autologous (carotid body, adrenal medullary tissue) cells, as well as xenotransplantation. However, there are multiple limitations related to these cell sources, including availability of adequate numbers of cells for transplant, heterogeneity within cells transplanted, imprecisely defined mechanisms of action, and poor cell survival after transplantation in some cases. Nonetheless, evidence has accrued from a subset of trials to support the rationale for such a regenerative approach. Recent rapid advances in stem cell technology may now overcome these prior limitations. For example, dopamine neuron precursor cells for transplant can be generated from induced pluripotent cells and human embryonic stem cells. The benefits of these innovative approaches include: the possibility of scalability; a high degree of quality control; and improved understanding of mechanisms of action with rigorous preclinical testing. In this review, we focus on the potential for cell-based therapies in Parkinson’s disease to restore the function of dopaminergic neurons, we critically review previous attempts to harness such strategies, we discuss potential benefits and predicted limitations, and we address how previous roadblocks may be overcome to bring a cell-based approach to the clinic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A regenerative medicine approach transplanting dopamine neuron progenitors to the striatum in Parkinson’s disease will potentially alleviate disabling dopa-dependent motor symptoms. |
The strongest evidence supporting cell transplant as a therapy in Parkinson’s disease is based upon human embryonic mesencephalic tissue as a cell source, but multiple drawbacks make this unlikely to be a feasible approach in the future. |
Recent strides in stem cell technology now serve as a platform for the development of scalable and high-quality cell sources that are entering early clinical trials in Parkinson’s disease. |
1 Introduction
There is a vigorous resurgence of interest in cell-based therapies and the potential of regenerative medicine to treat Parkinson’s disease (PD) as a result of recent rapid strides in stem cell technology [1, 2]. As these highly innovative approaches are transitioning to clinical trials, we focus on the potential of cell-based therapies in PD to restore function that is lost due to attrition of dopaminergic neurons, we critically review previous attempts to harness such therapies, and we address on-going and intensive efforts to bring a cell-based approach to the clinic.
1.1 Parkinson’s Disease and the Therapeutic Need
Parkinson’s disease is a common and incurable neurodegenerative disorder [3], leading to disabling motor signs and symptoms including bradykinesia, muscle rigidity, tremor, and imbalance [4]. The motor features are mostly due to degeneration of dopamine neurons within the midbrain substantia nigra pars compacta [2], associated with cytoplasmic aggregation of α-synuclein and the formation of Lewy bodies. This degeneration leads to the loss of projections to striatal medium spiny neurons, occurring more aggressively in the putamen than the caudate. In addition, multiple non-motor symptoms such as mild cognitive impairment and dementia, mood disorders, dysautonomia, and others develop as a result of dysfunction of multiple other nervous system pathways, involving several neurotransmitters [4].
Current pharmacologic treatments for motor symptoms are almost exclusively based upon restoring striatal dopaminergic input to improve motor function, most commonly using the “gold standard” levodopa, in addition to multiple other medications [5]. Unfortunately, these drugs have off-target effects and their temporal regulation is challenging. Therefore, they lead to side effects such as nausea, orthostasis, excessive daytime drowsiness, hallucinations, and others. Moreover, complications of therapy emerge over years, including motor fluctuations and levodopa-induced dyskinesia [5]. When medications prove insufficient, deep brain stimulation (DBS) provides significant relief in well-selected patients [6]. Non-incisional precisely targeted lesioning using magnetic resonance-guided focused ultrasound is approved in the USA for treating tremor-predominant PD and is being tested in randomized sham procedure-controlled clinical trials to examine more extensive motor benefits [7]. Other experimental surgical approaches include neurotrophic factor infusion, gene therapy [8], cell therapy based upon infusion of mesenchymal stem cells [9], and, as described in the following sections, cell-based therapies to directly restore striatal dopamine inputs that are lost in PD.
2 Potential Utility of Restoring Striatal Dopamine Inputs with Cell-Based Therapy
Using a regenerative medicine approach to replace dopaminergic inputs locally at their physiological site of action has a compelling rationale as a potentially superior treatment of levodopa responsive signs and symptoms, by providing the possibility of a single intervention that would deliver dopamine to its “normal” targets (Fig. 1). Although one potential mechanism of action is that transplanted cells would act as a constitutive local dopamine “pump”, evidence has accrued that transplanted cells are able in at least some cases to functionally integrate into the host neuronal networks [10, 12,13,14]. For example, in an animal model of PD, using a modified rabies virus for retrotracing has allowed precise mapping of synaptic connections formed to and from engrafted cells [15]. Once transplanted, an ideal cell source would thereby pseudo-normalize downstream circuits to improve dopamine-responsive symptoms such as bradykinesia and rigidity. Therefore, one can expect that graft recipients will experience greater “on” time, reduced “off” time, reduced severity of “off” symptoms, alleviation of diphasic dyskinesia (dyskinesia occurring as medication is either taking effect or wearing off), and potentially benefit from reduced medications and hence a reduction in their related side effects. In some cases, it is possible that there could be indirect benefits for non-motor symptoms, for example providing continuous relief of motor symptoms might help sleep. However, for certain motor and the vast majority of non-motor symptoms that arise outside of these dopaminergic pathways, dopaminergic cell replacement therapy is unlikely to suffice. This includes impairments such as dementia, falls, and incontinence, which have a dramatic impact and affect critically important facets of patients’ lives. For example, falls and dementia have been linked to cholinergic deficits [16, 17] and would not be expected to respond to a cell-based therapy focused upon replacing striatal dopamine inputs. Finally, whether and to what degree other potential benefits of cell transplants, such as neurotrophic effects, will be significant enough to provide benefit to patients remains conjectural at this stage.
Positron emission tomography (PET) demonstrates loss of dopaminergic neuron inputs from the substantia nigra pars compacta to the putamen more than caudate in Parkinson’s disease (PD). White arrows indicate the caudate and putamen in a healthy control (a) and in an individual with PD (b), with dashed white arrows at the site of input loss (b). Red arrows in (b) indicate the putamen as the cell transplantation target for the majority of cell-based therapy clinical trials in PD. PET imaging used the dopamine transporter ligand: [C-11]-PE2i (N-(3-iodoprop-2E-enyl)-2b-carbomethoxy-3b-(4-methyl-phenyl)nortropane). Courtesy of Mozley and Henchcliffe
3 Efficacy and Safety of Cell Transplantation in Clinical Trials in Parkinson’s Disease
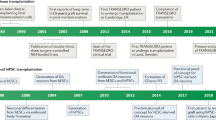
Groundbreaking preclinical work in the 1970s–1980s [18,19,20,21,22] was first translated into clinical trials with allogeneic grafts using donor cells derived from human embryonic ventral mesencephalic (hEM) tissue [23, 24]. During a similar time period, the first studies of autologous transplant of adrenal medullary cells occurred [25,26,27,28,29]. Multiple cell sources have now been tested, mostly in advanced PD (Table 1), resulting in a rich literature that, critically evaluated, should serve to enhance current and future development of cell-based therapeutics for PD (Tables 2, 3 and 4).
3.1 Human Embryonic Ventral Mesencephalic Tissue
The most extensive clinical experience has involved use of hEM tissue as a source of donor cells [1, 23, 24, 30,31,32,33,34,35,36,37]. Initial open-label studies in a small number of patients demonstrated that hEM tissue, when grafted into the striatum, had the capability to survive and function based upon neuroimaging and clinical outcomes [32, 35, 38,39,40]. A series of four patients with PD who received hEM transplants [23, 24, 30] led to cautious extension of this and other programs [31, 32, 34, 35, 38,39,40] (Table 2). Variability in outcomes, including a lack of any reported benefit for some [10, 34], may have occurred owing to differences in the donor tissue (age, dose, preparation, individual variability), surgical delivery, immunosuppression, and individual transplant recipient. Nonetheless, very long-term follow-ups have provided evidence not only of tissue survival, but of sustained benefits (in some cases robust) as well as relative safety and tolerability [41,42,43].
Two randomized, double-blind, sham surgery-controlled clinical trials were launched in the USA in the 1990s (Table 3) [44, 45]. First, an innovative, double-blind, randomized, sham surgery-controlled, participant- and evaluator-blinded phase II clinical trial of 40 individuals with severe PD studied the effects of cultured hEM tissue transplanted to the bilateral putamen [44] (Table 3). Participants were randomized 1:1 to receive dissected tissue from four donor embryos, delivered as strands of tissue rather than dissociated cells, or to a sham procedure in which the skull was drilled but the dura was not broken. No immunosuppressive agents were administered. At 1 year, the study did not meet its primary endpoint measured on a 7-point Likert-type scale (Table 3), despite 17/20 subjects having increased 18F-DOPA uptake measured by positron emission tomography (PET) [that continued to increase to 4 years]. However, a pre-specified sub-analysis of transplanted subjects was encouraging: those aged ≤ 60 years had a statistically significant improvement in the total Unified Parkinson Disease Rating Scale (UPDRS) score “off” medication of 28% compared with the sham surgery group (p = 0.01). Some, but not all, other endpoints demonstrated improvement in this “younger” group (Table 4), but tremor and PD diary scores did not significantly improve, and PD medications did not significantly differ between groups 1-year post-transplant. Unfortunately, 15% of the transplant recipients developed “off” state dyskinesia or dystonia, termed graft-induced dyskinesia (GID) over a 3-year period despite medication adjustment. Of nine serious adverse events, a subdural hematoma detected 6 weeks post-transplant was likely related to the intervention. At 3 years post-transplant, those in the original transplant arm maintained a 28% improvement in total UPDRS scores “off” medication, but improvements declined and differences based upon age were not maintained [46].
A widely acknowledged problem with this study was the use of a patient-centered global measure, with scores that changed when patients at the 1-year endpoint were shown a video of themselves prior to the intervention, thus limiting the scope of truly assessing what quality-of-life component improved or not. Using a more vigorously tested patient outcome measure such as the 36-item Short Form Health Survey (eight domains assessing both mental and physical symptoms) or the PD Quality of Life Score-39 (domains providing a global assessment of health-related quality of life) would now be preferable [47]. In addition, the fairly advanced state of disease with a mean disease duration of nearly 15 years is concerning as more non-motor features such as cognitive decline and autonomic dysfunction may play a larger role in patients’ overall quality of life as opposed to purely motor symptoms [48]. Autopsy data in two transplanted individuals demonstrated neurons staining positive for tyrosine hydroxylase up to 3 years post-transplant [44], but with fewer surviving cells than some other studies. It is unclear whether this is due to tissue preparation, absence of immunosuppression, or other reasons. Finally, based upon earlier open-label studies in which benefits accrued in some cases over a period of years, the 1-year study endpoint here dramatically limited the ability to detect later changes, despite some later follow-up studies in these patients.
A second rigorous, randomized, sham surgery-controlled, participant- and evaluator-blinded, multi-dose clinical trial of 34 individuals with advanced PD examined the effects of hEM cell transplant to the post-commissural putamen (Table 3) [45]. Subjects were randomized to receive a dose of one or four donors per hemisphere, or to undergo sham surgery with a partial burr hole that did not fully penetrate the skull. Cyclosporine was administered to provide immunosuppression for 2 weeks prior and 6 months following the surgery. Again, this study did not meet its primary endpoint of a change from baseline to 24 months in the UPDRS Part 3 score “off” medication: the sham surgery arm declined by 9.4 ± 4.25 points; the one-donor arm declined by 3.5 ± 4.23 points; and the four-donor arm improved by -0.72 ± 4.05 points. Despite not achieving the primary endpoint, there was a significant change in UPDRS Part 3 “off” scores in a subgroup with milder PD as defined by baseline UPDRS Part 3 “off” ≤ 49 (Table 4). No age effect was seen, unlike the previous study [44]. Questions have arisen over whether an apparent “inflection” in UPDRS Part 3 “off” scores at 6–12 months (meaning there was more improvement in the initial months of the study) could reflect withdrawal of cyclosporine at 6 months impacting upon cell function and/or survival. Although an intriguing idea, there is no evidence to support this. This study found robust graft survival based upon 18F-DOPA uptake to the bilateral putamen as measured by PET, and autopsy data in two transplanted individuals demonstrated engrafted tissue survival (Table 4). Graft-induced dyskinesia developed in 13/23 (56.5%) of the transplanted participants, requiring surgical treatment in three patients. The question (as in the previous study) is whether disease duration of at least 10 years suggests extensive degeneration with more severe motor and perhaps non-motor symptoms.
These two randomized controlled clinical trials, and the preceding open-label studies, therefore have provided some evidence of improvement in patient subgroups or certain individuals. However, they have also raised questions about optimal study design and how we might improve our ability to attain more consistent outcomes. This includes, for example, how the age of the cohort and severity of PD might impact on the potential for benefit from transplant. Moreover, further efforts to understand the development of GID have suggested that enrolling patients without significant levodopa-induced dyskinesia may help avoid GID development [49]. With this in mind, a recent multi-center clinical trial of open-label hEM transplantation was designed with optimization of clinical parameters, among others, based upon thoughtful re-analysis of previous data. This open-label study, TRANSEURO, enrolled individuals with early-to-moderate PD, randomly assigned to transplant and non-transplant arms, with a comparator “no intervention” arm [50]. Immunosuppression was achieved with cyclosporine, azathioprine, and prednisolone. Unfortunately, availability of hEM tissue was a significant concern that slowed down this study’s progress. Nonetheless, the investigators clearly lay out considerations in clinical trial design that will help support future studies with different cell sources. One further study is now recruiting 15 patients with severe PD for transplant with hEM tissue but as yet, with planned 5-year endpoints, there are no published results (NCT: 01860794).
Overall then, using hEM has paved the way for further trials of cell-based therapy in PD, but has certain major limitations. Concerns with this approach include availability and variability of the tissue, limitations in standardizing and escalating cell dosing, potential infection, and heterogeneity of cell types within the tissue. Moreover, GID development remains incompletely understood. While there are likely various factors involved, including graft effects in hosts with pre-existing levodopa-induced dyskinesia [49], the development of GID may also relate to the presence of serotonergic neuron precursors in the transplanted tissue [51]. Such limitations have prompted searches for other cell sources, described in Sects. 3.2–3.7.
3.2 Autologous Adrenal Medullary Tissue
As the first clinical studies described above using allogeneic grafts of hEM were starting, a distinct approach had already launched using autologous adrenal medullary tissue as a source of dopamine. The first clinical results reported in 1985 in two individuals with advanced PD demonstrated feasibility of the approach and a signal of potential clinical benefit [52]. Following this, remarkable results with dramatic improvement in two patients aged 35 and 39 years with severe PD (Table 2) [29] prompted multiple small open-label trials with variable outcomes. Unfortunately, a rigorous multi-center study in 16 men and three women with advanced PD found only modest improvements and significant morbidity [28] (Table 2). Subsequently, 13 centers participated in a United Parkinson Foundation Neurotransplantation Registry, comprising 13 centers collecting harmonized outcome measures over 2 years [53]. Unfortunately, deaths occurred in 18%, of which at least half were reported as attributable to the surgical procedures required to achieve these transplants. Moreover, psychiatric adverse effects persisted in a subset of participants, and benefits failed to attain the levels in the initial reports. In retrospect, the underlying rationale for transplanting adrenal medullary tissue has been questioned [54], and a post-mortem study at 30 months post-transplant, despite initial clinical improvement, demonstrated poor cell survival with necrosis and numerous macrophages [55].
3.3 Autologous Carotid Body Tissue
Carotid body tissue has been harvested as a source of dopaminergic cells, and activity as a dopamine source was originally postulated as its primary mechanism of action. However, this tissue also releases glial-derived neurotrophic factor (GDNF) and thus may have other effects than dopamine production [56]. A single phase I/II open-label clinical trial with a 1- to 3-year follow-up in 13 subjects with advanced PD used a harvesting and surgical implantation procedure carried out in a single surgical session targeting the bilateral putamen (and caudate in two, although only one of these received a full dose bilaterally) [56, 57]. The primary outcome, UPDRS motor “off” score, demonstrated variable changes of 5–74% improvement in 10/12 subjects evaluated at 1 year, with a mean change of 15 ± 21.5% (p = 0.034). One patient had a highly fibrous carotid body and derived no benefit. Changes in 18F-DOPA uptake measured by PET in a subset of seven subjects were not statistically significant. Further outcomes and adverse events (including symptomatic lacunar infarct, and seizure resulting from hemorrhage next to a burr hole) are summarized in Table 2. Patient selection was based upon testing emphasizing levodopa responsiveness, similar to DBS pre-surgical testing, but with difficulty obtaining appropriate carotid tissue because of vascular changes, the feasibility and reliability of this approach are questionable.
3.4 Xenografts
Embryonic porcine ventral mesencephalic tissue (12 million cells deposited in three tracks) was used as a donor cell source in this series of xenografts to the unilateral putamen and caudate of 12 patients with advanced PD, of whom six were administered cyclosporine and six received cells pretreated with an anti-major histocompatibility complex class 1 monoclonal antibody F(ab’) fragment [58, 59]. At the 1-year endpoint, total UPDRS “off” scores decreased by average 19%, and three subjects achieved a 30% decrease (five improved 11% or less). 18F-DOPA PET demonstrated no increase in uptake in the engrafted striatum. Scant cell survival (estimated 638 cells) was seen at autopsy at 7 months in one subject who had been administered cyclosporine (who died of pulmonary embolism) [60]. Although there is interest in this approach, testing has been extremely limited so far. The reasons for poor cell survival, and (likely related) the risk of rejection and immunosuppression requirements, need to be better understood, and management of potential xenotic infections is a consideration.
3.5 Retinal Pigment Epithelial Cells
Retinal pigment epithelial (RPE) cells are a source of levodopa and have been tested in clinical trials delivered on an excipient of cross-linked porcine gelatin microcarriers as Spheramine®. Such transplanted RPE cells were shown to improve symptoms in rodent and non-human primate models of PD. An open-label single-center clinical trial was undertaken [61,62,63] in six subjects with advanced PD with surgical delivery to the post-commissural putamen contralateral to the most affected side. There was an average 48% improvement in the UPDRS motor “off” score, the primary outcome, at 12 months, and no serious adverse events were deemed related to the intervention (Table 2). Based upon this encouraging open-label study, a subsequent phase II, multi-center, randomized, double-blind controlled study was undertaken in 35 individuals who received 325,000 RPE cells per side, and 36 individuals who underwent a sham procedure with a partial burr hole that did not penetrate the dura (Table 3) [64]. No immunosuppressive regimen was administered. The UPDRS motor score “off” medications, the primary endpoint, improved by − 10.5 ± 10.26 and − 10.1 ± 12.26 points in the transplant and sham procedure arm, respectively (p = 0.09). Other endpoints are detailed in Table 4. Unfortunately, not only did the study fail to demonstrate benefit, there were also more deaths in the transplant vs sham procedure group (seven vs two, respectively), with one of these deemed possibly related to the surgery or cells. At this point, it seems possible that a lack of benefit may have been due, at least in part, to suboptimal cell survival, based upon autopsy of a single individual at 6 months [65].
3.6 Neural Stem Cells
A single-center, open-label, dose-escalating clinical trial of human parthenogenetic neural stem cells (NSCs) [66,67,68,69] has taken place in in Australia (NCT02452723) (Table 2). Ascending doses of 30, 50, or 70 million cells (ISC-hpNSC®) were surgically delivered using stereotactic guidance to the bilateral caudate, putamen, and substantia nigra [70], with enrolment and procedures completed in early 2019. Interim results have been presented with a published abstract containing an overview of data for ten subjects transplanted, of whom eight had completed this 1-year study (with a planned 5-year follow-up). No serious adverse events were reported as related to the cell product, and specifically, no tumors and no infections were reported. In this small open-label study, a dose-dependent improvement was reported on the Hauser Motor Diary, PD Quality of Life Score-39, and Clinical Global Impression scale at 6 months [71] but publication of the full results is awaited. Although this study is included in our review, there have been critical questions raised about incomplete understanding of the mechanisms of action of these cells [72]. Although these NSCs can differentiate to dopamine neurons in rodent and non-human primate models of PD [67,68,69], recovery of dopaminergic inputs post-transplant is host derived, rather than from dopaminergic neuron replacement by engrafted cells. It has therefore been suggested that any recovery is more likely owing to neurotrophic support to the host from the engrafted cells [67, 68]. Interpretation of clinical results will be hampered until the mechanism of action of these NSCs is better understood. Other studies may aid in improving our understanding of the potential for such cells, such as a new clinical trial targeting 50 individuals with severe PD in China (NCT03119636) [73].
3.7 Human-Induced Pluripotent Cells
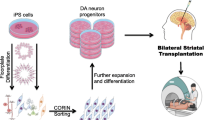
Induced pluripotent cells (iPSCs) may be derived from an individual’s cells, such as skin fibroblasts or blood cells [74, 75] and their fate programmed to become “authentic” midbrain dopamine neurons [76] that will not only survive robustly in preclinical models of PD, but will also ameliorate motor deficits [14, 77,78,79]. Such source iPSCs may be derived from patients themselves [80] or from allogeneic donors. In the case of allogeneic donors, it is possible to provide a degree of immunological matching, shown in animal models to confer a survival advantage on transplanted cells. Using this approach, and based upon highly robust preclinical data, in 2018, a clinical trial undertook the first in a planned series of surgical transplantations of allogeneic dopamine neuron precursors derived from hiPSCs for PD, performed in two stages implanting 2.4 million cells per hemisphere into the putamen bilaterally [77, 81]. No major adverse events were reported after the surgery [81], and published results are keenly awaited. These iPSCs were derived from skin fibroblasts of individuals homozygous for the human leukocyte antigen, so-called “super-donors”, thus facilitating a strategy based upon banking cells from multiple donors to potentially serve a majority of a population.
4 Challenges and Future Considerations in Cell Therapy for Parkinson’s Disease
Based on clinical trials undertaken so far, there is evidence that some individuals derived benefit, varying from minimal to robust. This provides a strong basis from which to examine what avenues are most promising. The optimal cell type for transplant is by no means yet determined. Because of the multiple limitations discussed earlier for hEM tissue transplants, including a lack of donor tissue availability, and heterogeneity between donors and within donor tissue, hEM tissue is highly unlikely to become an important treatment for PD despite its history. Use of other cell sources has been hampered by cell function, survival, and in some cases, incomplete understanding of their mechanism(s) of action. Neural stem cells are now in clinical trials but may have multiple mechanisms of action that remain to be better defined as human studies progress. Induced pluripotent cells (allogeneic or autologous) and hESCs are only just entering or are planned to enter clinical trials [1, 11, 77,78,79,80, 82, 83]. These novel approaches offer the potential to expand cells for banking and cryopreservation, and to rigorously assess quality and cell characteristics. These characteristics include markers of differentiation, cell function, and performance in preclinical models that pertain not only to efficacy but also to predicted safety. Such cells may also be engineered, for example, to deliver neurotrophic or other factors. There remain other core considerations to be addressed at preclinical stages, in clinical trials, and then in translation to clinical care.
4.1 Preclinical Models
Preclinical testing of the efficacy of transplanted cells has depended upon rodent and non-human primate models of PD that are based upon acute destruction of the nigrostriatal pathway by toxins, such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine or 6-hydroxydopamine. However, despite dopamine neuron loss in these animal models, the host environment is substantially different from that in a human with PD, in which multiple cellular pathways are disrupted [3]. Moreover, by the time of transplant in advanced PD, multiple anatomic locations and networks are affected in contrast to the animal models used. The complexity of PD phenotypes, pathology, and genetics in humans, well known to movement disorder clinicians, cannot be overstated and presents a substantial hurdle in translating preclinical findings from defined non-degenerative models into a heterogeneous clinical cohort. Positive results in animal models have therefore sometimes failed to translate to positive outcomes in clinical trials. New animal models, for example based upon alpha-synuclein, are predicted to address some, but not all of these prior limitations [84]. In addition, improved pathophysiological models of PD will support more complex approaches to cell therapy both in and outside of the dopaminergic system.
4.2 Clinical Trial Design
Previous clinical trials have highlighted challenges in terms of design and implementation: patient selection (including consideration of heterogeneity in PD that may be phenotypic, biomarker based, or genetic); variable tissue sources and surgical approaches; the role of immunosuppression; duration of follow-up; and, importantly, the relative lack of diversity in many cohorts studied to date.
4.2.1 Optimizing Clinical Outcome Measures
Selection of clinical batteries and rating scales appropriate to the needs of individual clinical trials is important, and previous guidance has been provided by expert investigators developing the “Core Assessment Program for Intracerebral Transplantations” (CAPIT) [85] and the “Core Assessment Program for Surgical Interventional Therapies in Parkinson’s Disease” (CAPSIT) [86] programs. Complementing previous guidance, incorporation of the Parkinson’s Kinetigraph and/or smart phone applications, which either actively or passively collect various data points regarding a patient’s symptomatology, may provide a more holistic picture of the response to cell-based therapy going forward [87].
4.2.2 Selecting Cell Dose
There is much work needed to identify optimal dose ranges for initial studies, given the limitations in extrapolating from preclinical studies. Investigators use estimates of surviving cells in previous studies, combined with knowledge of the numbers of cells lost in PD combined with cell survival after transplant in preclinical studies. Cautious dose ranging therefore seems to be advisable in early studies.
4.2.3 Identifying the Transplant Target
Although previous surgical approaches have overwhelmingly targeted the putamen, it remains to be determined whether adjusting delivery to a smaller area (for example defined by neuroimaging as having a higher level of dopamine depletion) or whether other targets (possibly in combination) would provide better results.
4.3 Translation to Clinical Care
Any discussion of potential benefits needs to be balanced by the risks to the patient. In the case of the cell-based approaches discussed above, there are risks associated with the surgical delivery of cells, such as hemorrhage, stroke, or infection; risks associated with immunosuppressive medications, such as an increased risk of infection, renal dysfunction, or an increased risk of certain cancers; and risks associated with the cells transplanted, such as cell overgrowth or tumorigenesis, or occurrence of GID. Previous clinical trials, with the exception of TRANSEURO [50], have therefore focused upon patients with later stages of PD. However, an important consideration is whether patients should be offered cell transplantation earlier should this approach prove successful. Previous studies of cell transplantation have suggested benefit for younger patients or for those with milder PD. The “EARLYSTIM” clinical trial provided some support for the use of earlier DBS when levodopa-induced motor fluctuations first develop [88] and might suggest earlier implementation of other “definitive” surgical interventions, such as cell therapies in the future. However, important criticisms of targeting earlier PD included the possibility that some patients would have slower progression and could be managed with pharmacotherapy alone thus avoiding unnecessary exposure to surgical risks. In earlier patients, there is also the risk of misdiagnosis, placing those with atypical parkinsonism at the risk of a surgical procedure unlikely to provide benefit [89]. In cell transplant surgeries, there are also additional risks associated with the cells themselves. Tumorigenesis or overgrowth of unwanted tissue remains a fear, and understanding how to minimize the potential for tumorigenesis is a current focus of research.
4.4 Limitations of a Dopamine-Replacement Strategy
The cell therapies discussed in this review are primarily focused on dopamine-responsive symptoms and therefore have predicted limitations. First, this approach is not predicted to ameliorate many of the disabling features of PD, particularly in later stages, such as dementia, psychosis, or postural instability and falls and their associated morbidities. Second, it does not mitigate the role of alpha-synuclein or other processes in the development of non-dopamine responsive symptoms (nor does it address the development of pathology seen in a few cases within the graft itself [90]). Thus, combining dopamine cell replacement therapy with alpha-synuclein-targeted therapy, such as monoclonal antibody treatments, of which several are in various stages of clinical trials, might be a more comprehensive approach to treat PD [91]. Combining cell-based therapy with gene or gene product-targeted therapy may also pave the way for “precision” treatments, and increasing interest in targeting individuals with genetic forms of PD is now demonstrated by recent and planned clinical trials, for example, in PD associated with a pathogenic mutation in the glucocerebrosidase gene [92]. Advanced targeting and non-invasive technologies such as the magnetic resonance-guided focused ultrasound can aid in the delivery of treatments by opening the blood–brain barrier and allowing not only chemotherapies and antibiotics, but potentially gene therapies and perhaps in the future cell-based therapies [93].
4.5 Cell-Based Therapies on Other Neurodegenerative Disorders
What have we learned from cell-based therapies in other neurodegenerative disorders? Cell replacement strategies have been tested in Huntington’s disease (HD) [94,95,96] and amyotrophic lateral sclerosis [97, 98]. Huntington’s disease is particularly relevant to PD as a progressive neurodegenerative movement disorder although with more prominent cognitive and psychiatric symptoms than PD. The initial loss of striatal GABAergic medium spiny neurons with later degeneration of other brain regions has made HD, like PD, a promising target for cell-based therapy. Open-label and randomized clinical trials focused on embryonic striatal tissue as a cell source demonstrated marked variability in graft survival and clinical effect [99, 100], owing at least in part to limitations already discussed for embryonic tissue used for transplant in PD. As in PD, host pathology has also been observed in human embryonic cell grafts in HD [101], although its clinical significance remains unclear. However, there have been some differences over and above those related to the disorders themselves and the mechanism of action of cells transplanted. The occurrence of subdural hematomas (likely related to surgery in individuals with marked brain atrophy) has prompted modification of the surgical procedure in HD, and rare graft rejection and formation of anti-human leukocyte antigen antibodies against the transplant have been observed in HD [94].
5 Conclusions and Future Prospects
We are in an exciting era in which intensive efforts are underway to develop an effective and competitive regenerative medicine approach to restore nigrostriatal inputs that are lost in PD, and to relieve associated disabling symptoms. Previous attempts to achieve a cell therapy that would replace dopaminergic inputs have been hampered by limitations of the cell sources, including limited donor tissue availability, poor survival post-transplant, and a lack of understanding of their mechanism of action. Nonetheless, transplantation of hEM cells has resulted in robust cell survival in most cases, and clinical benefit in some recipients. Stem cells now provide the potential to overcome limitations associated with previously available cell sources, as they provide high numbers of uniform cells that may be banked and subjected to rigorous testing prior to transplant. As multiple investigative teams begin cell therapy programs, a network called GForce-PD has launched to support communication and shared efforts between teams (http://www.gforce-pd.com) aiming to support more rapid advancement. Cell therapies that enter further development will face a broad competitive landscape, including oral drugs, infusions and injectables, DBS, magnetic resonance-guided focused ultrasound, and potentially gene therapies. Cell-based therapies will need to prove competitive in their efficacy and, importantly, more experience is needed to ascertain safety and tolerability of the various interventions being pursued. That being said, the potential benefits are enormous, with possibilities for one-time interventions that may alleviate (or avoid) patient burden from current drugs and surgical interventions. In the future, cell therapy if successful will likely be combined with other strategies to provide the best treatments possible for individual patients. It is early days, with much to learn, but the coming years will likely see a dramatic increase in clinical trials using cell-based approaches to treating PD.
References
Barker RA, Parmar M, Studer L, Takahashi J. Human trials of stem cell-derived dopamine neurons for Parkinson’s disease: dawn of a new era. Cell Stem Cell. 2017;21(5):569–73. https://doi.org/10.1016/j.stem.2017.09.014.
Henchcliffe C, Parmar M. Repairing the brain: cell replacement using stem cell-based technologies. J Parkinsons Dis. 2018;8(s1):S131–7. https://doi.org/10.3233/JPD-181488.
Simon DK, Tanner CM, Brundin P. Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clin Geriatr Med. 2020;36(1):11–2. https://doi.org/10.1016/j.cger.2019.08.002.
Balestrino R, Schapira AHV. Parkinson disease. Eur J Neurol. 2020;27(1):27–42. https://doi.org/10.1111/ene.14108.
Zesiewicz TA. Parkinson disease. Continuum (Minneap Minn). 2019;25(4):896–918. https://doi.org/10.1212/CON.0000000000000764.
Fox SH, Katzenschlager R, Lim SY, Barton B, de Bie RMA, Seppi K, et al. International Parkinson and Movement Disorder Society evidence-based medicine review: update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord. 2018;33(8):1248–66. https://doi.org/10.1002/mds.27372.
Fishman P, Lipsman N. Focused ultrasound as an evolving therapy for Parkinson’s disease. Mov Disord. 2019;34(9):1241–2. https://doi.org/10.1002/mds.27809.
Hitti FL, Yang AI, Gonzalez-Alegre P, Baltuch GH. Human gene therapy approaches for the treatment of Parkinson’s disease: an overview of current and completed clinical trials. Parkinsonism Relat Disord. 2019;66:16–24. https://doi.org/10.1016/j.parkreldis.2019.07.018.
Marques CR, Marote A, Mendes-Pinheiro B, Teixeira FG, Salgado AJ. Cell secretome based approaches in Parkinson’s disease regenerative medicine. Expert Opin Biol Ther. 2018;18(12):1235–45. https://doi.org/10.1080/14712598.2018.1546840.
Barker RA, Barrett J, Mason SL, Bjorklund A. Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson’s disease. Lancet Neurol. 2013;12(1):84–91. https://doi.org/10.1016/S1474-4422(12)70295-8.
Parmar M. Towards stem cell based therapies for Parkinson’s disease. Development. 2018;145(1):ev.156117. https://doi.org/10.1242/dev.156117.
Steinbeck JA, Choi SJ, Mrejeru A, Ganat Y, Deisseroth K, Sulzer D, et al. Optogenetics enables functional analysis of human embryonic stem cell-derived grafts in a Parkinson’s disease model. Nat Biotechnol. 2015;33(2):204–9. https://doi.org/10.1038/nbt.3124.
Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480(7378):547–51. https://doi.org/10.1038/nature10648.
Hallett PJ, Deleidi M, Astradsson A, Smith GA, Cooper O, Osborn TM, et al. Successful function of autologous iPSC-derived dopamine neurons following transplantation in a non-human primate model of Parkinson’s disease. Cell Stem Cell. 2015;16(3):269–74. https://doi.org/10.1016/j.stem.2015.01.018.
Adler AF, Cardoso T, Nolbrant S, Mattsson B, Hoban DB, Jarl U, et al. hESC-derived dopaminergic transplants integrate into basal ganglia circuitry in a preclinical model of Parkinson’s disease. Cell Rep. 2019;28(13):3462–73.e5. https://doi.org/10.1016/j.celrep.2019.08.058.
Bohnen NI, Muller ML, Koeppe RA, Studenski SA, Kilbourn MA, Frey KA, et al. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology. 2009;73(20):1670–6. https://doi.org/10.1212/WNL.0b013e3181c1ded6.
Bohnen NI, Kanel P, Muller M. Molecular imaging of the cholinergic system in Parkinson’s disease. Int Rev Neurobiol. 2018;141:211–50. https://doi.org/10.1016/bs.irn.2018.07.027.
Brundin P, Barbin G, Strecker RE, Isacson O, Prochiantz A, Bjorklund A. Survival and function of dissociated rat dopamine neurones grafted at different developmental stages or after being cultured in vitro. Brain Res. 1988;467(2):233–43. https://doi.org/10.1016/0165-3806(88)90027-2.
Brundin P, Isacson O, Gage FH, Prochiantz A, Bjorklund A. The rotating 6-hydroxydopamine-lesioned mouse as a model for assessing functional effects of neuronal grafting. Brain Res. 1986;366(1–2):346–9. https://doi.org/10.1016/0006-8993(86)91316-8.
Brundin P, Nilsson OG, Strecker RE, Lindvall O, Astedt B, Bjorklund A. Behavioural effects of human fetal dopamine neurons grafted in a rat model of Parkinson’s disease. Exp Brain Res. 1986;65(1):235–40. https://doi.org/10.1007/bf00243848.
Perlow MJ, Freed WJ, Hoffer BJ, Seiger A, Olson L, Wyatt RJ. Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science. 1979;204(4393):643–7. https://doi.org/10.1126/science.571147.
Bjorklund A, Stenevi U. Reconstruction of the nigrostriatal dopamine pathway by intracerebral nigral transplants. Brain Res. 1979;177(3):555–60. https://doi.org/10.1016/0006-8993(79)90472-4.
Lindvall O, Brundin P, Widner H, Rehncrona S, Gustavii B, Frackowiak R, et al. Grafts of fetal dopamine neurons survive and improve motor function in Parkinson’s disease. Science. 1990;247(4942):574–7.
Lindvall O, Rehncrona S, Brundin P, Gustavii B, Astedt B, Widner H, et al. Human fetal dopamine neurons grafted into the striatum in two patients with severe Parkinson’s disease: a detailed account of methodology and a 6-month follow-up. Arch Neurol. 1989;46(6):615–31.
Stromberg I, Herrera-Marschitz M, Hultgren L, Ungerstedt U, Olson L. Adrenal medullary implants in the dopamine-denervated rat striatum. I. Acute catecholamine levels in grafts and host caudate as determined by HPLC-electrochemistry and fluorescence histochemical image analysis. Brain Res. 1984;297(1):41–51. https://doi.org/10.1016/0006-8993(84)90541-9.
Herrera-Marschitz M, Stromberg I, Olsson D, Ungerstedt U, Olson L. Adrenal medullary implants in the dopamine-denervated rat striatum. II. Acute behavior as a function of graft amount and location and its modulation by neuroleptics. Brain Res. 1984;297(1):53–61. https://doi.org/10.1016/0006-8993(84)90542-0.
Morihisa JM, Nakamura RK, Freed WJ, Mishkin M, Wyatt RJ. Adrenal medulla grafts survive and exhibit catecholamine-specific fluorescence in the primate brain. Exp Neurol. 1984;84(3):643–53. https://doi.org/10.1016/0014-4886(84)90211-5.
Goetz CG, Olanow CW, Koller WC, Penn RD, Cahill D, Morantz R, et al. Multicenter study of autologous adrenal medullary transplantation to the corpus striatum in patients with advanced Parkinson’s disease. N Engl J Med. 1989;320(6):337–41. https://doi.org/10.1056/NEJM198902093200601.
Madrazo I, Drucker-Colin R, Diaz V, Martinez-Mata J, Torres C, Becerril JJ. Open microsurgical autograft of adrenal medulla to the right caudate nucleus in two patients with intractable Parkinson’s disease. N Engl J Med. 1987;316(14):831–4. https://doi.org/10.1056/NEJM198704023161402.
Lindvall O, Widner H, Rehncrona S, Brundin P, Odin P, Gustavii B, et al. Transplantation of fetal dopamine neurons in Parkinson’s disease: one-year clinical and neurophysiological observations in two patients with putaminal implants. Ann Neurol. 1992;31(2):155–65. https://doi.org/10.1002/ana.410310206.
Freed CR, Breeze RE, Rosenberg NL, Schneck SA, Wells TH, Barrett JN, et al. Transplantation of human fetal dopamine cells for Parkinson’s disease: results at 1 year. Arch Neurol. 1990;47(5):505–12. https://doi.org/10.1001/archneur.1990.00530050021007.
Freed CR, Breeze RE, Rosenberg NL, Schneck SA, Kriek E, Qi JX, et al. Survival of implanted fetal dopamine cells and neurologic improvement 12 to 46 months after transplantation for Parkinson’s disease. N Engl J Med. 1992;327(22):1549–55. https://doi.org/10.1056/NEJM199211263272202.
Molina H, Quinones R, Ortega I, Alvarez L, Munoz J, Gonzalez C, et al. Computer assisted CT-guided stereotactic transplantation of foetal ventral mesencephalon to the caudate nucleus and putamen in Parkinson’s disease. Acta Neurochir Suppl (Wien). 1993;58:17–9.
Spencer DD, Robbins RJ, Naftolin F, Marek KL, Vollmer T, Leranth C, et al. Unilateral transplantation of human fetal mesencephalic tissue into the caudate nucleus of patients with Parkinson’s disease. N Engl J Med. 1992;327(22):1541–8. https://doi.org/10.1056/NEJM199211263272201.
Peschanski M, Defer G, N’Guyen JP, Ricolfi F, Monfort JC, Remy P, et al. Bilateral motor improvement and alteration of L-dopa effect in two patients with Parkinson’s disease following intrastriatal transplantation of foetal ventral mesencephalon. Brain. 1994;117(Pt 3):487–99.
Mendez I, Dagher A, Hong M, Gaudet P, Weerasinghe S, McAlister V, et al. Simultaneous intrastriatal and intranigral fetal dopaminergic grafts in patients with Parkinson disease: a pilot study. Report of three cases. J Neurosurg. 2002;96(3):589–96. https://doi.org/10.3171/jns.2002.96.3.0589.
Barker RA, Drouin-Ouellet J, Parmar M. Cell-based therapies for Parkinson disease-past insights and future potential. Nat Rev Neurol. 2015;11(9):492–503. https://doi.org/10.1038/nrneurol.2015.123.
Wenning GK, Odin P, Morrish P, Rehncrona S, Widner H, Brundin P, et al. Short- and long-term survival and function of unilateral intrastriatal dopaminergic grafts in Parkinson’s disease. Ann Neurol. 1997;42(1):95–107. https://doi.org/10.1002/ana.410420115.
Hagell P, Schrag A, Piccini P, Jahanshahi M, Brown R, Rehncrona S, et al. Sequential bilateral transplantation in Parkinson’s disease: effects of the second graft. Brain. 1999;122(Pt 6):1121–32.
Brundin P, Pogarell O, Hagell P, Piccini P, Widner H, Schrag A, et al. Bilateral caudate and putamen grafts of embryonic mesencephalic tissue treated with lazaroids in Parkinson’s disease. Brain. 2000;123(Pt 7):1380–90.
Kefalopoulou Z, Politis M, Piccini P, Mencacci N, Bhatia K, Jahanshahi M, et al. Long-term clinical outcome of fetal cell transplantation for Parkinson disease: two case reports. JAMA Neurol. 2014;71(1):83–7. https://doi.org/10.1001/jamaneurol.2013.4749.
Hallett PJ, Cooper O, Sadi D, Robertson H, Mendez I, Isacson O. Long-term health of dopaminergic neuron transplants in Parkinson’s disease patients. Cell Rep. 2014;7(6):1755–61. https://doi.org/10.1016/j.celrep.2014.05.027.
Henchcliffe C, Carter J, Hanineva A, Kang Y, Babich J, Gollomp SM, et al. Clinical and neuroimaging outcomes up to 18 years after fetal tissue transplant for Parkinson’s disease [abstract]. Mov Disord. 2016;31(Suppl. 2).
Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;344(10):710–9. https://doi.org/10.1056/NEJM200103083441002.
Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol. 2003;54(3):403–14. https://doi.org/10.1002/ana.10720.
Ma Y, Tang C, Chaly T, Greene P, Breeze R, Fahn S, et al. Dopamine cell implantation in Parkinson’s disease: long-term clinical and (18)F-FDOPA PET outcomes. J Nucl Med. 2010;51(1):7–15. https://doi.org/10.2967/jnumed.109.066811.
Martinez-Martin P, Jeukens-Visser M, Lyons KE, Rodriguez-Blazquez C, Selai C, Siderowf A, et al. Health-related quality-of-life scales in Parkinson’s disease: critique and recommendations. Mov Disord. 2011;26(13):2371–80. https://doi.org/10.1002/mds.23834.
Titova N, Martinez-Martin P, Katunina E, Chaudhuri KR. Advanced Parkinson’s or “complex phase” Parkinson’s disease? Re-evaluation is needed. J Neural Transm (Vienna). 2017;124(12):1529–37. https://doi.org/10.1007/s00702-017-1799-3.
Lane EL, Winkler C. L-DOPA- and graft-induced dyskinesia following transplantation. Prog Brain Res. 2012;200:143–68. https://doi.org/10.1016/B978-0-444-59575-1.00007-7.
Barker RA, TRANSEURO Consortium. Designing stem-cell-based dopamine cell replacement trials for Parkinson’s disease. Nat Med. 2019;25(7):1045–53. https://doi.org/10.1038/s41591-019-0507-2.
Politis M, Wu K, Loane C, Quinn NP, Brooks DJ, Rehncrona S, et al. Serotonergic neurons mediate dyskinesia side effects in Parkinson’s patients with neural transplants. Sci Transl Med. 2010;2(38):38ra46. https://doi.org/10.1126/scitranslmed.3000976.
Backlund EO, Granberg PO, Hamberger B, Knutsson E, Martensson A, Sedvall G, et al. Transplantation of adrenal medullary tissue to striatum in parkinsonism: first clinical trials. J Neurosurg. 1985;62(2):169–73. https://doi.org/10.3171/jns.1985.62.2.0169.
Goetz CG, Stebbins GT 3rd, Klawans HL, Koller WC, Grossman RG, Bakay RA, et al. United Parkinson Foundation Neurotransplantation Registry on adrenal medullary transplants: presurgical, and 1- and 2-year follow-up. Neurology. 1991;41(11):1719–22.
Stoddard SL, Ahlskog JE, Kelly PJ, Tyce GM, van Heerden JA, Zinsmeister AR, et al. Decreased adrenal medullary catecholamines in adrenal transplanted parkinsonian patients compared to nephrectomy patients. Exp Neurol. 1989;104(3):218–22.
Kordower JH, Cochran E, Penn RD, Goetz CG. Putative chromaffin cell survival and enhanced host-derived TH-fiber innervation following a functional adrenal medulla autograft for Parkinson’s disease. Ann Neurol. 1991;29(4):405–12. https://doi.org/10.1002/ana.410290411.
Minguez-Castellanos A, Escamilla-Sevilla F, Hotton GR, Toledo-Aral JJ, Ortega-Moreno A, Mendez-Ferrer S, et al. Carotid body autotransplantation in Parkinson disease: a clinical and positron emission tomography study. J Neurol Neurosurg Psychiatry. 2007;78(8):825–31. https://doi.org/10.1136/jnnp.2006.106021.
Arjona V, Minguez-Castellanos A, Montoro RJ, Ortega A, Escamilla F, Toledo-Aral JJ, et al. Autotransplantation of human carotid body cell aggregates for treatment of Parkinson’s disease. Neurosurgery. 2003;53(2):321–8. https://doi.org/10.1227/01.neu.0000073315.88827.72(discussion 8–30).
Schumacher JM, Ellias SA, Palmer EP, Kott HS, Dinsmore J, Dempsey PK, et al. Transplantation of embryonic porcine mesencephalic tissue in patients with PD. Neurology. 2000;54(5):1042–50. https://doi.org/10.1212/wnl.54.5.1042.
Fink JS, Schumacher JM, Ellias SL, Palmer EP, Saint-Hilaire M, Shannon K, et al. Porcine xenografts in Parkinson’s disease and Huntington’s disease patients: preliminary results. Cell Transplant. 2000;9(2):273–8. https://doi.org/10.1177/096368970000900212.
Deacon T, Schumacher J, Dinsmore J, Thomas C, Palmer P, Kott S, et al. Histological evidence of fetal pig neural cell survival after transplantation into a patient with Parkinson’s disease. Nat Med. 1997;3(3):350–3. https://doi.org/10.1038/nm0397-350.
Bakay RA, Raiser CD, Stover NP, Subramanian T, Cornfeldt ML, Schweikert AW, et al. Implantation of spheramine in advanced Parkinson’s disease (PD). Front Biosci. 2004;9:592–602. https://doi.org/10.2741/1217.
Stover NP, Bakay RA, Subramanian T, Raiser CD, Cornfeldt ML, Schweikert AW, et al. Intrastriatal implantation of human retinal pigment epithelial cells attached to microcarriers in advanced Parkinson disease. Arch Neurol. 2005;62(12):1833–7. https://doi.org/10.1001/archneur.62.12.1833.
Stover NP, Watts RL. Spheramine for treatment of Parkinson’s disease. Neurotherapeutics. 2008;5(2):252–9. https://doi.org/10.1016/j.nurt.2008.02.006.
Gross RE, Watts RL, Hauser RA, Bakay RA, Reichmann H, von Kummer R, et al. Intrastriatal transplantation of microcarrier-bound human retinal pigment epithelial cells versus sham surgery in patients with advanced Parkinson’s disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2011;10(6):509–19. https://doi.org/10.1016/S1474-4422(11)70097-7.
Farag ES, Vinters HV, Bronstein J. Pathologic findings in retinal pigment epithelial cell implantation for Parkinson disease. Neurology. 2009;73(14):1095–102. https://doi.org/10.1212/WNL.0b013e3181bbff1c.
Gonzalez R, Garitaonandia I, Semechkin A, Kern R. Derivation of neural stem cells from human parthenogenetic stem cells. Methods Mol Biol. 2019;1919:43–57. https://doi.org/10.1007/978-1-4939-9007-8_4.
Gonzalez R, Garitaonandia I, Poustovoitov M, Abramihina T, McEntire C, Culp B, et al. Neural stem cells derived from human parthenogenetic stem cells engraft and promote recovery in a nonhuman primate model of Parkinson’s disease. Cell Transplant. 2016;25(11):1945–66. https://doi.org/10.3727/096368916X691682.
Gonzalez R, Garitaonandia I, Crain A, Poustovoitov M, Abramihina T, Noskov A, et al. Proof of concept studies exploring the safety and functional activity of human parthenogenetic-derived neural stem cells for the treatment of Parkinson’s disease. Cell Transplant. 2015;24(4):681–90. https://doi.org/10.3727/096368915X687769.
Gonzalez R, Garitaonandia I, Abramihina T, Wambua GK, Ostrowska A, Brock M, et al. Deriving dopaminergic neurons for clinical use: a practical approach. Sci Rep. 2013;3:1463. https://doi.org/10.1038/srep01463.
Garitaonandia I, Gonzalez R, Sherman G, Semechkin A, Evans A, Kern R. Novel approach to stem cell therapy in Parkinson’s disease. Stem Cells Dev. 2018;27(14):951–7. https://doi.org/10.1089/scd.2018.0001.
Kern R, Garitaonandia I, Gonzalez R, Sherman G, Semechkin A, Braine E, Nair G, Evans A. Results of an open label, dose escalating, phase 1 clinical trial evaluating the safety of a human neural stem cell based therapy in Parkinson’s disease. Neurology. 2019;92(15 Suppl.):P1.8-016.
Barker RA, Parmar M, Kirkeby A, Bjorklund A, Thompson L, Brundin P. Are stem cell-based therapies for Parkinson’s disease ready for the clinic in 2016? J Parkinsons Dis. 2016;6(1):57–63. https://doi.org/10.3233/JPD-160798.
Wang YK, Zhu WW, Wu MH, Wu YH, Liu ZX, Liang LM, et al. Human clinical-grade parthenogenetic ESC-derived dopaminergic neurons recover locomotive defects of nonhuman primate models of Parkinson’s disease. Stem Cell Rep. 2018;11(1):171–82. https://doi.org/10.1016/j.stemcr.2018.05.010.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. https://doi.org/10.1016/j.cell.2007.11.019.
Takahashi K, Yamanaka S. A decade of transcription factor-mediated reprogramming to pluripotency. Nat Rev Mol Cell Biol. 2016;17(3):183–93. https://doi.org/10.1038/nrm.2016.8.
Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27(3):275–80. https://doi.org/10.1038/nbt.1529.
Takahashi J. Strategies for bringing stem cell-derived dopamine neurons to the clinic: the Kyoto trial. Prog Brain Res. 2017;230:213–26. https://doi.org/10.1016/bs.pbr.2016.11.004.
Song B, Cha Y, Ko S, Jeon J, Lee N, Seo H, et al. Human autologous iPSC-derived dopaminergic progenitors restore motor function in Parkinson’s disease models. J Clin Invest. 2020;130(2):904–20. https://doi.org/10.1172/JCI130767.
Parmar M, Grealish S, Henchcliffe C. The future of stem cell therapies for Parkinson disease. Nat Rev Neurosci. 2020. https://doi.org/10.1038/s41583-019-0257-7.
Loring JF. Autologous induced pluripotent stem cell-derived neurons to treat Parkinson’s disease. Stem Cells Dev. 2018;27(14):958–9. https://doi.org/10.1089/scd.2018.0107.
‘Reprogrammed’ stem cells implanted into patient with Parkinson’s disease. Nature. 2018. https://www.nature.com/articles/d41586-018-07407-9. Accessed 4 Apr 2020.
Studer L. Strategies for bringing stem cell-derived dopamine neurons to the clinic: the NYSTEM trial. Prog Brain Res. 2017;230:191–212. https://doi.org/10.1016/bs.pbr.2017.02.008.
Kirkeby A, Parmar M, Barker RA. Strategies for bringing stem cell-derived dopamine neurons to the clinic: a European approach (STEM-PD). Prog Brain Res. 2017;230:165–90. https://doi.org/10.1016/bs.pbr.2016.11.011.
Koprich JB, Kalia LV, Brotchie JM. Animal models of alpha-synucleinopathy for Parkinson disease drug development. Nat Rev Neurosci. 2017;18(9):515–29. https://doi.org/10.1038/nrn.2017.75.
Langston JW, Widner H, Goetz CG, Brooks D, Fahn S, Freeman T, et al. Core assessment program for intracerebral transplantations (CAPIT). Mov Disord. 1992;7(1):2–13. https://doi.org/10.1002/mds.870070103.
Defer GL, Widner H, Marie RM, Remy P, Levivier M. Core assessment program for surgical interventional therapies in Parkinson’s disease (CAPSIT-PD). Mov Disord. 1999;14(4):572–84. https://doi.org/10.1002/1531-8257(199907)14:4%3c572:aid-mds1005%3e3.0.co;2-c.
Pahwa R, Isaacson SH, Torres-Russotto D, Nahab FB, Lynch PM, Kotschet KE. Role of the Personal KinetiGraph in the routine clinical assessment of Parkinson’s disease: recommendations from an expert panel. Expert Rev Neurother. 2018;18(8):669–80. https://doi.org/10.1080/14737175.2018.1503948.
Schuepbach WM, Rau J, Knudsen K, Volkmann J, Krack P, Timmermann L, et al. Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med. 2013;368(7):610–22. https://doi.org/10.1056/NEJMoa1205158.
Mestre TA, Espay AJ, Marras C, Eckman MH, Pollak P, Lang AE. Subthalamic nucleus-deep brain stimulation for early motor complications in Parkinson’s disease: the EARLYSTIM trial: early is not always better. Mov Disord. 2014;29(14):1751–6. https://doi.org/10.1002/mds.26024.
Brundin P, Kordower JH. Neuropathology in transplants in Parkinson’s disease: implications for disease pathogenesis and the future of cell therapy. Prog Brain Res. 2012;200:221–41. https://doi.org/10.1016/B978-0-444-59575-1.00010-7.
Brundin P, Dave KD, Kordower JH. Therapeutic approaches to target alpha-synuclein pathology. Exp Neurol. 2017;298(Pt B):225–35. https://doi.org/10.1016/j.expneurol.2017.10.003.
Sardi SP, Simuni T. New Era in disease modification in Parkinson’s disease: review of genetically targeted therapeutics. Parkinsonism Relat Disord. 2019;59:32–8. https://doi.org/10.1016/j.parkreldis.2018.10.025.
Timbie KF, Mead BP, Price RJ. Drug and gene delivery across the blood-brain barrier with focused ultrasound. J Control Release. 2015;219:61–75. https://doi.org/10.1016/j.jconrel.2015.08.059.
Bachoud-Levi AC. From open to large-scale randomized cell transplantation trials in Huntington’s disease: lessons from the multicentric intracerebral grafting in Huntington’s disease trial (MIG-HD) and previous pilot studies. Prog Brain Res. 2017;230:227–61. https://doi.org/10.1016/bs.pbr.2016.12.011.
Precious SV, Zietlow R, Dunnett SB, Kelly CM, Rosser AE. Is there a place for human fetal-derived stem cells for cell replacement therapy in Huntington’s disease? Neurochem Int. 2017;106:114–21. https://doi.org/10.1016/j.neuint.2017.01.016.
Maxan A, Mason S, Saint-Pierre M, Smith E, Ho A, Harrower T, et al. Outcome of cell suspension allografts in a patient with Huntington’s disease. Ann Neurol. 2018;84(6):950–6. https://doi.org/10.1002/ana.25354.
Glass JD, Hertzberg VS, Boulis NM, Riley J, Federici T, Polak M, et al. Transplantation of spinal cord-derived neural stem cells for ALS: analysis of phase 1 and 2 trials. Neurology. 2016;87(4):392–400. https://doi.org/10.1212/WNL.0000000000002889.
Baloh RH, Glass JD, Svendsen CN. Stem cell transplantation for amyotrophic lateral sclerosis. Curr Opin Neurol. 2018;31(5):655–61. https://doi.org/10.1097/WCO.0000000000000598.
Barker RA, Mason SL, Harrower TP, Swain RA, Ho AK, Sahakian BJ, et al. The long-term safety and efficacy of bilateral transplantation of human fetal striatal tissue in patients with mild to moderate Huntington’s disease. J Neurol Neurosurg Psychiatry. 2013;84(6):657–65. https://doi.org/10.1136/jnnp-2012-302441.
Hauser RA, Furtado S, Cimino CR, Delgado H, Eichler S, Schwartz S, et al. Bilateral human fetal striatal transplantation in Huntington’s disease. Neurology. 2002;58(5):687–95. https://doi.org/10.1212/wnl.58.5.687.
Cicchetti F, Lacroix S, Cisbani G, Vallieres N, Saint-Pierre M, St-Amour I, et al. Mutant hunting tin is present in neuronal grafts in Huntington disease patients. Ann Neurol. 2014;76(1):31–42. https://doi.org/10.1002/ana.24174.
Lindvall O, Sawle G, Widner H, Rothwell JC, Bjorklund A, Brooks D, et al. Evidence for long-term survival and function of dopaminergic grafts in progressive Parkinson’s disease. Ann Neurol. 1994;35(2):172–80. https://doi.org/10.1002/ana.410350208.
Sawle GV, Bloomfield PM, Bjorklund A, Brooks DJ, Brundin P, Leenders KL, et al. Transplantation of fetal dopamine neurons in Parkinson’s disease: PET [18F]6-L-fluorodopa studies in two patients with putaminal implants. Ann Neurol. 1992;31(2):166–73. https://doi.org/10.1002/ana.410310207.
Defer GL, Geny C, Ricolfi F, Fenelon G, Monfort JC, Remy P, et al. Long-term outcome of unilaterally transplanted parkinsonian patients. I. Clinical approach. Brain. 1996;119(Pt 1):41–50. https://doi.org/10.1093/brain/119.1.41.
Freeman TB, Olanow CW, Hauser RA, Nauert GM, Smith DA, Borlongan CV, et al. Bilateral fetal nigral transplantation into the postcommissural putamen in Parkinson’s disease. Ann Neurol. 1995;38(3):379–88. https://doi.org/10.1002/ana.410380307.
Acknowledgements
Claire Henchcliffe acknowledges funding from the C.V. Starr Foundation and New York State Stem Cell Science (NYSTEM). Harini Sarva acknowledges funding from the Daisy and Paul Soros Clinical Scholar Award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received by either author for the preparation of this manuscript.
Conflicts of interest
Dr Henchcliffe has received honoraria for consulting for the California Institute of Regenerative Medicine (CIRM) Grant Working Group and research funding as an investigator in a research consortium from New York State Stem Cell Science (NYSTEM). Unrelated to this manuscript she has the following disclosures: honorarium for lecture from the Houston Methodist Neurological Institute; honoraria for ad hoc advisory board participation from US WorldMeds, Adamas Pharmaceuticals, Mitsubishi Tanabe Pharma Inc., and Prevail Therapeutics; and funding from the Michael J. Fox Foundation, National Institute of Health, Insightec, and Prevail Therapeutics. Dr H Sarva has received funding from the Michael J Fox Foundation, and clinical trial support from Biogen, Insightec and Lundbeck Pharmaceuticals. She has received honoraria for participation in advisory boards for Merz and Amneal pharmaceuticals, and for serving as an independent video rater for Neurocrine Neurosciences.
Rights and permissions
About this article
Cite this article
Henchcliffe, C., Sarva, H. Restoring Function to Dopaminergic Neurons: Progress in the Development of Cell-Based Therapies for Parkinson’s Disease. CNS Drugs 34, 559–577 (2020). https://doi.org/10.1007/s40263-020-00727-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-020-00727-3