Abstract
Bipolar disorder (BD) is a mood disorder with genetic and neurobiological underpinnings, characterized primarily by recurrent episodes of mania and depression, with notable disruptions in rhythmic behaviors such as sleep, energy, appetite and attention. The chronobiological links to BD are further supported by the effectiveness of various treatment modalities such as bright light, circadian phase advance, and mood-stabilizing drugs such as lithium that have effects on the circadian clock. Over the past 30 years, the neurobiology of the circadian clock has been exquisitely described and there now exists a detailed knowledge of key signaling pathways, neurotransmitters and signaling mechanisms that regulate various dimensions of circadian clock function. With this new wealth of information, it is becoming increasingly plausible that new drugs for BD could be made by targeting molecular elements of the circadian clock. However, circadian rhythms are multidimensional and complex, involving unique, time-dependent factors that are not typically considered in drug development. We review the organization of the circadian clock in the central nervous system and briefly summarize data implicating the circadian clock in BD. We then consider some of the unique aspects of the circadian clock as a drug target in BD, discuss key methodological considerations and evaluate some of the candidate clock pathways and systems that could serve as potential targets for novel mood stabilizers. We expect this work will serve as a roadmap to facilitate the development of compounds acting on the circadian clock for the treatment of BD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Circadian rhythms are commonly disrupted in bipolar disorder. |
The molecular mechanisms underlying the circadian clock are now described in detail, and new drugs that target neurotransmitter receptors, protein kinases, transcription factors, nuclear receptors and protein degradation pathways have been developed that alter circadian rhythms. |
These clock-modifying drugs may be helpful in treating bipolar disorder. |
1 Introduction
Bipolar disorder (BD) is a common psychiatric disorder that affects 1–2% of the world’s population, causing severe and debilitating episodes of depression and mania, psychosis and persistently increased risk for suicide [1]. Among the defining features of BD are alterations in daily patterns of activity and sleep, reactivity to light and rhythmic physiological oscillations in melatonin and corticosteroids [2, 3]. Seasonal mood disturbances and changes in suicide risk are also common [4]. BD is clinically heterogeneous and likely arises from the complex interactions of numerous genetic and neurobiological factors [5, 6]. The rhythm disturbances observed in BD have led to the hypothesis that disturbances in the biological systems underlying circadian rhythms play an important role in the etiology of the disorder, particularly in patients with BD with illness phenotypes characterized by pronounced circadian rhythm disruption [7]. Multiple models have been proposed to explain the rhythm disruptions in BD, including genetic variation in biological timing systems affecting “clock genes”, desynchrony of internal clocks, differences in photosensitivity/light exposure, social rhythms and aberrant signaling inputs into the clock system [2, 8,9,10,11]. While important progress has been made in these areas, these circadian rhythm abnormalities in BD are not universal, and their underlying causes and relationship to other aspects of the illness remain unclear. For instance, it is not presently known how circadian disruption relates to other common schemes used to describe BD subphenotypes such as BD I versus BD II, rapid cycling and the presence of mixed or psychotic features. Nonetheless, the conceptual understanding of the circadian clock has now advanced to the point of understanding key mechanisms and may lend itself well to the development of novel drugs with effects on pathways and symptoms that could be therapeutically beneficial in BD.
1.1 Overview of the Circadian Clock
Most mammals show endogenous 24 h physiological rhythms (circadian rhythms) that direct recurring, daily physiological processes and behaviors to proceed in an organized manner that optimizes the efficient use of biological resources [12]. Central to this temporal coordination are biological timekeeping systems that synchronize daily physiological and behavioral processes with each other and environmental signals, i.e. the circadian clock. The biological basis of circadian rhythms is now well-understood. The master timekeeper in mammals is located in the suprachiasmatic nucleus (SCN) of the hypothalamus and responds primarily to blue/green light inputs from specialized, non-vision forming, retinal photoreceptors termed intrinsically photosensitive retinal ganglion cells (iPRGCs) [13, 14]. Loss of SCN function from anatomical lesions or genetic manipulation causes a complete loss of rhythms of sleep/wake activity in animals maintained under constant light (LL) or constant dark (DD) [13] and disrupts the normal timing of circadian behaviors [15]. Disruption of the SCN rhythms in mice also causes important changes in mood-related behaviors, leading to increases in helpless behaviors [16]. In addition to the SCN, virtually every cell in the body has an autonomous circadian clock, and most brain regions have circadian rhythms [17]. Inputs from ipRGCs project directly to the SCN and entrain rhythms [14] but also extend beyond the SCN and provide photic inputs to other brain regions in the peri-habenula and other subcortical structures, including the medial amygdala, ventrolateral preoptic area, lateral hypothalamus and others [18, 19]. Some of these inputs have effects on mood that may be independent of the SCN and circadian clock [19, 20]. With respect to mood regulation, the ventral tegmental (VTA) dopamine system is another important site regulated by the circadian clock, with indirect light input transmitted through the peri-habenula/lateral habenula [18], and endogenous rhythms present in dopamine synthesis, release [21, 22], and receptor availability [23]. Accordingly, genetic disruption of the circadian clock in the VTA leads to disruption of dopamine signaling and has been used to model bipolar mania [24, 25]. In the central nervous system (CNS), gene expression rhythms with distinct signatures have been detected in more than 60 distinct regions, indicating the widespread importance of timekeeping in the primate brain [26]. Rhythms in the mouse frontal cortex, hippocampus, dopamine projection neurons of the ventral tegmentum (VTA) and substantia nigra (SN), and subcortical limbic regions have been examined in detail and found to regulate reward-seeking behaviors, cognitive functions, escape/avoidance behaviors and memory [2, 3, 19, 27]. However, while some of these clocks may be able to function more autonomously under some circumstances, most of them are under direct control of the SCN master clock. Also of note, metabolic disorders are common in BD, either as primary comorbidities or induced by mood-stabilizer treatments. Therefore, rhythms in the pituitary, liver, pancreas and other peripheral organ systems may play important roles in endocrine function, metabolism and other critical processes that affect cardiometabolic health and weight gain over the course of psychiatric treatment [28, 29].
1.2 The Circadian Clock is Genetically Encoded
A transcriptional/translational feedback loop made up of ~ 20 “clock genes” exists to maintain essential functions underlying circadian rhythms [30]. At the center of this loop, CLOCK binds to the protein brain and muscle ARNT-like 1 (BMAL1) to form a heterodimeric transcriptional activator. The CLOCK/BMAL protein complex binds to E-box promoter elements, driving the expression of the period (PER) genes PER1/2/3, and cryptochrome (CRY) genes CRY1/2, transcriptional repressor proteins that—upon translocating from the cytosol to nucleus—inhibit their own expression to sustain a circadian oscillator with a period of ~ 24 h. Additional feedback loops have also been described that modulate amplitude, including the proteins REV-ERBα/β and retinoic acid-related orphan receptors (RORA/B/C). Many circadian network proteins are regulated through post-translational modification and/or protein–protein interactions. These modifications typically alter protein stability or function. For instance, the protein kinase glycogen synthase kinase 3 (GSK3) phosphorylates BMAL1, CLOCK, CRY2, REV-ERBα and PER2 to alter their stability [31,32,33,34]. Select clock proteins are also targets of a number of widely expressed signaling molecules such as the mitogen-activated protein kinases (MAPK) and protein kinase B (AKT) [35, 36]. As knowledge of the regulatory pathways and protein structures continues to improve, clock proteins are increasingly accessible as potential drug targets. Additional details covering specific clock gene functions and drug target features are covered in later sections, but an overview of the network and known sites for drug action is shown in Fig. 1.
Transcriptional, translational and post-translational components of the circadian clock as potential drug targets. The circadian clock is organized in dual negative feedback loops that maintain oscillations in gene expression over ~ 24 h periods. This activity establishes 24-h cycles in numerous cellular functions and coordinates their activities. Through binding at E-box elements in gene promoters, BMAL/CLOCK proteins (positive regulators, purple) drive the expression of CRY1/2 and PER1/2/3 (negative regulators, yellow) and the NR1D1/2 genes that encode REV-ERB nuclear receptors (green). REV-ERBs and RORs negatively and positively modulate rhythm amplitude through actions on RRE genomic elements. Various drugs have been developed that affect post-translational modification of clock proteins, modify protein–protein interactions, or serve as ligands for clock proteins with nuclear receptor functions. Kinases (orange) phosphorylate clock proteins. Ubiquitin ligases (blue) regulate the degradation of clock proteins. Light-sensitive inputs to the circadian clock and rhythmic output pathways may also amenable to drug modification. Specific drug classes mentioned in the review are indicated by red boxes. Additional details on each drug target are provided in the text. ARNT aryl hydrocarbon receptor nuclear translocator, BMAL1 brain and muscle ARNT-like 1, Ca2+ calcium, CK casein kinase, CK1D casein kinase 1 delta, CRY cryptochrome, FBXL F-box and leucine rich repeat proteins, GSK3 glycogen synthase kinase 3, ipRGC intrinsically photosensitive retinal ganglion cell, NR1D1 nuclear receptor subfamily 1 group D member 1, PACAP pituitary adenylate cyclase-activating polypeptide, PER period, ROR retinoic acid-related orphan receptor, RRE REV-ERB responsive element
1.3 Lithium as a Clock-Modifying Drug
Among the key findings that support a link between circadian rhythms and BD is the observation that the mood stabilizer lithium affects circadian rhythms. Lithium has a complex mechanism that involves the inhibition of both GSK3 and inositol mono-phosphatase (IMP) [37, 38]. Both mechanisms affect rhythms, with the GSK3 effect primarily altering amplitude, and the IMP inhibition affecting period, at least in part by actions on IP3 receptors [39]. This action of lithium on IMP distinguishes it in important ways from more selective GSK3 inhibitors: lithium typically lengthens period, whereas GSK3 inhibitors typically shorten it [40]. Pioneering studies in rats revealed that lithium extended the period of wheel-running behaviors and facilitated entrainment to longer (27–28 h) photoperiods [41, 42]. These effects were replicated in healthy human volunteers living in standard environmental conditions, with lithium delaying the sleep/wake rhythm by an average of 14 minutes [43]. Studies in nonhuman primates maintained under controlled LL conditions also revealed dose-dependent, period-lengthening effects of lithium [44]. More recent studies employing molecular reporter genes have examined the effects of lithium on SCN neurons and BD patient skin fibroblasts and found consistent period-lengthening effects but at concentrations five to ten times higher than those used clinically in humans (0.5–1.0 mM) [45, 46]. The effects of lithium on amplitude have also been reported but are more variable. In mouse SCN neurons, lithium had no effect on amplitude [46]. In fibroblast cultures from healthy controls, lithium increased amplitude at concentrations closer to the therapeutic range (1.0 mM) that are too low to affect period [45]. Interestingly, the amplitude effect was absent in fibroblast cultures from patients with BD, possibly due to differences in calcium channel and extracellular signal-regulated kinase (ERK) signaling [45, 47, 48]. A study of fibroblast cultures from patients with BD determined to be lithium responders or nonresponders found that the period-lengthening effects of lithium were generally associated with nonresponsiveness. Lithium responders typically had shorter periods and/or were phase advanced at baseline and typically showed a weaker period-lengthening response to lithium, whereas nonresponders had longer period/phase delays at baseline and were more likely to show period-lengthening effects. It is not yet clear from human studies whether an action on the circadian clock is essential for lithium’s therapeutic effects. However, in the forced swim test model of depression, genetic disruption of clock genes (especially Cry1) is essential to confer a benefit from lithium treatment, indicating the circadian clock may be a key element of the therapeutic mechanism [49].
2 Behavioral Rhythm Disturbances in Bipolar Disorder
The most easily observed rhythmic behaviors in humans are the 24 h sleep/wake cycle. Disturbances in these sleep and activity rhythms are central diagnostic features of BD. Additional differences in chronotype, social patterns and seasonality are also present in patients with BD versus healthy subjects.
2.1 Sleep and Insomnia
Sleep disturbance is a hallmark of BD during symptomatic states (i.e., mania and depression) and relatively symptom-free euthymic periods [50, 51]. It has been proposed that sleep disturbances may be a heritable endophenotype that genetically co-segregates with BD. For instance, higher rates of sleep disturbance have been reported in the high-risk offspring of patients with BD compared with controls [52]. In children and adolescents not yet diagnosed with BD, sleep disturbances, including lack of sleep, decreased sleep, insomnia and poor sleep efficiency may be an initial prodrome for both mania and depression; in adults with BD, insomnia predicts the onset of mood relapse [50, 53,54,55].
Some sleep disturbances may be specifically associated with mood states. Variability in sleep latency has been associated with depressive symptoms, and lower sleep efficiency has been associated with more lifetime depressive episodes [56]. Inducing sleep loss in a controlled setting induces manic symptoms at rates similar to antidepressants, suggesting sleep loss may be causally involved in the emergence of mania [57]. Manic symptoms have been associated with decreased sleep efficiency and the duration of rapid eye movement (REM) and slow-wave sleep [56, 58]. Somnographic findings in both manic and depressed patients with BD included disruption in sleep continuity, increased time spent in stage 1 sleep, shortened REM latency and increased REM sleep density [59].
In long-term studies, sleep disturbances in BD have demonstrated an association with clinical and course-of-illness characteristics. Short sleep duration is associated with more severe symptoms, whereas sleep abnormalities in either direction (i.e., short or long) were associated with poor functioning and quality of life [60]. Sleep disruptions have been associated with a worse course of illness, increased symptom severity and impairments in functioning and quality of life [50].
2.2 Psychomotor Activity
Low-amplitude activity rhythms, typified by higher variability of activity and fragmentation of psychomotor activity patterns have been noted in patients with BD [61,62,63]. In a large study of euthymic patients with BD and unaffected relatives, the patients with BD demonstrated later onset of activity, greater variability, less stable circadian patterns, and a greater fragmentation of activity patterns; increased movement during sleep; and a lower activity amplitude than controls [64]. Interestingly, genetic linkage peaks associated with activity abnormalities were often co-transmitted with BD [64]. Similar conclusions were reached by an analysis of accelerometry data from > 90,000 subjects from the UK Biobank community sample. In this study, low rhythm amplitudes predicted BD, among other indicators of poor mental health [65].
Disturbances in the levels of locomotor activity may be informative biomarkers for both manic and depressive phases of BD [66]. Patients with BD showed less activity when depressed [66] and greater locomotor activity with less coherent rhythms when manic [62, 66]. In one of these studies, the severity of manic symptoms directly correlated with the loss of locomotor activity rhythm [62]. Relationships were most pronounced between the rhythm disruption and decreased need for sleep, disturbances in thought content, pressured speech, and increased motor activity/energy.
2.3 Chronotype
Chronotype refers to the stable preferences of people over time to favor morning or evening activity. Morningness is genetically influenced and heritable and overlaps to some extent with traits underlying psychiatric disorders [8, 67, 68]. Studies suggest that patients with BD have, on average, lower “morningness” and show a general preference for evening activities [69, 70], suggesting a circadian phase delay. Importantly, the delays in sleep offset/activity onset are commonly seen in patients with BD even during euthymic periods when mood symptoms are relatively well controlled, indicating they are less likely state related and caused by symptoms of BD [64]. In patients with BD, greater eveningness has been associated with earlier age of illness onset, rapid cycling, greater recurrence rates of mood episodes [71], increased suicidality and poor response to lithium [39].
2.4 Social Rhythms
The social zeitgeber theory suggests that personal interactions might be an environmental cue that coordinates rhythmic behaviors among humans [11, 72]. Patients with BD demonstrate delays in social contact compared with healthy controls and were more likely to miss meals, be late for work and not exercise [61, 73]. Moreover, disruption of social rhythms could destabilize biological rhythms, thereby risking exacerbation of manic mood episodes [74].
2.5 Seasonality
Light is the most prominent entrainment signal for circadian rhythms but also serves as an annual marker that directs seasonal rhythms. The light-responsive systems involved in annual cycles include genetic and neurobiological pathways that overlap with the circadian clock [75]. It has been proposed that seasonality may also be a heritable trait that can be co-transmitted with BD [76]. Compared with controls, subjects with BD report a greater degree of seasonal change in mood disturbances and sleep, and—in twins with BD—concordance for seasonal variability in these traits is higher than in healthy matched twin pairs [77]. Indeed, a subset of patients with BD demonstrate a seasonal pattern to their mood episodes, with the type of mood episode typically varying by season: depression peaked in autumn, mixed mania in late summer and mania in early spring [78,79,80], and this seasonality may be one factor that differentiates patients with BD from patients with unipolar depression [80]. Other phase shifts in photoperiod, such as those induced by long-distance travel, may also provoke mood episodes in patients with BD [81]. Taken together, these studies suggest that patients with BD may demonstrate an altered sensitivity to light and photoperiod that can destabilize mood.
3 Biological and Physiological Markers of Rhythm Disruption
The rhythm disturbances commonly observed clinically in BD have led to a search for one or more pathophysiological markers of rhythm disturbances that can be used to track rhythm in the illness. In particular, research has explored the potential links between BD, rhythmic melatonin secretion and corticosteroid secretion as potential biomarkers, and/or as a means to phenotypically differentiate subcategories of patients with BD.
3.1 Melatonin Secretion
Melatonin released from the pineal gland is a key output of the circadian clock and one of several signals that synchronizes rhythms throughout the body with the central circadian pacemaker and seasonal changes in photoperiod. Melatonin is a biological marker of darkness and is released overnight with an onset a few hours before the start of sleep. Melatonin is produced and secreted by the pineal gland in a diurnal fashion that is influenced by the endogenous circadian rhythm and ocular light exposure [82]. Light inhibits the production of melatonin in a dose-dependent fashion, and so the hormone is sometimes considered a biological marker of darkness. It has been suggested that dysfunction in the rhythmic secretion of melatonin may be linked with BD [83]. Studies in BD commonly report lower levels of overnight melatonin [84] and delays in dim-light melatonin onset (DLMO), both in BD versus unipolar depressed patients and compared with controls [85]. Patients with BD have also demonstrated significantly lower peak nocturnal melatonin levels [84,85,86,87]. The mechanisms underlying the melatonin differences observed in BD are not well-established but may involve the transmission of light by melanopsin-containing iPRGCs in the retina. In healthy subjects, hypomanic traits were positively associated with the post-illumination pupil response (PIPR) to light [88], whereas in patients with seasonal depression, the PIPR to blue light was diminished compared with controls [89]. Therefore, retinal light sensitivity may correlate with mood state. However, to the extent that they have been examined, the differences in peak nocturnal melatonin levels are typically similar across mood states [87], indicating a stable trait phenotype. Studies in cells from healthy subjects have shown that individual variability in intracellular signaling pathways terminating on the cyclic adenosine monophosphate (cAMP) response element-binding protein (CREB) correlate with the physiological effects of light on melatonin secretion [90]. The same study found elevated CREB activity in BD cells compared with controls [90], a finding supported by genome-wide association studies of BD that have revealed enrichment in light-sensitive signaling pathways [8]. These latter findings may be more relevant for BD-related trait abnormalities in melatonin secretion.
3.2 Hypothalamic Pituitary Adrenal Axis
The hypothalamic pituitary adrenal (HPA) axis is under tight regulation by the circadian clock, and systemic levels of cortisol show a clear circadian rhythm. Dysregulation of the HPA axis, including hypercortisolemia, increased adrenocorticotropic hormone (ACTH) levels and impaired glucocorticoid receptor feedback mechanisms have all been suggested as possible mechanisms underlying BD [91] and confirmed in large meta-analyses [92]. Using 24-h blood sampling, overall higher mean and peak overnight cortisol levels were observed as well as an early nadir of cortisol secretion in patients with BD during both depressive and manic states [93, 94]. In longer sampling periods over 72 h, cortisol rhythms in patients with BD showed greater variability than those in controls [95]. In terms of impaired HPA axis feedback, dexamethasone suppression impairments were also reported in a meta-analysis of four studies conducted at various timepoints across the day [92]. Notably, cortisol-release hormone (CRH) levels were not consistently altered in BD [92].
In spite of the theoretical implications for circadian rhythms, it has not been clearly demonstrated that the BD-associated rhythm disturbances in the HPA axis result from primary dysfunction of the circadian timing system or caused instead by a more proximal cause, including genetic variation in the HPA axis itself.
4 Candidate Circadian Systems as Novel Drug Targets for Bipolar Disorder
4.1 Therapeutic Endpoints of Circadian Clock Targets
Given the evidence implicating circadian rhythm abnormalities in BD and related mood disorders, it is reasonable to conclude that drugs targeted to block or stimulate circadian clock components may be beneficial to restoring rhythms. For instance, inducing sleep (e.g., with sedative hypnotics) or promoting activity (e.g., with stimulants) are well-established examples of beneficial therapeutic outcomes that arise from augmenting rhythmic behaviors. However, while these endpoints clearly relate to behavioral output of the circadian clock, they are presented as examples of imprecise endpoints. Stimulants and sedatives do not target the underlying mechanisms that directly control rhythms, and they generally cause similar effects at any time (i.e., a stimulant will increase activity day or night). In this section, we specifically consider drug actions that more precisely target circadian clock mechanisms underlying period, amplitude and entrainment mechanisms and address how these drugs might affect therapeutic outcomes. A summary of the candidate systems is presented in Table 1. We then discuss the various methods that could be considered in assessing human subjects in a clinical trial.
4.1.1 Period
Among the circadian parameters, period is most accurately quantified and best studied as a drug target. Drugs that both shorten and lengthen circadian period have been developed, including some already in clinical use (e.g., lithium). Previous reports have indicated that period can be lengthened to a greater extent than it can be shortened. The small molecule longdaysin, identified in a high-throughput circadian screen, caused > 10 h lengthening in period [96]. More recently, we have shown that inhibition of the P38 kinase in immortalized mouse fibroblasts caused an even greater period lengthening of > 20 h [47]. In contrast, the largest period shortening effects from pharmacological manipulations are in the 2–3 h range [40], suggesting “speed limits” placed upon the circadian oscillator may put biological constraints on the size of the period shortening effect that may be induced with a drug. Considerable heterogeneity of circadian phenotypes exists in BD, and there are perhaps situations where period lengthening may support a beneficial outcome [39, 97]. However, there is general consensus that delayed circadian activity is the bigger risk factor for most mental health outcomes, including depression, poor response to treatment, substance use disorders, social impairment and metabolic disorders [39, 97]. Therefore, drugs that shorten period might be predicted to have generally more therapeutic benefit in these conditions. For instance, chronic fluoxetine shortens circadian activity periods in mice [98]. By accelerating the turnover of the circadian cycle, they might offset some of the biological factors leading to circadian delay.
4.1.2 Amplitude
As described, BD has been associated with low amplitude in many studies. Amplitude-increasing drugs might therefore be useful, particularly in consolidating fragmented sleep and activity patterns into more regular cycles. Higher amplitude rhythms may also be beneficial in situations where environmental timekeepers are attenuated, and weak rhythms may become prone to incoherence (e.g., light inputs during seasonal mood episodes). By maintaining robust intrinsic rhythms, the circadian mechanisms underlying mood stability may be preserved, even in the absence of consistent external reinforcement. One potential risk of amplitude modulation is that by strengthening the rhythm, the clock may become less flexible and less likely to re-set in response to entrainment stimuli, perhaps making adjustments more difficult for shift workers or long-distance travelers adjusting to jet lag.
4.1.3 Entrainment
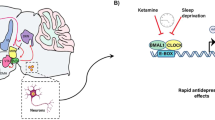
The mechanisms underlying circadian entrainment in the retino-hypothalamic pathway are well-described. In SCN synapses, light-induced glutamate is released from ipRGCs and triggers calcium signaling and MAPK activation of clock genes in the SCN neurons that either advance or delay the circadian cycle in a phase-dependent manner [99,100,101]. Mutant mice lacking the L-type calcium channel show impairments in phase advances [102], whereas mice lacking the protein kinase G (PKG) show impairments in phase delays [103], suggesting that these known drug targets may be co-opted to modify circadian entrainment. Small molecules have been developed that target other mechanisms. Serotonin (5HT) receptors play an important role in this regard, especially 5HT1A, 5HT1B and 5HT7 receptors. In animals, acute administration of BMY7378, a mixed 5-HT1A agonist/antagonist, causes dramatic facilitation of light-induced phase advances [104]. In humans, lurasidone—a 5HT7 antagonist with approved use for BD depression—has been shown to have effects on improving sleep when dosed at night in a phase advance model of insomnia [105]. These data are consistent with the effects of 5HT7 antagonism in facilitating phase advance. When used in conjunction with sleep deprivation, phase advance has been shown to be an effective antidepressant treatment in BD. It is tempting to speculate that drugs that facilitate phase advancing might have a role in augmenting this process.
4.2 Melatonin
Melatonin onset is a marker of circadian phase, and exogenous melatonin given before onset of sleep can be used to phase advance circadian rhythms. At higher doses, melatonin has a sedative effect. MT1 and MT2 melatonin receptors are Gi-protein coupled and located throughout the brain and periphery [106]. Melatonin may also bind other targets, including the circadian transcription factor RORA [107]. In the CNS, MT1 is highly expressed in the hypothalamus, cerebellum, hippocampus and midbrain dopamine neurons. MT2 is involved in phase setting of endogenous rhythms and is more limited in expression with enrichment in light-sensitive tissues such as the retina and SCN [106]. In the periphery, MT1 receptors are expressed in the cardiovascular system, immune cells, reproductive organs, endocrine organs, skin, liver, kidney and placenta, whereas MT2 is expressed in immune cells, skin, pituitary, blood vessels, testes, kidney, gastrointestinal system and adipose [106]. MT1 receptors have a more conclusive role than MT2 in underlying depressive mood states, whereas both MT1 and MT2 receptors appear to be involved in sleep regulation [108,109,110]. Given the key roles that melatonin plays in the shifting of circadian rhythms and seasonal behaviors, drugs targeting the melatonin system may be of clinical benefit. Accordingly, both exogenous melatonin and melatonin-acting small molecules have been developed as therapeutic agents for mood symptoms in BD. Two small open-label adjunctive-treatment studies examined the therapeutic effects of melatonin on mood and sleep symptoms in BD and reported mixed results [111, 112]. Agomelatine is a mixed melatonin receptor agonist and 5HT2C receptor antagonist. In healthy individuals, agomelatine increased REM sleep and caused a phase advance of circadian rhythms [113]. Agomelatine has also shown efficacy in the treatment of unipolar depression, leading to improved sleep quality, sleep efficiency and normalization of non-REM sleep [113,114,115,116]. In BD depression, open-label studies showed early promise [117, 118], but randomized placebo-controlled trials have been less successful [119]. Ramelteon, another melatonin receptor agonist, showed promise in preventing relapses in BD maintenance [120, 121]. In addition to mood symptoms, melatonin receptor agonists may also be useful as adjunctive agents to mitigate metabolic disturbances caused by antipsychotics [122], drugs commonly used to treat BD. While melatonin-acting drugs may be a productive line of drug development, additional questions remain. For instance, it remains unclear whether engagement with the circadian clock is a necessary component of the therapeutic mechanism or whether/how the sleep-modulating properties of melatonin receptor agonists contribute to mood improvements. Ligands selectively targeting both MT1 and MT2 have been developed and studied in preclinical models, making this question resolvable with further study [108, 109].
4.3 Hypothalamic Neuropeptides
The SCN is composed of a large number of neuronal subpopulations that utilize distinct neuropeptides to integrate light information and regulate circadian rhythms [123, 124]. Three notable neuropeptide systems are vasoactive intestinal polypeptide (VIP)/pituitary adenylate cyclase-activating polypeptide (PACAP), vasopressin (AVP), and neuromedin S (NMS). In the SCN, VIP neurons play a dominant role in synchronization and phase regulation, whereas AVP neurons are the principal determinant of circadian period generated by the SCN network [125]. The NMS is a more recently described neuropeptide system with a limited expression profile that may allow for specific targeting of the SCN clock. Each system is discussed in the following.
PACAP is released, together with glutamate, in response to light by ipRGC terminals in the retinorecipient region of the SCN and regulates circadian phase [126]. Neurons expressing VIP are located in the ventral region of the SCN and receive direct projections from ipRGCs. VIP plays a key role in photic phase shift of the SCN. In the absence of VIP, SCN neurons display desynchronized rhythms and a lower propensity for sustained cellular oscillations [127, 128]. PACAP and VIP act via G-protein coupled receptors: PAC1, which is selective for PACAP, and VPAC2, which is responsive to both VIP and PACAP [129]. VPAC2 is highly expressed in the SCN. Transgenic mice overexpressing the human VPAC2 receptor have an altered circadian phenotype, whereas mice carrying a null mutation of the VPAC2 receptor (Vipr2−/−) are incapable of sustaining circadian rhythms of sleep/wake behavior [129]. These null mutant mice also fail to exhibit rhythms in clock gene and AVP expression in the SCN [129]. No data directly implicate PACAP/VIP in BD, but human genetic studies have found that a duplication of chromosome 7q36.3 (encompassing VIPR2) is associated with schizophrenia and high levels of PACAP-PAC1 signaling are associated with posttraumatic stress disorder [130, 131]. In animal models, upregulation of PACAP has been implicated in stress-induced anxiety behaviors [132]. Small-molecule ligands targeting PACAP/VIP receptors have been developed and used in vivo. For instance, administration of the VPAC2 agonist Ro25-1553 causes pronounced phase advances, which may be advantageous in BD, but chronic administration in mice causes deficits in pre-pulse inhibition, a physiological marker associated with a number of psychiatric disorders [133, 134].
One-third of SCN neurons rhythmically express AVP and show projection within the SCN, paraventricular nuclei and dorsomedial hypothalamus. There are three types of AVP receptors, V1A, V1B and V2, but only V1A and V1B are expressed in the brain [125]. The V1B receptor is located in the pituitary and in the amygdala, whereas V1A is expressed in the SCN and many other areas throughout the brain. Deletion of the gene encoding V1A in mice lengthens the circadian period in DD situations by approximately 100 minutes, and a portion of these mice are arrhythmic [135]. Double-mutant mice lacking both V1A and V1B can immediately re-entrain to phase-shifted light–dark cycles [136]. This suggests that AVP might contribute to making the SCN resistant to environmental perturbations, such as jet lag, by mediating neuronal communication within the SCN. Outside the SCN, AVP and the V1A receptor have important roles in affiliative social bonding [137]. Of note, reductions of both synthesis and release of AVP have been reported in the SCN of patients with depression [138]. Therefore, molecules that act as agonists for VIP or AVP receptors might be helpful in BD where circadian desynchronization is present.
NMS expression is restricted to the SCN, representing an appealing pharmacological target for selectively manipulating circadian rhythms in the SCN. Previous studies showed that intracerebroventricular administration of NMS in rats activated SCN neurons and induced phase-shift of circadian rhythms [139], suppressed feeding [140] and stimulated oxytocin release [141]. Recently, NMS neurons were shown to act through synaptic transmission as SCN pacemakers that control network synchrony and circadian rhythms [142]. NMS binds to two different type of G-protein coupled receptors, NMU1R and NMU2R. The NMU1R is widely distributed in peripheral tissues, whereas NMU2R is highly expressed in the hypothalamic paraventricular nucleus (PVN) and SCN [139, 143]. A subset of NMU2R-expressing PVN dopamine neurons receive NMS input from the SCN. Bath application of NMS stimulated these cells, suggesting that excitatory NMS neurons in the SCN might regulate the activity of the dopamine system in the PVN [144]. Dysfunction of dopaminergic neurons in the PVN is associated with depression and anxiety-like behavior in rodents and winter depression in humans [145, 146]. Taken together with its circadian effects, NMS neurons and NMU2R may provide a future target for therapies for mood disorders such as BD. Further investigation is needed to develop selective small molecules targeting NMS receptors and determine their effects in vivo.
4.4 Casein Kinases
The casein kinase I delta (CSNK1D) phosphorylates PER2 and regulates protein turnover. Genetic studies of families with advanced sleep phase syndrome (ASPS) and mutant mice identified the gene encoding CSNK1D as a potent regulator of circadian period, and mice that overexpress CSNK1D show alterations in dopamine receptors and hyperactive behaviors that may model some aspects of mania [147]. Other studies, large-scale screens of small-molecule libraries, have identified inhibitors of CSNK1D and, to a lesser extent, CSNK1E and casein kinase II (CSNK2) as potential regulators of the circadian clock [148, 149]. In mouse SCN slices, the CSNK1D inhibitor PF-670462 showed period-lengthening effects in reporter gene rhythm assays. However, in arrhythmic, null mutant Vipr2 SCN-slices, PF-670462 had a pronounced effect on amplitude and was able to restore 24 h rhythms. When studied in vivo, arrhythmic mice carrying the null Vipr2 mutation were tested under 12/12 LD conditions. Behavioral rhythms in these mice were restored by PF-670462 and maintained under both DD and LL conditions [150]. In other studies, PF-670462 regulated α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamate receptor phosphorylation and reduced amphetamine-induced locomotor activity, suggesting a possible antimanic effect [151]. Using chronic administration of a drug similar to PF-670462 in mice, depressive and anxiety-like behaviors were attenuated in the CLOCKΔ19 mouse, an arrhythmic mutant used extensively to model bipolar mania [152], whereas wild-type mice with an intact clock showed no behavioral or toxic effects from the drug. CSNKI inhibition serves as an interesting example of how there may be complex drug actions on the clock that affect multiple rhythm parameters. Cell-based and SCN slice rhythm assays highlight the effects of CSNKI inhibitors on period, but this feature of the drug may not be the one that restores rhythms in arrhythmic mouse models. Instead, by slowing PER turnover in the arrhythmic mutants, the main effect of this drug may be to restore rhythms by increasing the amplitude of PER expression. Therefore, it may be that a putative therapeutic effect of this drug class in BD would be most notable in subjects where rhythms were severely disturbed (from amplitude increase), but perhaps it would be less effective when rhythms are largely intact and phase delayed (from period lengthening).
4.5 Nuclear Receptors REV-ERBs/RORs
The REV-ERB and ROR nuclear receptors act as transcription factors that regulate the expression of BMAL1 and CLOCK and therefore act as critical regulators of circadian rhythms [153]. Considerable experimental data suggest the REV-ERB/ROR pathway intersects with a number of mood-related behaviors and could be a viable target for future drug development. In mice, loss of the REV-ERBα gene causes time-dependent elevations in dopamine and alterations in mood-related behaviors that overlap with BD symptoms [154]. Interestingly, protein stability REV-ERBα is regulated in cells by lithium through GSK3B inhibition [32], and genetic variation in REV-ERBα and GSK3B predicted lithium responsiveness in BD patients [155]. This suggests that modulation of REV-ERB may partly explain some of lithium’s therapeutic action in BD. Previously classified as “orphan receptors”, the ligands for the REV-ERB/ROR receptors are now increasingly well-characterized. REV-ERBα/β both bind heme, RORA binds 24-hydroxycholesterol (24-OHC) as an inverse agonist and RORB binds all-trans retinoic acid and other retinoids [156]. RORC (also called RORγ) is thought to bind the cholesterol precursor desmosterol but has isoforms with potentially distinct binding profiles [157]. In recent years, small-molecule modulators of REV-ERBs/RORs have been developed and show promise as modulators of the immune system and metabolism [158]. In cells transfected with circadian reporter genes, the REV-ERB drugs primarily modulate amplitude, with agonists blunting the expression of Bmal-luc in both peripheral cell lines and SCN slices [158]. Small molecules targeting the RORs are less well-characterized with respect to circadian rhythms but include examples of other amplitude-enhancing drugs such as nobiletin [159]. With respect to behavioral effects, the REV-ERB/ROR drugs remain incompletely characterized. However, early studies did show that REV-ERB agonists have CNS effects, causing increased wakefulness, reduced anxiety-like behaviors and reduced behavioral response to cocaine [160], indicating that the REV-ERB system could be targeted in BD to alter some aspects of the illness, perhaps with actions on depression. However, REV-ERB/ROR proteins are highly tissue specific and may act differently in some brain regions than in peripheral cells, and there remains some question as to whether the effects of REV-ERB-acting drugs are nuclear receptor specific, mediated by the circadian clock or through pleiotropic effects on other target systems [161]. Accordingly, there remains a need to develop additional molecules that specifically target REV-ERB/ROR proteins. As yet, no clinical trials of REV-ERBs/ROR-acting drugs have been conducted in humans. However, the REV-ERB agonist SR9009 is sold without regulatory approval as an exercise supplement. It remains to be seen whether there are clinically important effects, either favorable or unfavorable, among users of the drug.
4.6 Glycogen Synthase Kinase 3
At least one of the mechanisms by which lithium affects circadian rhythms and stabilizes mood is inhibition of GSK3. GSK3 inhibition has been associated with a number of effects that could potentially be therapeutic in BD, including dendritic sprouting, neuroprotection, adult neurogenesis and modulation of circadian rhythms. Large-scale drug library screens have shown that GSK3 inhibitors have potent effects on the circadian clock, typically causing period shortening and increased amplitude [40], effects on rhythms that are similar to those of lithium on rhythms in fibroblast cultures from lithium-responsive patients with BD [39, 162]. The mood stabilizer valproic acid has also been identified as an inhibitor of GSK3B and has similar effects on period in both mouse models of mania and peripheral cells from patients with BD [163]. In the CLOCKΔ19 mutant model of mania, selective small-molecule GSK3 inhibitors normalized the novelty-induced hyperactivity phenotype in this arrhythmic mutant [164]. The same drugs attenuated the effects of amphetamine on locomotor behaviors and pre-pulse inhibition on wild-type mice, with effects comparable to those of another mood stabilizer, valproic acid. Despite the considerable promise around GSK3 inhibition as a drug mechanism in BD and perhaps other neurological disorders such as Alzheimer’s dementia, the widespread nature of GSK3 expression makes development of drugs targeting this system difficult, largely because of the high propensity for off-target effects on bone, the gall bladder and blood glucose levels [165]. Nonetheless, GSK3 inhibitors remain an active area of research and are in active clinical trials.
4.7 Cryptochromes
The CRY proteins participate directly in circadian clock mechanism, serving as negative feedback inhibitors of CLOCK/BMAL transcriptional activators. Small-molecule CRY activators have been developed that stabilize the CRY protein by inhibiting FBXL3-dependent degradation of the protein and extend the duration of CRY repression of CLOCK/BMAL [166, 167]. These compounds also reduce the expression of Per messenger RNAs (mRNAs) through a mechanism that is not well-described. The net effect on clock function is to lengthen the period of the circadian cycle, both in peripheral cells and SCN slices. In hepatocytes, a CRY activator (KL001) reduced gluconeogenesis, indicating that the effects on the molecular clock translate into physiological outcomes [168]. Working through a distinct mechanism, CRY inhibitors (e.g., KS15) have also been developed that block the inhibitory CRY-BMAL protein interaction and subsequently increase the activity of CLOCK/BMAL transcriptional activation on E-box elements, modulating rhythm amplitude and minimally affecting period [169]. CRY1 proteins have also been shown to modulate G-protein coupled receptor activity in the cytosol, inhibiting the ability of the Gαs subunit to stimulate adenylyl cyclase [168]. This inhibition leads to downstream reductions in cAMP and inactivation of the transcription factor CREB. Work on CRY proteins lags behind the other mechanisms covered previously. To date, no data investigating whether treatment with a KL001-like drug could affect the ability of CRY to negatively regulate Gs-protein and its downstream pathways have been published. Additionally, no published studies have demonstrated in vivo activity of these drugs, either in humans or animals, nor studied their potential for toxic effects, and the mechanism of CRY modulation remains unstudied in the brain. Nonetheless, small-molecule modulators of CRY warrant further investigation as drugs targeting CRY proteins directly may have greater specificity than drugs that target clock inputs (e.g., protein kinases) that typically have off-target effects. Drugs targeting the FBXL E3-ubiquitin ligase proteins may be another promising mechanism by which to target CRY proteins to obtain additional control over circadian period. Loss of the Fbxl3 gene lengthens period, whereas loss of Fbxl21 shortens period [170]. One can imagine situations where directing the circadian period is advantageous, and FBXL proteins could offer a potential “switch” whereby this could be accomplished. Efforts are underway to develop modulators of E3-ubiquitin ligase for cancer [171]. Interestingly, these early efforts have identified the antidepressant clomipramine as an E3-ubiquitin ligase inhibitor, showing the plausibility of CNS uses for these drugs [171].
4.8 Pharmacological Modulation of Retinal Photoreceptors
Finally, it is worth considering how pharmacological approaches to BD could make use of light-dependent, mood-regulatory mechanisms that include both circadian and noncircadian mechanisms. These pathways contribute to light-induced antidepressant (or manic) actions and/or dark-induced antimanic (or depressive) actions. Recent work in mice indicates that photic pathways into the brain from ipRGCs target additional brain regions beyond the SCN and have effects on mood independent of the circadian clock [19]. The retinal photoreceptors responsible for sending light information to the SCN are relatively accessible compared with other CNS tissues and may be amenable to direct application of drugs that would otherwise be difficult to administer systemically (e.g., antagonists of the melanopsin-mediated signaling pathway [172]) and/or that make use of targeted newly developed molecular approaches that precisely target circuits in an anatomically restricted manner that targets only relevant cells. Preliminary work using chemogenetic approaches and optogenetic stimulation have shown some potential for translation [173, 174]. It may also be possible to use light-based somatic therapies such as bright light stimulation to augment the action of systemic drugs preferentially in brain regions that receive strong light inputs.
4.9 Other Targets
A variety of other signaling pathways have shown links to the circadian clock but are not as well-studied or have complex mechanisms of action (e.g., longdaysin [96]) or toxic drug effects (e.g. chemotherapeutic agents [40]) that may not be practical in clinical use for BD. For instance, we have inhibited DUSP6, a gene encoding the MAPK phosphatase (MKP) 1 to enhance rhythm amplitude in human cell lines [47]. MKP inhibitors are in development as chemotherapeutic agents in cancer [175]. However, given the ubiquitous expression of MAPKs, it remains unclear whether the tolerability profile of these drugs, even if they were successful at rhythm modulation, would make them suitable for use in BD. Among the more promising candidates in this group is the AMP-dependent kinase (AMPK), which is expressed in the brain, regulates CRY stability and may be involved in regulation of the response of calcium channels to photic inputs. Both activators and inhibitors of AMPK have been developed that may have effects on the circadian clock. Inositol pyrophosphates are brain molecules synthesized by the actions of inositol hexakisphosphate (IP6) kinases (IP6Ks). IP6Ks stimulate GSK3B signaling and regulate motor and social behaviors as well as the response to psychostimulants [176]. In peripherally derived cell lines, IP6K inhibitors increase circadian rhythm amplitude, similar to the effects of GSK3B inhibition [162].
5 Clinical Trial Methods to Assess Circadian Rhythms
To approve a new drug for use in humans, clinical trials demonstrating both safety and efficacy are essential. Circadian rhythms can be assessed in a variety of ways, each of which has different strengths and weaknesses and is more or less suitable to assessing various dimensions of rhythms. Melatonin assays using biological fluids such as blood, urine and saliva are often considered the gold standard but often require biochemical analysis and repeated measurements over time and specialized lighting conditions that can affect results obtained outside a laboratory environment. For this reason, there has been an ongoing search among chronobiologists to identify alternative means to measure rhythms in human subjects. Fundamental hurdles faced by many of these methods are masking (i.e., loss of circadian signal due to interference from light or other factors driving sleep) and distinguishing state versus trait aspects of activity patterns. For humans living in standard environmental conditions, the light–dark cycle imposes a ~ 24 h diurnal cycle on subjects that may make it difficult to resolve changes in the underlying biological rhythm. Mood symptoms of BD such as insomnia and hyperactivity may disrupt typical sleep/activity patterns and further mask underlying circadian rhythm traits. Masking can be overcome to some extent by using forced desynchrony or constant routine protocols, methods that require long periods of observation in special laboratories [177, 178]. However, in BD it may also be necessary to perform long-term observations that extend beyond the duration of a mood episode. Other factors that may affect the utility of circadian rhythm measures include cost, scalability (i.e., how many subjects can be conveniently sampled), logistical factors and sensitivity to nonspecific clinical variables (e.g., age). Fortunately, in the age of mobile technology, widespread connectivity, wearable sensors and high-throughput biology, there is good reason to expect many of these methods will be improved and/or made more cost effective in coming years. For instance, wrist actigraph measurements of sleep and activity in patients with BD reveal specific differences in circadian phase that correlate with mood state, cortisol rhythms and cellular clock gene expression. Depressive episodes were associated with phase delays, whereas manic episodes were associated with phase advances, and both normalized after recovery [179]. This study provides an important proof-of principle, indicating that circadian biomarkers have mood correlates that may be practically studied in clinical trials. Other work has shown that it may be possible to impute chronotype genetically, based on a polygenic score of trait morningness-associated variants [67, 68, 180] or from the gene expression profile of a biological sample taken at a single timepoint to estimate the circadian phase of a subject [181,182,183]. The latter method shows particular promise as a convenient point-of-care test that might allow for the reliable assessment of rhythms and circadian organization with a single test, potentially diminishing the need for continuous measurement over an extended time period. Methods are summarized in Table 2.
The US FDA has approved relatively few drugs targeting circadian rhythms and no drugs for a psychiatric disorder such as BD, in which the mechanism of action relies upon the circadian clock but the primary endpoint reflects changes in mood state or related symptoms. Tasimelteon, a melatonin receptor agonist, was approved in 2013 for non-24 h circadian rhythm disorder (non-24 CRD) in blind people. The primary endpoints of the clinical trials were related to synchronization, as determined by urinary 6-hydroxymelatonin sulfate, a metabolite of endogenous melatonin and biomarker of circadian phase [184]. A standardized clinical inventory was also used to assess function in patients [184]. Press reports from the time made it clear that the FDA had not entirely determined which biomarkers, clinical endpoints and statistical methods were most appropriate in evaluating a circadian drug [185]. The issues have not been addressed in intervening years and remain mostly unresolved. Therefore, as clinical trials examining clock compounds are completed, it will be essential for industry and the FDA to standardize the units and accepted norms for potential endpoint markers and develop guidelines for which circadian biomarkers should be used. This issue may be particularly complicated when the clinical indication for a drug is a mood disorder such as BD, with clinical endpoints (e.g., depression, mania, suicidality) that may not necessarily directly relate to a circadian parameter (e.g., phase or amplitude). There may be debate as to whether the circadian effect of the drug should be considered in terms of efficacy, safety or even at all or whether to focus exclusively on mood-related outcomes.
6 Conclusions
Existing treatments for BD are often ineffective and/or poorly tolerated because of side effects and the burdens placed on patients to maintain compliance. Therefore, the demand is great for improved mood-stabilizing pharmacotherapies. A major challenge to treating BD and other psychiatric disorders remains our incomplete understanding of the brain systems involved. However, the disruption of rhythmic behaviors and physiology commonly observed in BD perhaps holds important clues indicating that disturbances in the circadian clock may be an important pathophysiological mechanism contributing to the disorder. The success of lithium in treating a substantial number of patients with BD, and the use of circadian biomarkers to more readily identify lithium-responsive patients, illustrates how drugs acting upon the clock may have an important place in therapy for BD. The more recent success of melatonin agonists and 5HT7 antagonists as therapeutic agents in BD further underscore the potential value of this approach. As the role of circadian rhythms in human health continues to be revealed, considerable effort has been made in developing small-molecule modulators of the clock proteins. Many of these efforts are in disease areas other than BD, particularly immunology and metabolism (two areas increasingly recognized as adjacent and relevant to psychiatric disorders). Therefore, the CNS effects and clinical implications of targeting some clock proteins remain to be fully elucidated and certainly not all will be applicable or practical for clinical use. However, the lessons learned from using clock-acting drugs, either in BD or adjacent health fields, will provide important lessons, further elucidating new disease mechanisms and providing practical experience for clinicians, researchers and regulators on the various aspects of conducting therapeutic interventions affecting the clock. This review has taken a deliberately broad view of identifying potential drug targets for BD, recognizing that there will be many failures and false starts but in the hope that further research in these areas will stimulate further innovation and succeed in helping patients and families affected by BD. Additional effort in this rapidly emerging area is warranted.
References
Merikangas KR, Jin R, He JP, Kessler RC, Lee S, Sampson NA, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68(3):241–51. https://doi.org/10.1001/archgenpsychiatry.2011.12.
McCarthy MJ, Welsh DK. Cellular circadian clocks in mood disorders. J Biol Rhythms. 2012;27(5):339–52. https://doi.org/10.1177/0748730412456367.
Landgraf D, McCarthy MJ, Welsh DK. Circadian clock and stress interactions in the molecular biology of psychiatric disorders. Curr Psychiatry Rep. 2014;16(10):483. https://doi.org/10.1007/s11920-014-0483-7.
Maruani J, Anderson G, Etain B, Lejoyeux M, Bellivier F, Geoffroy PA. The neurobiology of adaptation to seasons: relevance and correlations in bipolar disorders. Chronobiol Int. 2018;35(10):1335–53. https://doi.org/10.1080/07420528.2018.1487975.
Charney AW, Ruderfer DM, Stahl EA, Moran JL, Chambert K, Belliveau RA, et al. Evidence for genetic heterogeneity between clinical subtypes of bipolar disorder. Transl Psychiatry. 2017;7(1):e993. https://doi.org/10.1038/tp.2016.242.
Malhi GS, Fritz K, Elangovan P, Irwin L. Mixed States: modelling and management. CNS Drugs. 2019;33(4):301–13. https://doi.org/10.1007/s40263-019-00609-3.
Gonzalez R, Suppes T, Zeitzer J, McClung C, Tamminga C, Tohen M, et al. The association between mood state and chronobiological characteristics in bipolar I disorder: a naturalistic, variable cluster analysis-based study. Int J Bipolar Disord. 2018;6(1):5. https://doi.org/10.1186/s40345-017-0113-5.
McCarthy MJ. Missing a beat: assessment of circadian rhythm abnormalities in bipolar disorder in the genomic era. Psychiatr Genet. 2019;29(2):29–36. https://doi.org/10.1097/YPG.0000000000000215.
Frank E, Kupfer DJ, Thase ME, Mallinger AG, Swartz HA, Fagiolini AM, et al. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Arch Gen Psychiatry. 2005;62(9):996–1004. https://doi.org/10.1001/archpsyc.62.9.996.
Roecklein KA, Wong PM, Miller MA, Donofry SD, Kamarck ML, Brainard GC. Melanopsin, photosensitive ganglion cells, and seasonal affective disorder. Neurosci Biobehav Rev. 2013;37(3):229–39. https://doi.org/10.1016/j.neubiorev.2012.12.009.
Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23(1–2):497–509. https://doi.org/10.1080/07420520500545979.
Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–62. https://doi.org/10.1146/annurev-neuro-060909-153128.
Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–77. https://doi.org/10.1146/annurev-physiol-021909-135919.
Schmidt TM, Chen SK, Hattar S. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 2011;34(11):572–80. https://doi.org/10.1016/j.tins.2011.07.001.
Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, et al. Mutagenesis and mapping of a mouse gene, clock, essential for circadian behavior. Science. 1994;264(5159):719–25.
Landgraf D, Long JE, Proulx CD, Barandas R, Malinow R, Welsh DK. Genetic disruption of circadian rhythms in the suprachiasmatic nucleus causes helplessness, behavioral despair, and anxiety-like behavior in mice. Biol Psychiatry. 2016;80(11):827–35. https://doi.org/10.1016/j.biopsych.2016.03.1050.
Paul JR, Davis JA, Goode LK, Becker BK, Fusilier A, Meador-Woodruff A, et al. Circadian regulation of membrane physiology in neural oscillators throughout the brain. Eur J Neurosci. 2019. https://doi.org/10.1111/ejn.14343.
LeGates TA, Fernandez DC, Hattar S. Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci. 2014;15(7):443–54. https://doi.org/10.1038/nrn3743.
Fernandez DC, Fogerson PM, Lazzerini Ospri L, Thomsen MB, Layne RM, Severin D, et al. Light affects mood and learning through distinct retina–brain pathways. Cell. 2018;175(1):71–84. https://doi.org/10.1016/j.cell.2018.08.004 (e18).
LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H, et al. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 2012;491(7425):594–8. https://doi.org/10.1038/nature11673.
Sidor MM, Spencer SM, Dzirasa K, Parekh PK, Tye KM, Warden MR, et al. Daytime spikes in dopaminergic activity drive rapid mood-cycling in mice. Mol Psychiatry. 2015;20(11):1406–19. https://doi.org/10.1038/mp.2014.167.
Ferris MJ, Espana RA, Locke JL, Konstantopoulos JK, Rose JH, Chen R, et al. Dopamine transporters govern diurnal variation in extracellular dopamine tone. Proc Natl Acad Sci USA. 2014;111(26):E2751–9. https://doi.org/10.1073/pnas.1407935111.
Ozburn AR, Falcon E, Twaddle A, Nugent AL, Gillman AG, Spencer SM, et al. Direct regulation of diurnal Drd3 expression and cocaine reward by NPAS2. Biol Psychiatry. 2015;77(5):425–33. https://doi.org/10.1016/j.biopsych.2014.07.030.
Mukherjee S, Coque L, Cao JL, Kumar J, Chakravarty S, Asaithamby A, et al. Knockdown of clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biol Psychiatry. 2010;68(6):503–11. https://doi.org/10.1016/j.biopsych.2010.04.031.
Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, et al. Mania-like behavior induced by disruption of clock. Proc Natl Acad Sci USA. 2007;104(15):6406–11. https://doi.org/10.1073/pnas.0609625104.
Mure LS, Le HD, Benegiamo G, Chang MW, Rios L, Jillani N, et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science. 2018. https://doi.org/10.1126/science.aao0318.
Landgraf D, Long JE, Welsh DK. Depression-like behaviour in mice is associated with disrupted circadian rhythms in nucleus accumbens and periaqueductal grey. Eur J Neurosci. 2016;43(10):1309–20. https://doi.org/10.1111/ejn.13085.
Freyberg Z, McCarthy MJ. Dopamine D2 receptors and the circadian clock reciprocally mediate antipsychotic drug-induced metabolic disturbances. NPJ Schizophr. 2017;3:17. https://doi.org/10.1038/s41537-017-0018-4.
Barandas R, Landgraf D, McCarthy MJ, Welsh DK. Circadian clocks as modulators of metabolic comorbidity in psychiatric disorders. Curr Psychiatry Rep. 2015;17(12):98. https://doi.org/10.1007/s11920-015-0637-2.
Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014;24(2):90–9. https://doi.org/10.1016/j.tcb.2013.07.002.
Harada Y, Sakai M, Kurabayashi N, Hirota T, Fukada Y. Ser-557-phosphorylated mCRY2 is degraded upon synergistic phosphorylation by glycogen synthase kinase-3 beta. J Biol Chem. 2005;280(36):31714–21. https://doi.org/10.1074/jbc.M506225200.
Yin L, Wang J, Klein PS, Lazar MA. Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock. Science. 2006;311(5763):1002–5. https://doi.org/10.1126/science.1121613.
Sahar S, Zocchi L, Kinoshita C, Borrelli E, Sassone-Corsi P. Regulation of BMAL1 protein stability and circadian function by GSK3beta-mediated phosphorylation. PLoS One. 2010;5(1):e8561. https://doi.org/10.1371/journal.pone.0008561.
Iitaka C, Miyazaki K, Akaike T, Ishida N. A role for glycogen synthase kinase-3beta in the mammalian circadian clock. J Biol Chem. 2005;280(33):29397–402. https://doi.org/10.1074/jbc.M503526200.
Luciano AK, Zhou W, Santana JM, Kyriakides C, Velazquez H, Sessa WC. Clock phosphorylation by AKT regulates its nuclear accumulation and circadian gene expression in peripheral tissues. J Biol Chem. 2018;293(23):9126–36. https://doi.org/10.1074/jbc.RA117.000773.
Sanada K, Harada Y, Sakai M, Todo T, Fukada Y. Serine phosphorylation of mCRY1 and mCRY2 by mitogen-activated protein kinase. Genes Cells. 2004;9(8):697–708. https://doi.org/10.1111/j.1356-9597.2004.00758.x.
Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA. 1996;93(16):8455–9.
Berridge MJ, Downes CP, Hanley MR. Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989;59(3):411–9.
McCarthy M, Wei H, Nievergelt C, Stautland A, Maihofer A, Welsh DK. Chronotype and cellular circadian rhythms predict the clinical response to lithium maintenance treatment in patients with bipolar disorder. Neuropsychopharmacology. 2019;44:620–8.
Hirota T, Lewis WG, Liu AC, Lee JW, Schultz PG, Kay SA. A chemical biology approach reveals period shortening of the mammalian circadian clock by specific inhibition of GSK-3beta. Proc Natl Acad Sci USA. 2008;105(52):20746–51. https://doi.org/10.1073/pnas.0811410106.
McEachron DL, Kripke DF, Wyborney VG. Lithium promotes entrainment of rats to long circadian light-dark cycles. Psychiatry Res. 1981;5(1):1–9.
Kripke DF, Wyborney VG. Lithium slows rat circadian activity rhythms. Life Sci. 1980;26(16):1319–21.
Kripke DF, Judd LL, Hubbard B, Janowsky DS, Huey LY. The effect of lithium carbonate on the circadian rhythm of sleep in normal human subjects. Biol Psychiatry. 1979;14(3):545–8.
Welsh DK, Moore-Ede MC. Lithium lengthens circadian period in a diurnal primate, Saimiri sciureus. Biol Psychiatry. 1990;28(2):117–26.
McCarthy MJ, Wei H, Marnoy Z, Darvish RM, McPhie DL, Cohen BM, et al. Genetic and clinical factors predict lithium’s effects on PER2 gene expression rhythms in cells from bipolar disorder patients. Transl Psychiatry. 2013;3:e318. https://doi.org/10.1038/tp.2013.90.
Abe M, Herzog ED, Block GD. Lithium lengthens the circadian period of individual suprachiasmatic nucleus neurons. Neuroreport. 2000;11(14):3261–4.
McCarthy MJ, Wei H, Landgraf D, Le Roux MJ, Welsh DK. Disinhibition of the extracellular-signal-regulated kinase restores the amplification of circadian rhythms by lithium in cells from bipolar disorder patients. Eur Neuropsychopharmacol. 2016;26(8):1310–9. https://doi.org/10.1016/j.euroneuro.2016.05.003.
McCarthy MJ, LeRoux M, Wei H, Beesley S, Kelsoe JR, Welsh DK. Calcium channel genes associated with bipolar disorder modulate lithium’s amplification of circadian rhythms. Neuropharmacology. 2015. https://doi.org/10.1016/j.neuropharm.2015.10.017.
Schnell A, Sandrelli F, Ranc V, Ripperger JA, Brai E, Alberi L, et al. Mice lacking circadian clock components display different mood-related behaviors and do not respond uniformly to chronic lithium treatment. Chronobiol Int. 2015;32(8):1075–89. https://doi.org/10.3109/07420528.2015.1062024.
Harvey AG. Sleep and circadian rhythms in bipolar disorder: seeking synchrony, harmony, and regulation. Am J Psychiatry. 2008;165(7):820–9. https://doi.org/10.1176/appi.ajp.2008.08010098.
Harvey AG, Schmidt DA, Scarna A, Semler CN, Goodwin GM. Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. Am J Psychiatry. 2005;162(1):50–7. https://doi.org/10.1176/appi.ajp.162.1.50.
Duffy A, Alda M, Hajek T, Sherry SB, Grof P. Early stages in the development of bipolar disorder. J Affect Disord. 2010;121(1–2):127–35. https://doi.org/10.1016/j.jad.2009.05.022.
Jackson A, Cavanagh J, Scott J. A systematic review of manic and depressive prodromes. J Affect Disord. 2003;74(3):209–17.
Skjelstad DV, Malt UF, Holte A. Symptoms and signs of the initial prodrome of bipolar disorder: a systematic review. J Affect Disord. 2010;126(1–2):1–13. https://doi.org/10.1016/j.jad.2009.10.003.
Rucklidge JJ. Retrospective parent report of psychiatric histories: do checklists reveal specific prodromal indicators for postpubertal-onset pediatric bipolar disorder? Bipolar Disord. 2008;10(1):56–66. https://doi.org/10.1111/j.1399-5618.2008.00533.x.
Eidelman P, Talbot LS, Gruber J, Harvey AG. Sleep, illness course, and concurrent symptoms in inter-episode bipolar disorder. J Behav Ther Exp Psychiatry. 2010;41(2):145–9. https://doi.org/10.1016/j.jbtep.2009.11.007.
Colombo C, Benedetti F, Barbini B, Campori E, Smeraldi E. Rate of switch from depression into mania after therapeutic sleep deprivation in bipolar depression. Psychiatry Res. 1999;86(3):267–70.
Eidelman P, Talbot LS, Gruber J, Hairston I, Harvey AG. Sleep architecture as correlate and predictor of symptoms and impairment in inter-episode bipolar disorder: taking on the challenge of medication effects. J Sleep Res. 2010;19(4):516–24. https://doi.org/10.1111/j.1365-2869.2010.00826.x.
Hudson JI, Lipinski JF, Keck PE Jr, Aizley HG, Lukas SE, Rothschild AJ, et al. Polysomnographic characteristics of young manic patients Comparison with unipolar depressed patients and normal control subjects. Arch Gen Psychiatry. 1992;49(5):378–83.
Gruber J, Harvey AG, Wang PW, Brooks JO 3rd, Thase ME, Sachs GS, et al. Sleep functioning in relation to mood, function, and quality of life at entry to the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). J Affect Disord. 2009;114(1–3):41–9. https://doi.org/10.1016/j.jad.2008.06.028.
Jones SH, Hare DJ, Evershed K. Actigraphic assessment of circadian activity and sleep patterns in bipolar disorder. Bipolar Disord. 2005;7(2):176–86. https://doi.org/10.1111/j.1399-5618.2005.00187.x.
Gonzalez R, Tamminga CA, Tohen M, Suppes T. The relationship between affective state and the rhythmicity of activity in bipolar disorder. J Clin Psychiatry. 2014;75(4):e317–22. https://doi.org/10.4088/JCP.13m08506.
McKenna BS, Drummond SP, Eyler LT. Associations between circadian activity rhythms and functional brain abnormalities among euthymic bipolar patients: a preliminary study. J Affect Disord. 2014;164:101–6. https://doi.org/10.1016/j.jad.2014.04.034.
Pagani L, St Clair PA, Teshiba TM, Service SK, Fears SC, Araya C, et al. Genetic contributions to circadian activity rhythm and sleep pattern phenotypes in pedigrees segregating for severe bipolar disorder. Proc Natl Acad Sci USA. 2016;113(6):E754–61. https://doi.org/10.1073/pnas.1513525113.
Lyall LM, Wyse CA, Graham N, Ferguson A, Lyall DM, Cullen B, et al. Association of disrupted circadian rhythmicity with mood disorders, subjective wellbeing, and cognitive function: a cross-sectional study of 91 105 participants from the UK Biobank. Lancet Psychiatry. 2018;5(6):507–14. https://doi.org/10.1016/S2215-0366(18)30139-1.
Teicher MH. Actigraphy and motion analysis: new tools for psychiatry. Harv Rev Psychiatry. 1995;3(1):18–35.
Hu Y, Shmygelska A, Tran D, Eriksson N, Tung JY, Hinds DA. GWAS of 89,283 individuals identifies genetic variants associated with self-reporting of being a morning person. Nat Commun. 2016;7:10448. https://doi.org/10.1038/ncomms10448.
Jones SE, Tyrrell J, Wood AR, Beaumont RN, Ruth KS, Tuke MA, et al. Genome-wide association analyses in 128,266 individuals identifies new morningness and sleep duration loci. PLoS Genet. 2016;12(8):e1006125. https://doi.org/10.1371/journal.pgen.1006125.
Wood J, Birmaher B, Axelson D, Ehmann M, Kalas C, Monk K, et al. Replicable differences in preferred circadian phase between bipolar disorder patients and control individuals. Psychiatry Res. 2009;166(2–3):201–9. https://doi.org/10.1016/j.psychres.2008.03.003.
Ahn YM, Chang J, Joo YH, Kim SC, Lee KY, Kim YS. Chronotype distribution in bipolar I disorder and schizophrenia in a Korean sample. Bipolar Disord. 2008;10(2):271–5. https://doi.org/10.1111/j.1399-5618.2007.00573.x.
Mansour HA, Wood J, Chowdari KV, Dayal M, Thase ME, Kupfer DJ, et al. Circadian phase variation in bipolar I disorder. Chronobiol Int. 2005;22(3):571–84. https://doi.org/10.1081/CBI-200062413.
Ehlers CL, Frank E, Kupfer DJ. Social zeitgebers and biological rhythms. A unified approach to understanding the etiology of depression. Arch Gen Psychiatry. 1988;45(10):948–52.
Ashman SB, Monk TH, Kupfer DJ, Clark CH, Myers FS, Frank E, et al. Relationship between social rhythms and mood in patients with rapid cycling bipolar disorder. Psychiatry Res. 1999;86(1):1–8.
Malkoff-Schwartz S, Frank E, Anderson B, Sherrill JT, Siegel L, Patterson D, et al. Stressful life events and social rhythm disruption in the onset of manic and depressive bipolar episodes: a preliminary investigation. Arch Gen Psychiatry. 1998;55(8):702–7.
Wood S, Loudon A. Clocks for all seasons: unwinding the roles and mechanisms of circadian and interval timers in the hypothalamus and pituitary. J Endocrinol. 2014;222(2):R39–59. https://doi.org/10.1530/JOE-14-0141.
Kripke DF, Elliott JA, Welsh DK, Youngstedt SD. Photoperiodic and circadian bifurcation theories of depression and mania. F1000Res. 2015;4:107. https://doi.org/10.12688/f1000research.6444.1.
Hakkarainen R, Johansson C, Kieseppa T, Partonen T, Koskenvuo M, Kaprio J, et al. Seasonal changes, sleep length and circadian preference among twins with bipolar disorder. BMC Psychiatry. 2003;3:6. https://doi.org/10.1186/1471-244X-3-6.
Cassidy F, Carroll BJ. Seasonal variation of mixed and pure episodes of bipolar disorder. J Affect Disord. 2002;68(1):25–31.
Silverstone T, Romans S, Hunt N, McPherson H. Is there a seasonal pattern of relapse in bipolar affective disorders? A dual northern and southern hemisphere cohort study. Br J Psychiatry. 1995;167(1):58–60. https://doi.org/10.1192/bjp.167.1.58.
Shin K, Schaffer A, Levitt AJ, Boyle MH. Seasonality in a community sample of bipolar, unipolar and control subjects. J Affect Disord. 2005;86(1):19–25. https://doi.org/10.1016/j.jad.2004.11.010.
Inder ML, Crowe MT, Porter R. Effect of transmeridian travel and jetlag on mood disorders: evidence and implications. Aust N Z J Psychiatry. 2016;50(3):220–7. https://doi.org/10.1177/0004867415598844.
Macchi MM, Bruce JN. Human pineal physiology and functional significance of melatonin. Front Neuroendocrinol. 2004;25(3–4):177–95. https://doi.org/10.1016/j.yfrne.2004.08.001.
Pacchierotti C, Iapichino S, Bossini L, Pieraccini F, Castrogiovanni P. Melatonin in psychiatric disorders: a review on the melatonin involvement in psychiatry. Front Neuroendocrinol. 2001;22(1):18–32. https://doi.org/10.1006/frne.2000.0202.
Nurnberger JI Jr, Adkins S, Lahiri DK, Mayeda A, Hu K, Lewy A, et al. Melatonin suppression by light in euthymic bipolar and unipolar patients. Arch Gen Psychiatry. 2000;57(6):572–9.
Robillard R, Naismith SL, Rogers NL, Scott EM, Ip TK, Hermens DF, et al. Sleep-wake cycle and melatonin rhythms in adolescents and young adults with mood disorders: comparison of unipolar and bipolar phenotypes. Eur Psychiatry. 2013;28(7):412–6. https://doi.org/10.1016/j.eurpsy.2013.04.001.
Lam RW, Berkowitz AL, Berga SL, Clark CM, Kripke DF, Gillin JC. Melatonin suppression in bipolar and unipolar mood disorders. Psychiatry Res. 1990;33(2):129–34.
Kennedy SH, Kutcher SP, Ralevski E, Brown GM. Nocturnal melatonin and 24-hour 6-sulphatoxymelatonin levels in various phases of bipolar affective disorder. Psychiatry Res. 1996;63(2–3):219–22.
Bullock B, McGlashan EM, Burns AC, Lu BS, Cain SW. Traits related to bipolar disorder are associated with an increased post-illumination pupil response. Psychiatry Res. 2019;278:35–41. https://doi.org/10.1016/j.psychres.2019.05.025.
Roecklein K, Wong P, Ernecoff N, Miller M, Donofry S, Kamarck M, et al. The post illumination pupil response is reduced in seasonal affective disorder. Psychiatry Res. 2013;210(1):150–8. https://doi.org/10.1016/j.psychres.2013.05.023.
Gaspar L, van de Werken M, Johansson AS, Moriggi E, Owe-Larsson B, Kocks JW, et al. Human cellular differences in cAMP–CREB signaling correlate with light-dependent melatonin suppression and bipolar disorder. Eur J Neurosci. 2014;40(1):2206–15. https://doi.org/10.1111/ejn.12602.
Daban C, Vieta E, Mackin P, Young AH. Hypothalamic-pituitary-adrenal axis and bipolar disorder. Psychiatr Clin N Am. 2005;28(2):469–80. https://doi.org/10.1016/j.psc.2005.01.005.
Belvederi Murri M, Prestia D, Mondelli V, Pariante C, Patti S, Olivieri B, et al. The HPA axis in bipolar disorder: systematic review and meta-analysis. Psychoneuroendocrinology. 2016;63:327–42. https://doi.org/10.1016/j.psyneuen.2015.10.014.
Linkowski P, Kerkhofs M, Van Onderbergen A, Hubain P, Copinschi G, L’Hermite-Baleriaux M, et al. The 24-hour profiles of cortisol, prolactin, and growth hormone secretion in mania. Arch Gen Psychiatry. 1994;51(8):616–24.
Linkowski P, Mendlewicz J, Leclercq R, Brasseur M, Hubain P, Golstein J, et al. The 24-hour profile of adrenocorticotropin and cortisol in major depressive illness. J Clin Endocrinol Metab. 1985;61(3):429–38. https://doi.org/10.1210/jcem-61-3-429.
Prossin AR, Chandler M, Ryan KA, Saunders EF, Kamali M, Papadopoulos V, et al. Functional TSPO polymorphism predicts variance in the diurnal cortisol rhythm in bipolar disorder. Psychoneuroendocrinology. 2018;89:194–202. https://doi.org/10.1016/j.psyneuen.2018.01.013.
Hirota T, Lee JW, Lewis WG, Zhang EE, Breton G, Liu X, et al. High-throughput chemical screen identifies a novel potent modulator of cellular circadian rhythms and reveals CKIalpha as a clock regulatory kinase. PLoS Biol. 2010;8(12):e1000559. https://doi.org/10.1371/journal.pbio.1000559.
Takaesu Y, Inoue Y, Ono K, Murakoshi A, Futenma K, Komada Y, et al. Circadian rhythm sleep-wake disorders predict shorter time to relapse of mood episodes in euthymic patients with bipolar disorder: a prospective 48-week study. J Clin Psychiatry. 2018. https://doi.org/10.4088/jcp.17m11565.
Possidente B, Lumia AR, McEldowney S, Rapp M. Fluoxetine shortens circadian period for wheel running activity in mice. Brain Res Bull. 1992;28(4):629–31.
Obrietan K, Impey S, Storm DR. Light and circadian rhythmicity regulate MAP kinase activation in the suprachiasmatic nuclei. Nat Neurosci. 1998;1(8):693–700. https://doi.org/10.1038/3695.
Dziema H, Oatis B, Butcher GQ, Yates R, Hoyt KR, Obrietan K. The ERK/MAP kinase pathway couples light to immediate-early gene expression in the suprachiasmatic nucleus. Eur J Neurosci. 2003;17(8):1617–27.
Gamble KL, Ciarleglio CM. Ryanodine receptors are regulated by the circadian clock and implicated in gating photic entrainment. J Neurosci. 2009;29(38):11717–9. https://doi.org/10.1523/JNEUROSCI.3820-09.2009.
Schmutz I, Chavan R, Ripperger JA, Maywood ES, Langwieser N, Jurik A, et al. A specific role for the REV-ERBalpha-controlled L-type voltage-gated calcium channel CaV1.2 in resetting the circadian clock in the late night. J Biol Rhythms. 2014;29(4):288–98. https://doi.org/10.1177/0748730414540453.
Oster H, Werner C, Magnone MC, Mayser H, Feil R, Seeliger MW, et al. cGMP-dependent protein kinase II modulates mPer1 and mPer2 gene induction and influences phase shifts of the circadian clock. Curr Biol. 2003;13(9):725–33.
Byku M, Gannon RL. Effects of the 5HT1A agonist/antagonist BMY 7378 on light-induced phase advances in hamster circadian activity rhythms during aging. J Biol Rhythms. 2000;15(4):300–5. https://doi.org/10.1177/074873000129001404.
Krystal AD, Zammit G. The sleep effects of lurasidone: a placebo-controlled cross-over study using a 4-h phase-advance model of transient insomnia. Hum Psychopharmacol. 2016;31(3):206–16. https://doi.org/10.1002/hup.2533.
Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol. 2012;351(2):152–66. https://doi.org/10.1016/j.mce.2012.01.004.
Missbach M, Jagher B, Sigg I, Nayeri S, Carlberg C, Wiesenberg I. Thiazolidine diones, specific ligands of the nuclear receptor retinoid Z receptor/retinoid acid receptor-related orphan receptor alpha with potent antiarthritic activity. J Biol Chem. 1996;271(23):13515–22. https://doi.org/10.1074/jbc.271.23.13515.
Comai S, Gobbi G. Unveiling the role of melatonin MT2 receptors in sleep, anxiety and other neuropsychiatric diseases: a novel target in psychopharmacology. J Psychiatry Neurosci. 2014;39(1):6–21. https://doi.org/10.1503/jpn.130009.
Comai S, Lopez-Canul M, De Gregorio D, Posner A, Ettaoussi M, Guarnieri FC, et al. Melatonin MT1 receptor as a novel target in neuropsychopharmacology: MT1 ligands, pathophysiological and therapeutic implications, and perspectives. Pharmacol Res. 2019;144:343–56. https://doi.org/10.1016/j.phrs.2019.04.015.
Comai S, Ochoa-Sanchez R, Dominguez-Lopez S, Bambico FR, Gobbi G. Melancholic-like behaviors and circadian neurobiological abnormalities in melatonin MT1 receptor knockout mice. Int J Neuropsychopharmacol. 2015. https://doi.org/10.1093/ijnp/pyu075.
Bersani G, Garavini A. Melatonin add-on in manic patients with treatment resistant insomnia. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24(2):185–91.
Leibenluft E, Feldman-Naim S, Turner EH, Wehr TA, Rosenthal NE. Effects of exogenous melatonin administration and withdrawal in five patients with rapid-cycling bipolar disorder. J Clin Psychiatry. 1997;58(9):383–8.
Zupancic M, Guilleminault C. Agomelatine: a preliminary review of a new antidepressant. CNS Drugs. 2006;20(12):981–92. https://doi.org/10.2165/00023210-200620120-00003.
Yu YM, Gao KR, Yu H, Shen YF, Li HF. Efficacy and safety of agomelatine vs paroxetine hydrochloride in chinese han patients with major depressive disorder: a multicentre, double-blind, noninferiority, randomized controlled trial. J Clin Psychopharmacol. 2018;38(3):226–33. https://doi.org/10.1097/JCP.0000000000000878.
Gupta K, Gupta R, Bhatia MS, Tripathi AK, Gupta LK. Effect of agomelatine and fluoxetine on HAM-D score, serum brain-derived neurotrophic factor, and tumor necrosis factor-alpha level in patients with major depressive disorder with severe depression. J Clin Pharmacol. 2017;57(12):1519–26. https://doi.org/10.1002/jcph.963.
Kennedy SH, Avedisova A, Belaidi C, Picarel-Blanchot F, de Bodinat C. Sustained efficacy of agomelatine 10 mg, 25 mg, and 25–50 mg on depressive symptoms and functional outcomes in patients with major depressive disorder. A placebo-controlled study over 6 months. Eur Neuropsychopharmacol. 2016;26(2):378–89. https://doi.org/10.1016/j.euroneuro.2015.09.006.
Fornaro M, McCarthy MJ, De Berardis D, De Pasquale C, Tabaton M, Martino M, et al. Adjunctive agomelatine therapy in the treatment of acute bipolar II depression: a preliminary open label study. Neuropsychiatr Dis Treat. 2013;9:243–51. https://doi.org/10.2147/NDT.S41557.
Calabrese JR, Guelfi JD, Perdrizet-Chevallier C. Agomelatine adjunctive therapy for acute bipolar depression: preliminary open data. Bipolar Disord. 2007;9(6):628–35. https://doi.org/10.1111/j.1399-5618.2007.00507.x.
Yatham LN, Vieta E, Goodwin GM, Bourin M, de Bodinat C, Laredo J, et al. Agomelatine or placebo as adjunctive therapy to a mood stabiliser in bipolar I depression: randomised double-blind placebo-controlled trial. Br J Psychiatry. 2016;208(1):78–86. https://doi.org/10.1192/bjp.bp.114.147587.
McElroy SL, Winstanley EL, Martens B, Patel NC, Mori N, Moeller D, et al. A randomized, placebo-controlled study of adjunctive ramelteon in ambulatory bipolar I disorder with manic symptoms and sleep disturbance. Int Clin Psychopharmacol. 2011;26(1):48–53. https://doi.org/10.1097/YIC.0b013e3283400d35.
Norris ER, Karen B, Correll JR, Zemanek KJ, Lerman J, Primelo RA, et al. A double-blind, randomized, placebo-controlled trial of adjunctive ramelteon for the treatment of insomnia and mood stability in patients with euthymic bipolar disorder. J Affect Disord. 2013;144(1–2):141–7. https://doi.org/10.1016/j.jad.2012.06.023.
Wang HR, Woo YS, Bahk WM. The role of melatonin and melatonin agonists in counteracting antipsychotic-induced metabolic side effects: a systematic review. Int Clin Psychopharmacol. 2016;31(6):301–6. https://doi.org/10.1097/YIC.0000000000000135.
Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001;916(1–2):172–91. https://doi.org/10.1016/s0006-8993(01)02890-6.
Lee JE, Atkins N Jr, Hatcher NG, Zamdborg L, Gillette MU, Sweedler JV, et al. Endogenous peptide discovery of the rat circadian clock: a focused study of the suprachiasmatic nucleus by ultrahigh performance tandem mass spectrometry. Mol Cell Proteom. 2010;9(2):285–97. https://doi.org/10.1074/mcp.M900362-MCP200.
Mieda M. The network mechanism of the central circadian pacemaker of the SCN: do AVP neurons play a more critical role than expected? Front Neurosci. 2019;13:139. https://doi.org/10.3389/fnins.2019.00139.
Harrington ME, Hoque S, Hall A, Golombek D, Biello S. Pituitary adenylate cyclase activating peptide phase shifts circadian rhythms in a manner similar to light. J Neurosci. 1999;19(15):6637–42.
Evans JA, Leise TL, Castanon-Cervantes O, Davidson AJ. Dynamic interactions mediated by nonredundant signaling mechanisms couple circadian clock neurons. Neuron. 2013;80(4):973–83. https://doi.org/10.1016/j.neuron.2013.08.022.
Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8(4):476–83. https://doi.org/10.1038/nn1419.
Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, et al. The VPAC(2) receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell. 2002;109(4):497–508. https://doi.org/10.1016/s0092-8674(02)00736-5.
Dias BG, Ressler KJ. PACAP and the PAC1 receptor in post-traumatic stress disorder. Neuropsychopharmacology. 2013;38(1):245–6. https://doi.org/10.1038/npp.2012.147.
Vacic V, McCarthy S, Malhotra D, Murray F, Chou HH, Peoples A, et al. Duplications of the neuropeptide receptor gene VIPR2 confer significant risk for schizophrenia. Nature. 2011;471(7339):499–503. https://doi.org/10.1038/nature09884.
King SB, Lezak KR, O’Reilly M, Toufexis DJ, Falls WA, Braas K, et al. The effects of prior stress on anxiety-like responding to intra-BNST pituitary adenylate cyclase activating polypeptide in male and female rats. Neuropsychopharmacology. 2017;42(8):1679–87. https://doi.org/10.1038/npp.2017.16.
Ago Y, Condro MC, Tan YV, Ghiani CA, Colwell CS, Cushman JD, et al. Reductions in synaptic proteins and selective alteration of prepulse inhibition in male C57BL/6 mice after postnatal administration of a VIP receptor (VIPR2) agonist. Psychopharmacology (Berl). 2015;232(12):2181–9. https://doi.org/10.1007/s00213-014-3848-z.
Reed HE, Meyer-Spasche A, Cutler DJ, Coen CW, Piggins HD. Vasoactive intestinal polypeptide (VIP) phase-shifts the rat suprachiasmatic nucleus clock in vitro. Eur J Neurosci. 2001;13(4):839–43.
Li JD, Burton KJ, Zhang C, Hu SB, Zhou QY. Vasopressin receptor V1a regulates circadian rhythms of locomotor activity and expression of clock-controlled genes in the suprachiasmatic nuclei. Am J Physiol Regul Integr Comp Physiol. 2009;296(3):R824–30. https://doi.org/10.1152/ajpregu.90463.2008.
Yamaguchi Y, Suzuki T, Mizoro Y, Kori H, Okada K, Chen Y, et al. Mice genetically deficient in vasopressin V1a and V1b receptors are resistant to jet lag. Science. 2013;342(6154):85–90. https://doi.org/10.1126/science.1238599.
Young LJ, Nilsen R, Waymire KG, MacGregor GR, Insel TR. Increased affiliative response to vasopressin in mice expressing the V1a receptor from a monogamous vole. Nature. 1999;400(6746):766–8. https://doi.org/10.1038/23475.
Zhou JN, Riemersma RF, Unmehopa UA, Hoogendijk WJ, van Heerikhuize JJ, Hofman MA, et al. Alterations in arginine vasopressin neurons in the suprachiasmatic nucleus in depression. Arch Gen Psychiatry. 2001;58(7):655–62.
Mori K, Miyazato M, Ida T, Murakami N, Serino R, Ueta Y, et al. Identification of neuromedin S and its possible role in the mammalian circadian oscillator system. Embo J. 2005;24(2):325–35. https://doi.org/10.1038/sj.emboj.7600526.
Ida T, Mori K, Miyazato M, Egi Y, Abe S, Nakahara K, et al. Neuromedin s is a novel anorexigenic hormone. Endocrinology. 2005;146(10):4217–23. https://doi.org/10.1210/en.2005-0107.
Sakamoto T, Mori K, Miyazato M, Kangawa K, Sameshima H, Nakahara K, et al. Involvement of neuromedin S in the oxytocin release response to suckling stimulus. Biochem Biophys Res Commun. 2008;375(1):49–53. https://doi.org/10.1016/j.bbrc.2008.07.124.
Lee IT, Chang AS, Manandhar M, Shan Y, Fan J, Izumo M, et al. Neuromedin s-producing neurons act as essential pacemakers in the suprachiasmatic nucleus to couple clock neurons and dictate circadian rhythms. Neuron. 2015;85(5):1086–102. https://doi.org/10.1016/j.neuron.2015.02.006.
Mitchell JD, Maguire JJ, Davenport AP. Emerging pharmacology and physiology of neuromedin U and the structurally related peptide neuromedin S. Br J Pharmacol. 2009;158(1):87–103. https://doi.org/10.1111/j.1476-5381.2009.00252.x.
Romanov RA, Zeisel A, Bakker J, Girach F, Hellysaz A, Tomer R, et al. Molecular interrogation of hypothalamic organization reveals distinct dopamine neuronal subtypes. Nat Neurosci. 2017;20(2):176–88. https://doi.org/10.1038/nn.4462.
Dulcis D, Jamshidi P, Leutgeb S, Spitzer NC. Neurotransmitter switching in the adult brain regulates behavior. Science. 2013;340(6131):449–53. https://doi.org/10.1126/science.1234152.
Aumann TD. Environment- and activity-dependent dopamine neurotransmitter plasticity in the adult substantia nigra. J Chem Neuroanat. 2016;73:21–32. https://doi.org/10.1016/j.jchemneu.2015.12.009.
Zhou M, Rebholz H, Brocia C, Warner-Schmidt JL, Fienberg AA, Nairn AC, et al. Forebrain overexpression of CK1delta leads to down-regulation of dopamine receptors and altered locomotor activity reminiscent of ADHD. Proc Natl Acad Sci USA. 2010;107(9):4401–6. https://doi.org/10.1073/pnas.0915173107.
Walton KM, Fisher K, Rubitski D, Marconi M, Meng QJ, Sladek M, et al. Selective inhibition of casein kinase 1 epsilon minimally alters circadian clock period. J Pharmacol Exp Ther. 2009;330(2):430–9. https://doi.org/10.1124/jpet.109.151415.
Isojima Y, Nakajima M, Ukai H, Fujishima H, Yamada RG, Masumoto KH, et al. CKIepsilon/delta-dependent phosphorylation is a temperature-insensitive, period-determining process in the mammalian circadian clock. Proc Natl Acad Sci USA. 2009;106(37):15744–9. https://doi.org/10.1073/pnas.0908733106.
Meng QJ, Maywood ES, Bechtold DA, Lu WQ, Li J, Gibbs JE, et al. Entrainment of disrupted circadian behavior through inhibition of casein kinase 1 (CK1) enzymes. Proc Natl Acad Sci USA. 2010;107(34):15240–5. https://doi.org/10.1073/pnas.1005101107.
Li D, Herrera S, Bubula N, Nikitina E, Palmer AA, Hanck DA, et al. Casein kinase 1 enables nucleus accumbens amphetamine-induced locomotion by regulating AMPA receptor phosphorylation. J Neurochem. 2011;118(2):237–47. https://doi.org/10.1111/j.1471-4159.2011.07308.x.
Arey R, McClung CA. An inhibitor of casein kinase 1 epsilon/delta partially normalizes the manic-like behaviors of the ClockDelta19 mouse. Behav Pharmacol. 2012;23(4):392–6. https://doi.org/10.1097/FBP.0b013e32835651fd.
Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, et al. Regulation of circadian behaviour and metabolism by REV-ERB-alpha and REV-ERB-beta. Nature. 2012;485(7396):123–7. https://doi.org/10.1038/nature11048.
Chung S, Lee EJ, Yun S, Choe HK, Park SB, Son HJ, et al. Impact of circadian nuclear receptor REV-ERBalpha on midbrain dopamine production and mood regulation. Cell. 2014;157(4):858–68. https://doi.org/10.1016/j.cell.2014.03.039.
McCarthy MJ, Nievergelt CM, Shekhtman T, Kripke DF, Welsh DK, Kelsoe JR. Functional genetic variation in the Rev-Erbalpha pathway and lithium response in the treatment of bipolar disorder. Genes Brain Behav. 2011;10(8):852–61. https://doi.org/10.1111/j.1601-183X.2011.00725.x.
Stehlin-Gaon C, Willmann D, Zeyer D, Sanglier S, Van Dorsselaer A, Renaud JP, et al. All-trans retinoic acid is a ligand for the orphan nuclear receptor ROR beta. Nat Struct Biol. 2003;10(10):820–5. https://doi.org/10.1038/nsb979.
Hu X, Wang Y, Hao LY, Liu X, Lesch CA, Sanchez BM, et al. Sterol metabolism controls T(H)17 differentiation by generating endogenous RORgamma agonists. Nat Chem Biol. 2015;11(2):141–7. https://doi.org/10.1038/nchembio.1714.
Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature. 2012;485(7396):62–8. https://doi.org/10.1038/nature11030.
He B, Nohara K, Park N, Park YS, Guillory B, Zhao Z, et al. The small molecule nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metab. 2016;23(4):610–21. https://doi.org/10.1016/j.cmet.2016.03.007.
Banerjee S, Wang Y, Solt LA, Griffett K, Kazantzis M, Amador A, et al. Pharmacological targeting of the mammalian clock regulates sleep architecture and emotional behaviour. Nat Commun. 2014;5:5759. https://doi.org/10.1038/ncomms6759.
Dierickx P, Emmett MJ, Jiang C, Uehara K, Liu M, Adlanmerini M, et al. SR9009 has REV-ERB-independent effects on cell proliferation and metabolism. Proc Natl Acad Sci USA. 2019;116(25):12147–52. https://doi.org/10.1073/pnas.1904226116.
Wei H, Landgraf D, Wang G, McCarthy MJ. Inositol polyphosphates contribute to cellular circadian rhythms: Implications for understanding lithium’s molecular mechanism. Cell Signal. 2018;44:82–91. https://doi.org/10.1016/j.cellsig.2018.01.001.
Landgraf D, Joiner WJ, McCarthy MJ, Kiessling S, Barandas R, Young JW, et al. The mood stabilizer valproic acid opposes the effects of dopamine on circadian rhythms. Neuropharmacology. 2016;107:262–70. https://doi.org/10.1016/j.neuropharm.2016.03.047.
Kozikowski AP, Gunosewoyo H, Guo S, Gaisina IN, Walter RL, Ketcherside A, et al. Identification of a glycogen synthase kinase-3beta inhibitor that attenuates hyperactivity in CLOCK mutant mice. ChemMedChem. 2011;6(9):1593–602. https://doi.org/10.1002/cmdc.201100188.
Bhat RV, Andersson U, Andersson S, Knerr L, Bauer U, Sundgren-Andersson AK. The conundrum of GSK3 inhibitors: is it the dawn of a new beginning? J Alzheimers Dis. 2018;64(s1):S547–54. https://doi.org/10.3233/JAD-179934.
Hirota T, Lee JW, St John PC, Sawa M, Iwaisako K, Noguchi T, et al. Identification of small molecule activators of cryptochrome. Science. 2012;337(6098):1094–7. https://doi.org/10.1126/science.1223710.
Lee JW, Hirota T, Kumar A, Kim NJ, Irle S, Kay SA. Development of small-molecule cryptochrome stabilizer derivatives as modulators of the circadian clock. ChemMedChem. 2015;10(9):1489–97. https://doi.org/10.1002/cmdc.201500260.
Zhang EE, Liu Y, Dentin R, Pongsawakul PY, Liu AC, Hirota T, et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med. 2010;16(10):1152–6. https://doi.org/10.1038/nm.2214.
Jang J, Chung S, Choi Y, Lim HY, Son Y, Chun SK, et al. The cryptochrome inhibitor KS15 enhances E-box-mediated transcription by disrupting the feedback action of a circadian transcription-repressor complex. Life Sci. 2018;200:49–55. https://doi.org/10.1016/j.lfs.2018.03.022.
Yoo SH, Mohawk JA, Siepka SM, Shan Y, Huh SK, Hong HK, et al. Competing E3 ubiquitin ligases govern circadian periodicity by degradation of CRY in nucleus and cytoplasm. Cell. 2013;152(5):1091–105. https://doi.org/10.1016/j.cell.2013.01.055.
Landre V, Rotblat B, Melino S, Bernassola F, Melino G. Screening for E3-ubiquitin ligase inhibitors: challenges and opportunities. Oncotarget. 2014;5(18):7988–8013. https://doi.org/10.18632/oncotarget.2431.
Jones KA, Hatori M, Mure LS, Bramley JR, Artymyshyn R, Hong SP, et al. Small-molecule antagonists of melanopsin-mediated phototransduction. Nat Chem Biol. 2013;9(10):630–5. https://doi.org/10.1038/nchembio.1333.
Keenan WT, Fernandez DC, Shumway LJ, Zhao H, Hattar S. Eye-drops for activation of DREADDs. Front Neural Circuits. 2017;11:93. https://doi.org/10.3389/fncir.2017.00093.
De Silva SR, Barnard AR, Hughes S, Tam SKE, Martin C, Singh MS, et al. Long-term restoration of visual function in end-stage retinal degeneration using subretinal human melanopsin gene therapy. Proc Natl Acad Sci USA. 2017;114(42):11211–6. https://doi.org/10.1073/pnas.1701589114.
Vogt A, Cooley KA, Brisson M, Tarpley MG, Wipf P, Lazo JS. Cell-active dual specificity phosphatase inhibitors identified by high-content screening. Chem Biol. 2003;10(8):733–42.
Chakraborty A, Latapy C, Xu J, Snyder SH, Beaulieu JM. Inositol hexakisphosphate kinase-1 regulates behavioral responses via GSK3 signaling pathways. Mol Psychiatry. 2014;19(3):284–93. https://doi.org/10.1038/mp.2013.21.
Dijk DJ, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1992;1(2):112–7.
Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284(5423):2177–81.
Moon JH, Cho CH, Son GH, Geum D, Chung S, Kim H, et al. Advanced circadian phase in mania and delayed circadian phase in mixed mania and depression returned to normal after treatment of bipolar disorder. EBioMedicine. 2016;11:285–95. https://doi.org/10.1016/j.ebiom.2016.08.019.
Lane JM, Vlasac I, Anderson SG, Kyle SD, Dixon WG, Bechtold DA, et al. Genome-wide association analysis identifies novel loci for chronotype in 100,420 individuals from the UK Biobank. Nat Commun. 2016;7:10889. https://doi.org/10.1038/ncomms10889.
Anafi RC, Francey LJ, Hogenesch JB, Kim J. CYCLOPS reveals human transcriptional rhythms in health and disease. Proc Natl Acad Sci USA. 2017;114(20):5312–7. https://doi.org/10.1073/pnas.1619320114.
Braun R, Kath WL, Iwanaszko M, Kula-Eversole E, Abbott SM, Reid KJ, et al. Universal method for robust detection of circadian state from gene expression. Proc Natl Acad Sci USA. 2018;115(39):E9247–56. https://doi.org/10.1073/pnas.1800314115.
Wu G, Ruben MD, Schmidt RE, Francey LJ, Smith DF, Anafi RC, et al. Population-level rhythms in human skin with implications for circadian medicine. Proc Natl Acad Sci USA. 2018;115(48):12313–8. https://doi.org/10.1073/pnas.1809442115.
Lockley SW, Dressman MA, Licamele L, Xiao C, Fisher DM, Flynn-Evans EE, et al. Tasimelteon for non-24-hour sleep-wake disorder in totally blind people (SET and RESET): two multicentre, randomised, double-masked, placebo-controlled phase 3 trials. Lancet. 2015;386(10005):1754–64. https://doi.org/10.1016/S0140-6736(15)60031-9.
Anderson P. FDA panel gives nod to circadian rhythm disorder drug. Medscape. 2013.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This work was conducted in accordance with all pertinent standards for ethical research.
Funding
MJM is supported by a VA Merit Award (U.S. Department of Veterans Affairs; BX003431) and a research award from the Prentiss Foundation. The funders had no role in the preparation of the manuscript or decision to publish.
Conflict of interest
MJM has received consulting fees from Janssen Pharmaceuticals in the past 12 months. AP and RG have no conflicts to report.
Rights and permissions
About this article
Cite this article
Porcu, A., Gonzalez, R. & McCarthy, M.J. Pharmacological Manipulation of the Circadian Clock: A Possible Approach to the Management of Bipolar Disorder. CNS Drugs 33, 981–999 (2019). https://doi.org/10.1007/s40263-019-00673-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-019-00673-9