Abstract
Background
In the treatment of pediatric epilepsy, there is a critical demand for effective and safe therapeutic options to address patients’ unmet clinical needs. Eslicarbazepine acetate is a novel once-daily antiepileptic drug and a third-generation single enantiomer member of the dibenzazepine family.
Objective
The objective of this study was to evaluate the efficacy and safety of eslicarbazepine acetate as add-on treatment for focal-onset seizures in pediatric patients using meta-analytical techniques.
Methods
Randomized, placebo-controlled, single- or double-blinded add-on trials of eslicarbazepine acetate in patients < 18 years of age with focal-onset seizures uncontrolled by concomitant stable antiepileptic drug regimens were identified through a systematic literature search. The assessed outcomes included the mean relative change and ≥ 50% reduction in the baseline seizure frequency, the incidence of treatment withdrawal, serious adverse events, and treatment-emergent adverse events. Risk ratio and weighted mean difference with 95% confidence intervals were estimated for dichotomous/continuous outcomes.
Results
Two trials were included involving 386 participants (age range 2–18 years), 217 for eslicarbazepine acetate and 169 for placebo groups, respectively. At the dosage of 30 mg/kg/day, eslicarbazepine acetate-treated patients had a significantly greater reduction in baseline seizure frequency (weighted mean difference − 21.67, 95% confidence interval − 40.87 to − 2.46; p = 0.027) and 58 patients (44.6%) were seizure responders compared with 27 controls (29.7%) [risk ratio 1.48, 95% confidence interval 0.99–2.20; p = 0.055]. There were no differences in treatment withdrawal (risk ratio 1.24, 95% confidence interval 0.65–2.37; p = 0.513), serious adverse events (risk ratio 1.40, 95% confidence interval 0.69–2.86; p = 0.350), and treatment-emergent adverse events (risk ratio 1.07, 95% confidence interval 0.94–1.22; p = 0.313).
Conclusions
Adjunctive eslicarbazepine acetate could be an effective well-tolerated option in children and adolescents with focal-onset seizures uncontrolled by one or more concomitant anti-epileptic drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Eslicarbazepine acetate is a novel, once-daily, third-generation antiepileptic drug. |
Adjunctive eslicarbazepine acetate could be an effective treatment option in pediatric patients with focal epilepsy. |
Add-on eslicarbazepine acetate demonstrated favorable safety and tolerability profiles in children and adolescents. |
1 Introduction
Epilepsy is one of the most common neurologic disorders in the pediatric population, affecting 0.5–1% of all children [1, 2]. Worldwide, more than 10.5 million boys and girls aged younger than 15 years have active epilepsy, and they represent about one quarter of the global epilepsy population [3, 4]. Of the 3.5 million people diagnosed with epilepsy every year, nearly half are aged younger than 15 years; cumulative incidence studies show that up to the age of 15 years, 1–2% of children will have at least one unprovoked seizure, and approximately 1% will have repeated seizures [5, 6].
Overall, 20–30% of the pediatric population affected by epilepsy continue to have seizures despite treatment with antiepileptic drugs (AEDs) used in mono- or polytherapy [7, 8]. The unpredictable nature of the episodes can disrupt the lives of the young patients and their families and friends. Moreover, refractory seizures are a risk factor for poor intellectual, psychological, and social outcomes in the long term [9], and can increase the likelihood of sudden unexpected death in epilepsy [10]. Hence, there is a critical demand for additional therapeutic options to address patients’ unmet clinical needs.
Eslicarbazepine acetate (ESL) is a novel once-daily AED; it is a third-generation single enantiomer member of the dibenzazepine family, which includes carbamazepine and oxcarbazepine, and acts as a competitive blocker of voltage-gated sodium channels [11]. Unlike traditional sodium channel blockers, which affect the fast inactivation of the channel, ESL selectively enhances the slow inactivation, similarly to lacosamide. This mechanism can result in stabilization of hyperexcitable neuronal membranes, inhibition of sustained repetitive firing of neurons characteristic of epilepsy, and a reduction in long-term channel availability with a low propensity to disturb physiologic function [12,13,14].
Eslicarbazepine acetate has been approved as mono- and adjunctive therapy for focal-onset seizures (FOS) in adults and it has been recently authorized as adjunctive treatment in pediatric patients by the European Medicines Agency and the US Food and Drug Administration. The aim of our study was to systematically evaluate the efficacy and safety of ESL as add-on therapy in children and adolescents affected by focal epilepsy uncontrolled by one or more concomitant AEDs at optimal stable dosages.
2 Materials and Methods
2.1 Search Strategy
The study results were reported according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement [15]. We systematically searched the databases (week 4, October 2017) MEDLINE (accessed through PubMed), CENTRAL (Cochrane Central Register of Controlled Trials), and the US National Institutes of Health Clinical Trials Registry (http://www.clinicaltrials.gov/) [search strategies are outlined in e-Appendix I of the Electronic Supplementary Material (ESM)]. Additional data were sought in the Assessment Reports of ESL by the European Medicines Agency/Committee for Medicinal Products for Human Use (http://www.ema.europa.eu/). There were no date limitations or language restrictions. The manufacturer of ESL was contacted for information about unpublished or ongoing studies. The protocol was not registered previously.
2.2 Eligibility Criteria
Studies were selected when they met the following entry criteria: randomized, single- or double-blinded, placebo-controlled, parallel-group or crossover, add-on studies with active and control groups receiving oral ESL and matching placebo, respectively, in addition to conventional AED treatment. Participants had to meet the following criteria: any sex, any ethnicity, age up to 18 years, and diagnosis of epilepsy with refractory FOS (simple partial, complex partial, secondary generalized tonic-clonic seizures). Seizures were considered refractory if uncontrolled by one or more concomitant AEDs at optimal stable dosages.
2.3 Outcome Measures
The efficacy outcomes were the mean relative change and the proportion of patients with a 50% or greater reduction in the standardized 4-week seizure frequency from the pre-randomization baseline to the treatment maintenance period. The safety endpoints included the proportions of patients withdrawing from the treatment for any reason and for treatment-emergent adverse events (TEAEs). We also assessed the proportions of participants who experienced any serious adverse event (SAE), any TEAE, and any of the following adverse events (AEs) considered by the review authors as common and important AED-related side effects: headache, somnolence, dizziness, ataxia, diplopia, nausea, vomiting, fatigue, anxiety, insomnia, and irritability. The effects on vital signs, height, weight, head circumference, hematology, and biochemistry laboratory tests were narratively reviewed.
2.4 Study Selection, Data Extraction, and Assessment of the Risk of Bias
Two review authors (EG and CC) independently assessed trials for inclusion and extracted the information from included trials. Any disagreement was resolved by discussion with a third review author (SL). The risk of bias of the identified studies was assessed in accordance with the recommendations of the Cochrane Collaboration [16].
2.5 Statistical Analysis
Heterogeneity among the trials was assessed by the chi-squared test and the I2 statistics for heterogeneity [16,17,18]. Provided no significant heterogeneity was present (p > 0.05), results were synthesized using a fixed-effect model; if the probability value was ≤ 0.05, heterogeneity determined the choice of a fixed- or random-effects model for I2 < 40% or ≥ 40%, respectively [19,20,21,22]. The risk ratio (RR) and weighted mean difference (WMD), with 95% confidence intervals (CIs), were the measures of associations between treatment and dichotomous/continuous outcomes. The intent-to-treat population data were used for the analysis. We planned to perform sub-group analyses by ESL maintenance dose (20 and 30 mg/kg/day) and patient age (younger and older than 6 years). Reported probability values were two sided, with significance set at < 0.05. Data analysis was performed using the STATA/IC 13.1 statistical package (StataCorp LP, College Station, TX, USA).
3 Results
3.1 Search Results
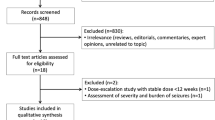
Two hundred and seventy-nine records were identified by searches of the databases and trial registers. Six randomized controlled trials were retrieved for detailed assessment, of which three recruited only adult patients. In one trial, patients were eligible if aged ≥ 16 years, but only a few participants (ESL n = 8, placebo n = 3) were aged 16–18 years and no outcome data were explicitly provided for this age cohort. Accordingly, two studies [23, 24] were considered in the review, both of which were included in the meta-analysis (Fig. 1).
3.2 Characteristics and Risk of Bias of Included Studies
Both studies were randomized, double-blind, placebo-controlled, multicentre, parallel-group trials; details of the studies are given in Table 1. In Study 305 [23], the recommended ‘target’ dose of the 12-week maintenance period was 20 mg/kg/day (up to a maximum of 1200 mg/day), and further titration up to 30 mg/kg/day (up to a maximum of 1200 mg/day) was allowed if tolerability was acceptable but therapeutic response judged unsatisfactory. In Study 208 [24], patients received ESL 30 mg/kg/day with a maximum dose of 1200 mg/day during the 8-week maintenance period.
The studies included 386 participants, 217 allocated to ESL and 169 to placebo. Overall, 199 (51.6%) were male and 362 (93.8%) of Caucasian ethnicity (n = 362, 93.8%); other pertinent characteristics of study participants are provided in Table 2.
Both trials applied centralized randomization procedures with adequate methods of sequence generation and allocation concealment based on a randomization list or code generated by means of computerized techniques. We rated both trials with a low risk of performance and detection bias because blinding was ensured by matching placebo, and neither the investigators nor the patients knew the identity of the study treatment being administered. The risks of attrition and selective reporting bias were judged low because patients lost to follow-up and withdrawals were documented, and there was no suspicion of selective outcome reporting. Both trials were sponsored by the ESL manufacturer.
3.3 Relative Change in Standardized Seizure Frequency and Response Rate at any Dose
There was no significant difference in the mean relative change in the standardized seizure frequency from the baseline to treatment maintenance period between the active and placebo arms across the trials [WMD − 11.68 (95% CI − 26.36 to 3.00); p = 0.119] (Chi-squared = 0.36, df = 1, p = 0.548; I2 = 0.0%). A total of 83 (38.2%) and 50 (29.6%) participants taking ESL and matching placebo achieved ≥ 50% seizure reduction across the trials, respectively; the estimated overall RR was 1.36 [(95% CI 0.68–2.75); p = 0.388] (Chi-squared = 4.30, df = 1, p = 0.038; I2 = 76.8%).
3.4 Dose Sub-Group Analysis
At the 20-mg/kg daily maintenance dose, there were no differences between the treatment arms in the mean relative change in the standardized seizure frequency [WMD − 2.10 (95% CI − 25.62 to 21.42); p = 0.861], while ESL-treated patients had a significantly greater reduction in baseline seizure frequency at the dosage of 30 mg/kg/day [WMD −21.67 (95% CI − 40.87 to − 2.46); p = 0.027] (Chi-squared = 0.00, df = 1, p = 0.961; I2 = 0.0%) [test for sub-groups difference: Chi-squared = 1.60, df = 1, p = 0.207) [Fig. e-1 of the ESM]. At the 20-mg/kg/day dose, the rate of ≥ 50% reduction in seizure frequency did not differ between treated patients and controls [RR 1.12 (95% CI 0.68–1.86); p = 0.654] and at the dosage of 30 mg/kg/day, 58 (44.6%) ESL-treated patients were seizure responders compared with 27 (29.7%) controls, with a trend in favor of the active drug [RR 1.48 (95% CI 0.99–2.20); p = 0.055] (Chi-squared = 2.83, df = 1, p = 0.092; I2 = 64.7%) [test for sub-groups difference: chi-squared = 0.69, df = 1, p = 0.41] (Fig. e-2 of the ESM). Data from patients of Study 208 and those of Study 305 who were up-titrated to the 30-mg/kg/day maintenance dose (ESL n = 47, placebo n = 51) were combined to estimate the drug efficacy at the highest dose.
3.5 Age Sub-Group Analysis
The mean relative change in seizure frequency did not differ between the treatment arms in patients aged from 2 to 6 years [WMD 0.04 (95% CI − 0.39 to 0.47); p = 0.857], while it was significantly greater in the ESL arm among the older patients [WMD −13.92 (95% CI − 15.59 to − 12.25); p < 0.001] (Chi-squared = 0.17, df = 1, p = 0.681; I2 = 0.0%)] (test for sub-groups difference: Chi-squared = 250.89, df = 1, p < 0.001) [Fig. e-3 of the ESM]. The seizure response during the maintenance phase was not different between the ESL and placebo arms among patients aged 2–6 years [RR 0.48 (95% CI 0.19–1.23); p = 0.129]; in the older age group, a higher rate of ≥ 50% seizure reduction was observed among the ESL-treated participants compared with controls [RR 1.43 (95% CI 1.03–1.98); p = 0.034] (Chi-squared = 2.45, df = 1, p = 0.117; I2 = 59.2%) [test for sub-groups difference: Chi-squared = 4.55, df = 1, p = 0.03] (Fig. e-4 of the ESM). Strata II + III participants of Study 305 and patients of Study 208 were combined to estimate the efficacy of ESL in the older age group.
3.6 Treatment Withdrawal
Across the trials, treatment was discontinued in 22 (10.1%) and 14 (8.2%) cases in the ESL and placebo groups, respectively; the overall RR for withdrawal for any reason was 1.24 (95% CI 0.65–2.37; p = 0.513) [Chi-squared = 0.00, df = 1, p = 0.950; I2 = 0.0%]. Drug discontinuation because of TEAEs occurred in 12 (5.5%) and 3 (1.8%) patients in the active and control arm, respectively; the corresponding RR was 2.81 (95% CI 0.84–9.42; p = 0.094) [Chi-squared = 0.30, df = 1, p = 0.581; I2 = 0.0%]. In Study 305, the only TEAE leading to discontinuation reported more than once was convulsion (ESL n = 1, placebo n = 3). In Study 208, five participants in the ESL arm withdrew drug because of TEAEs; two of five patients experienced cutaneous events (moderate rash and mild allergic dermatitis).
3.7 Serious Adverse Events
Serious adverse events were reported by 18 (8.3%) and 11 (6.5%) patients treated with ESL and placebo, respectively; the overall RR to develop SAEs during ESL treatment was 1.40 [(95% CI 0.69–2.86); p = 0.350] (Chi-squared = 0.66, df = 1, p = 0.416; I2 = 0.0%). In Study 305, the most common SAEs were status epilepticus (ESL n = 3, placebo n = 0), convulsion (ESL n = 2, placebo n = 2), and pneumonia (ESL n = 1, placebo n = 3). In Study 208, five patients reported at least one SAE (ESL n = 3, placebo n = 2), of which one was a complex partial status epilepticus occurring in the active arm. Two deaths occurred in Study 305, one in each treatment arm, and none was deemed related to the trial medication by the investigator.
3.8 Treatment-Emergent Adverse Events
The overall RR to develop at least one TEAE, not including SAEs, during treatment was 1.07 [(95% CI 0.94–1.22); p = 0.313] (Chi-squared = 3.24, df = 1, p = 0.072; I2 = 69.1%). The incidence rates of the selected TEAEs among the ESL-treated participants were: headache 12.0%, somnolence 9.2%, dizziness 3.7%, ataxia 1.2%, diplopia 6.0%, nausea 4.1%, vomiting 6.0%, fatigue 2.3%, anxiety 1.2%, insomnia 1.2%, and irritability 1.2%. The RRs with corresponding CIs are reported in Table 3; no AEs were significantly associated with ESL treatment. The mean variations from baseline in vital signs, height, weight, and head circumference did not differ across visits between ESL- and placebo-treated patients. Overall, there were only few changes in hematology and biochemistry laboratory parameters in either treatment groups.
In Study 305, the highest incidence of subjects with laboratory abnormalities considered significant by the investigator was reported for gamma-glutamyltransferase: three subjects in the ESL group and one in the placebo arm experienced clinically significant increased gamma-glutamyltransferase values. In Study 208, a slight reduction in serum thyroid hormone levels, with most of the values falling within the normal range, and one event of hypothyroidism were reported in the ESL and placebo groups, respectively; no TEAEs of hyponatremia were reported [25].
4 Discussion
The main findings of the current systematic review and meta-analysis were the overall efficacy, tolerability, and safety of ESL when prescribed as add-on treatment in pediatric patients presenting with focal seizures, with or without secondary generalization, despite stable therapy with one or more concomitant AEDs.
At the daily dosage of 30 mg/kg, and up to a maximum of 1200 mg, adjunctive ESL was associated with a relative reduction in standardized seizure frequency of about 33% compared with 11% in the placebo arm, implying a 22% real reduction not attributable to the placebo effect. A tendency in favor of ESL was also observed toward the 50% or greater seizure reduction, with near to 45% of the treated patients being responders in comparison to approximately 30% in the placebo group.
Conversely, the efficacy of the 20-mg/kg daily dose has not been convincingly demonstrated. In this regard, it is noteworthy underlying that the dose sub-analysis may be difficult to interpret when the drug titration is based on clinical response instead of pre-planned randomization. A possible explanation is that the study participants who were not up-titrated to the highest recommended dose were those less susceptible to seizures, and the larger effect size observed in the placebo arm may have contributed to reduce the difference in the seizure frequency change between treated patients and controls. Accordingly, no definitive conclusions can be drawn and, although the available evidence to clearly support the efficacy of the 20-mg/kg/day dosage is limited, it cannot be excluded that it may represent a valuable therapeutic option in some patients.
The overall low response observed at the 20-mg/kg/daily dose may have also diluted the treatment effect and explained the lack of a statistically significant difference between the ESL and placebo groups in the pooled analysis, irrespective of the targeted dose. Moreover, approximately one third of the participants enrolled in Study 305 were treated with ESL at the 30-mg/kg daily dose: the low rate of dose up-titration, joined with the greater disease severity compared with the Study 208 population, as suggested by the higher AED burden and seizure frequency at baseline, may have further contributed to downsize the overall estimate of the treatment effect.
The age of patients can also affect the response to ESL. The sub-analysis by patient age suggests greater efficacy of ESL among children aged above 6 years, and lower, no statistically significant treatment effect in the group of children aged 2–6 years. Although difficulties in identifying seizures may have biased the findings and influenced outcome in the youngest cohort of patients, pharmacokinetic issues and drug suboptimal or under-dosing may have also played a role. A population pharmacokinetic model demonstrated comparable exposure between the 20- and 30-mg/kg daily doses in children aged older than 6 years and the established effective maintenance dosages of 800 and 1200 mg/day in adults, respectively. Conversely, in children aged 2–6 years, systemic exposure is lower and plasma clearance is higher than in older patients, and this is most likely related to faster drug metabolism [25, 26]. Pharmacokinetic simulations suggest that a minimum dose of 27.5 mg/kg/day is required in the youngest age group to have the exposure achieved with 20 mg/kg/day in patients aged above 6 years [25]. Accordingly, the required dose in patients aged 2–6 years to match ESL exposure associated with a 30-mg/kg daily dose in older children should necessarily exceed such a dosage that, however, was the highest to be allowed across the trials. Bearing in mind all the above issues, the indication and dosing schedule approved by the regulatory authorities, namely a starting dose of 10 mg/kg/day and up-titration to 30 mg/kg/day based on individual response in children aged above 6 years (4 years in USA), seems reasonable.
Across phase II and III trials, adjunctive ESL demonstrated a favorable safety and tolerability profile in children and adolescents with drug-resistant FOS. Treatment-emergent adverse events were observed in approximately 70% of the patients in both the treatment and placebo arms, and they were mostly mild to moderate in intensity. There were no meaningful differences in the incidence of any of the considered AEs across treated patients and controls. Headache, somnolence, nausea, and dizziness were among the most frequent side effects observed in the ESL cohort; they represent common AEs and substantially overlap the safety profile of the vast majority of the AEDs [27, 28]. The discontinuation rate owing to side effects was low, and it was even inferior to the incidence observed in the randomized controlled trials involving adult patients [29]. The most common TEAEs leading to ESL discontinuation were convulsion, dermatitis allergic, and rash. Allergic reactions and skin disorders are known to occur while taking AEDs, and they have also been associated with ESL in adults [30]. There were few changes in hematology and biochemistry laboratory parameters, and hyponatremia was reported at a lower rate compared with the adult population. The incidence of SAEs was overall low, and no major safety concerns arose.
Our systematic review and meta-analysis is the first to synthetize the evidence coming from randomized controlled trials about the efficacy and safety of ESL as adjunctive treatment for uncontrolled FOS in pediatric patients. In addition, the sub-analyses we performed according to drug dosage and patient age could have clinical relevance and practical implications. Nonetheless, different caveats need to be considered while interpreting the findings. Only two trials met the eligibility criteria and both were sponsored by a pharmaceutical company. Evidence for the 20-mg/kg daily dosage and 2–6 years age group was derived from one single trial and a small sample size, and should be cautiously interpreted; further, the use of aggregate rather than individual participant-level data could have limited the strength of the results. Owing to the short double-blind treatment period of the included trials, this meta-analysis cannot provide information about the long-term efficacy and safety of ESL, the frequency estimation of rare but serious AEs, which may be seen with AEDs (i.e. Steven Johnson syndrome or aplastic anemia), and the occurrence of phenomena such as habituation and tolerance [31, 32]. Data collated for up to 3 years through the open-label extension phases of the controlled trials suggested that ESL maintains or improves efficacy and tolerability over time, and does not have detrimental consequences for attention, information processing, and working or episodic memory [25]. However, the open-label design and the limited size of the pediatric database do not allow the drawing of definitive conclusions on the long-term effects on growth, brain development and learning, endocrine function, puberty, and childbearing potential. Continued post-marketing surveillance and longer term studies will be needed to address all these issues.
5 Conclusion
In the last decades, many different novel second- and third-generation AEDs have emerged to treat epilepsy. No direct head-to-head trials exist, and scant evidence suggests how and in which order drugs should be chosen as an adjunct and combined with each other to design the so-called ‘rational polytherapy’. In this regard, although only two trials met the inclusion criteria and no comparative studies exist, the current integrated analysis of pediatric studies suggests that ESL could be an effective, well-tolerated, once-daily treatment option to manage refractory FOS, but it could not directly compare ESL to other therapies. The application field of ESL in the care of children and adolescents presenting with focal epilepsy remains to be established and should be explored in future research.
References
Oka E, Ohtsuka Y, Yoshinaga H, Murakami N, Kobayashi K, Ogino T. Prevalence of childhood epilepsy and distribution of epileptic syndromes: a population-based survey in Okayama, Japan. Epilepsia. 2006;47:626–30.
Cagnetti C, Lattanzi S, Foschi N, Provinciali L, Silvestrini M. Seizure course during pregnancy in catamenial epilepsy. Neurology. 2014;83:339–44.
Guerrini R. Epilepsy in children. Lancet. 2006;367:499–524.
Forsgren L. Incidence and prevalence. In: Wallace SJ, Farrell K, editors. Epilepsy in children. 2nd ed. London: Arnold; 2004. p. 21–5.
Tsuboi T. Prevalence and incidence of epilepsy in Tokyo. Epilepsia. 1988;29:103–10.
Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993;34:453–68.
Rosati A, De Masi S, Guerrini R. Antiepileptic drug treatment in children with epilepsy. CNS Drugs. 2015;29:847–63.
Wirrell EC. Predicting pharmacoresistance in pediatric epilepsy. Epilepsia. 2013;54(Suppl. 2):19–22.
Dwivedi R, Ramanujam B, Chandra PS, Sapra S, Gulati S, Kalaivani M, Garg A, Bal CS, Tripathi M, Dwivedi SN, Sagar R, Sarkar C, Tripathi M. Surgery for drug-resistant epilepsy in children. N Engl J Med. 2017;377:1639–47.
Donner EJ, Camfield P, Brooks L, Buchhalter J, Camfield C, Loddenkemper T, Wirrell E. Understanding death in children with epilepsy. Pediatr Neurol. 2017;70:7–15.
Lattanzi S, Cagnetti C, Foschi N, Lorusso A, Provinciali L, Silvestrini M. Eslicarbazepine acetate as adjunctive treatment in partial-onset epilepsy. Acta Neurol Scand. 2018;137(1):29–32.
Lattanzi S, Cagnetti C, Foschi N, Provinciali L, Silvestrini M. Lacosamide monotherapy for partial onset seizures. Seizure. 2015;27:71–4.
Doty P, Hebert D, Mathy FX, Byrnes W, Zackheim J, Simontacchi K. Development of lacosamide for the treatment of partial-onset seizures. Ann N Y Acad Sci. 2013;1291:56–68.
Lattanzi S, Cagnetti C, Foschi N, Provinciali L, Silvestrini M. Lacosamide during pregnancy and breastfeeding. Neurol Neurochir Pol. 2017;51:266–9.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. Higgins JPT, Green S, editors. The Cochrane Collaboration, 2011. Available from: http://handbook-5-1.cochrane.org/. Accessed Oct 2017.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Lattanzi S, Cagnetti C, Foschi N, Provinciali L, Silvestrini M. Brivaracetam add-on for refractory focal epilepsy: a systematic review and meta-analysis. Neurology. 2016;86:1344–52.
Lattanzi S, Grillo E, Brigo F, Silvestrini M. Efficacy and safety of perampanel in Parkinson’s disease: a systematic review with meta-analysis. J Neurol. 2017. https://doi.org/10.1007/s00415-017-8681-y (epub ahead of print).
Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. How should we lower blood pressure after cerebral hemorrhage? A systematic review and meta-analysis. Cerebrovasc Dis. 2017;43:207–13.
Lattanzi S, Cagnetti C, Danni M, Provinciali L, Silvestrini M. Oral and intravenous steroids for multiple sclerosis relapse: a systematic review and meta-analysis. J Neurol. 2017;264:1697–704.
ClinicalTrials.gov. Eslicarbazepine acetate as therapy for refractory partial seizures in children. Available from: https://clinicaltrials.gov/ct2/NCT00988156. Accessed 28 Feb 2018.
ClinicalTrials.gov. Effects of eslicarbazepine acetate on cognitive function in children with partial onset seizures. Available from: https://clinicaltrials.gov/ct2/NCT01527513. Accessed 28 Feb 2018.
European Medicines Agency. Assessment report: Zebinix. International non-proprietary name: eslicarbazepine acetate. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000988/WC500218805.pdf. Accessed 28 Feb 2018.
Almeida L, Minciu I, Nunes T, Butoianu N, Falcão A, Magureanu SA, Soares-da-Silva P. Pharmacokinetics, efficacy, and tolerability of eslicarbazepine acetate in children and adolescents with epilepsy. J Clin Pharmacol. 2008;48:966–77.
Sarco DP, Bourgeois BF. The safety and tolerability of newer antiepileptic drugs in children and adolescents. CNS Drugs. 2010;24:399–430.
Zaccara G, Gangemi PF, Cincotta M. Central nervous system adverse effects of new antiepileptic drugs: a meta-analysis of placebo-controlled studies. Seizure. 2008;17:405–21.
Elger C, Koepp M, Trinka E, Villanueva V, Chaves J, Ben-Menachen E, Kowacs PA, Gil-Nagel A, Moreira J, Gama H, Rocha JF, Soares-da-Silva P. Pooled efficacy and safety of eslicarbazepine acetate as add-on treatment in patients with focal-onset seizures: data from four double-blind placebo-controlled pivotal phase III clinical studies. CNS Neurosci Ther. 2017;23:961–72.
Gama H, Vieira M, Costa R, Graça J, Magalhães LM, Soares-da-Silva P. Safety profile of eslicarbazepine acetate as add-on therapy in adults with refractory focal-onset seizures: from clinical studies to 6 years of post-marketing experience. Drug Saf. 2017;40:1231–40.
Gaitatzis A, Sander JW. The long-term safety of antiepileptic drugs. CNS Drugs. 2013;27:435–55.
Guerrini R, Zaccara G, la Marca G, Rosati A. Safety and tolerability of antiepileptic drug treatment in children with epilepsy. Drug Saf. 2012;35:519–33.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding has been received for the conduct of this study.
Conflict of interest
Simona Lattanzi, Claudia Cagnetti, Alberto Verrotti, and Mauro Silvestrini have no conflicts of interest directly relevant to the content of this study. Francesco Brigo acted as a consultant for Eisai. Elisabetta Grillo is an employee of Eisai s.r.l. Gaetano Zaccara has received speaker’s or consultancy fees from Eisai, Sanofi-Aventis, and UCB Pharma.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lattanzi, S., Brigo, F., Grillo, E. et al. Adjunctive Eslicarbazepine Acetate in Pediatric Patients with Focal Epilepsy: A Systematic Review and Meta-Analysis. CNS Drugs 32, 189–196 (2018). https://doi.org/10.1007/s40263-018-0504-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-018-0504-x