Abstract
Eslicarbazepine acetate (Zebinix®), a voltage-gated sodium channel blocker, is a once-daily, orally administered anti-seizure medication available in the EU for use as monotherapy in adults with newly diagnosed focal-onset seizures and as adjunctive therapy in adults, adolescents and children aged > 6 years with focal-onset seizures. In adult patients, adjunctive eslicarbazepine acetate was generally associated with a significant decrease in seizure frequency and an increase in responder rate compared with placebo. The drug was also an effective monotherapy agent in adult patients, demonstrating noninferiority to controlled-release carbamazepine, in terms of seizure freedom rates. In paediatric patients, eslicarbazepine acetate provided seizure control when administered as adjunctive therapy, with the benefits appearing to be dependent on age and dose. The antiepileptic efficacy of eslicarbazepine acetate as adjunctive therapy or as monotherapy was maintained during longer-term extension studies, with each extension study period being up to 2 years. Oral eslicarbazepine acetate was generally well tolerated when administered as adjunctive therapy or monotherapy in adult patients and when administered as adjunctive therapy in paediatric patients, with most adverse events being of mild or moderate intensity. In conclusion, with the convenience of once-daily administration, eslicarbazepine acetate is an effective and generally well-tolerated treatment option for adults, adolescents and children aged > 6 years with focal-onset seizures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A once-daily, orally administered voltage-gated sodium channel blocker |
Provides effective seizure control as adjunctive therapy in adult and paediatric patients and as monotherapy in adult patients |
Antiepileptic efficacy maintained in the longer term |
Generally well tolerated, with most adverse events being of mild or moderate intensity |
1 Introduction
Epilepsy is one of the most common neurological diseases, affecting ≈ 70 million people worldwide, with focal-onset seizures (previously known as partial-onset seizures [1]) being the most frequent seizure type in patients with epilepsy [2]. Anti-seizure medications (ASMs) are the mainstay of epilepsy treatment, with the goal being achieving seizure control whilst minimizing adverse events (AEs) associated with ASMs [3]. However, despite a substantial increase in the number of ASMs being available in the last few decades, approximately one-third of patients remain treatment-resistant [2, 3]. The newer generation of ASMs have been developed in an attempt to provide better efficacy and safety profiles than those of older ASMs [4].
Eslicarbazepine acetate [Zebinix® (EU), Aptiom® (USA)], a voltage-gated sodium channel blocker, is a once-daily, orally administered, third-generation ASM for the treatment of focal-onset seizures that is approved in several countries, including those in the EU [5] and the USA [6]. The efficacy and safety of eslicarbazepine acetate for the treatment of focal-onset seizures is well established and its use as adjunctive therapy or monotherapy in adult patients with epilepsy has been extensively reviewed previously [7, 8]. With the expanded indication of eslicarbazepine acetate use in paediatric patients [5, 6], this article provides an updated review of the clinical use of eslicarbazepine acetate for the treatment of focal-onset seizures in adults, adolescents and children aged > 6 years from an EU perspective. The pharmacological properties of oral eslicarbazepine acetate are summarized in Table 1.
2 Therapeutic Efficacy of Eslicarbazepine Acetate
2.1 In Adult Populations
2.1.1 Adjunctive Therapy
The efficacy of oral eslicarbazepine acetate as adjunctive therapy to other ASMs for the treatment of focal-onset seizures was evaluated in four pivotal randomized, double-blind, placebo-controlled, multicentre phase III trials of similar study design and patient population (studies 301 [9], 302 [10], 303 [11] and 304 [12]). Across the trials, enrolled patients were aged ≥ 16 [12] or ≥ 18 [9,10,11] years, were diagnosed with simple or complex partial seizures (i.e. focal seizures) with or without secondary generalization in the past 12 months, were on stable dosages of 1–2 [9, 11, 12] or 1–3 [10] ASMs for ≥ 1 [12] or ≥ 2 [9,10,11] months prior to screening and experienced ≥ 4 focal-onset seizures in the one [12] or two [10, 11] 4-week periods prior to screening. Patients who were treated with oxcarbazepine or with a serum sodium level of < 130 mEq/L were among those excluded [9,10,11,12].
Following screening, patients entered a 8-week observation baseline period and those who had ≥ 4 focal-onset seizures per 4-week period were eligible to enter a double-blind phase [9,10,11,12]. Eligible patients were randomized to receive eslicarbazepine acetate 400 mg [9, 10], 800 mg [9,10,11,12], 1200 mg [9,10,11,12] or placebo once daily [9,10,11,12]. In studies 301, 303 and 304, the target doses of eslicarbazepine acetate were achieved during a 2-week titration period and were then maintained for 12 weeks (i.e. maintenance period) [9, 11, 12]. In study 302, however, only eslicarbazepine acetate 1200 mg/day recipients underwent a 2-week titration period while all other eslicarbazepine acetate recipients received their full target doses without titration throughout the 14-week treatment period [10].
At baseline, patient and disease characteristics were generally similar between the treatment groups within each trial [9,10,11,12]. More than half of patients (60–79%) were concurrently taking two ASMs, with the most common concomitant ASMs including carbamazepine (34–69%), valproic acid (13–35%), lamotrigine (11–27%) and levetiracetam (7–30%). The primary efficacy endpoint in studies 301–304 was the standardized seizure frequency over the maintenance/treatment period, assessed in the intent-to-treat (ITT) population (i.e. randomized patients who received ≥ 1 dose of study drug and had ≥ 1 post-baseline seizure frequency assessment) [9,10,11,12]. Discussion focuses on the approved maintenance dosage of eslicarbazepine acetate (800 or 1200 mg/day), with data from the 400 mg/day treatment arm tabulated for completeness.
In studies 301–304, adjunctive eslicarbazepine acetate provided effective seizure control in patients with refractory focal-onset seizures, with 77–82% of patients completing the maintenance/treatment period [9,10,11,12]. Relative to placebo, eslicarbazepine acetate 1200 mg/day significantly (p < 0.05) reduced the standardized seizure frequency during the maintenance/treatment period, with eslicarbazepine acetate 800 mg/day also providing significant benefit over placebo for this endpoint in studies 301, 302 and 303 (primary endpoint; Table 2). Supportive analyses of the primary endpoint conducted in the per-protocol (PP) populations yielded results similar to those observed in the ITT populations [9,10,11,12].
The responder rate (i.e. patients achieving a ≥ 50% reduction from baseline in seizure frequency) and the median relative reduction in seizure frequency were generally greater in eslicarbazepine acetate than placebo recipients [9,10,11,12]. The responder rate was significantly (p < 0.05) greater with eslicarbazepine acetate 1200 mg/day than with placebo in all four studies, but the rate with eslicarbazepine acetate 800 mg/day versus placebo was significantly greater only in studies 301 and 302 (Table 2) [9,10,11,12]. The median relative reduction in seizure frequency was significantly greater with eslicarbazepine acetate 1200 mg/day than placebo recipients in the two studies that tested for statistical significance and with eslicarbazepine acetate 800 mg/day over placebo in one of these two studies (Table 2) [10, 12]. Eslicarbazepine acetate 800 and 1200 mg/day also reduced the severity of seizure compared with placebo in a dose-dependent manner (as measured by the seizure severity questionnaire in study 304) [13]. During the maintenance/treatment period, 2.0–8.0% of patients receiving eslicarbazepine acetate 800 or 1200 mg/day and 0.9–2.0% of patients receiving placebo achieved seizure freedom [9,10,11,12].
The antiepileptic efficacy of adjunctive eslicarbazepine acetate was further supported by several pooled analyses of data from the pivotal phase III studies [14,15,16]. In the pooled analysis of studies 301–304, relative to placebo, eslicarbazepine acetate 800 or 1200 mg/day had a significantly (p < 0.0001) lower least squares mean (LSM) standardized seizure frequency (6.5 and 6.1 vs 8.0), a significantly (p < 0.0001) higher responder rate (33.8% and 43.1% vs 22.2%) and a significantly (p ≤ 0.0005) greater median relative reduction in standardized seizure frequency (33.4% and 37.8% vs 17.6%) during the maintenance/treatment period [14]. The clinical benefits of eslicarbazepine acetate 800 or 1200 mg/day over placebo were consistently seen regardless of age, gender, geographical region, epilepsy duration, seizure type and the type of concomitant ASMs [14]. Results of 1-year, open-label extension studies of studies 301 [17] and 302 [18] showed that the antiepileptic efficacy of adjunctive eslicarbazepine acetate was maintained during longer-term treatment (Table 2).
In general, patients who responded to eslicarbazepine acetate treatment showed greater improvements in health-related quality of life (HR-QOL), as assessed by the Quality of Life in Epilepsy Inventory-31 (QOLIE-31) questionnaires, compared with non-responders [19]. In a pooled analysis of patients who received eslicarbazepine acetate 800 or 1200 mg/day in studies 301, 302 and 303 (n = 530), treatment responders with ≥ 50% seizure frequency reduction had a significantly (p ≤ 0.018) greater mean change in all subscales (except emotional well-being) and total scores of QOLIE-31 than non-responders, with two subscales (medication effects and overall quality of life) exceeding the established minimal clinically important differences. Significantly (p ≤ 0.021) greater improvements in all subscales and total scores of QOLIE-31 were also seen in treatment responders with ≥ 75% seizure frequency reduction than in non-responders, with the total score and five subscales (cognitive functioning, medication effects, overall quality of life, seizure worry and social functioning) exceeding the established minimal clinically important differences [19]. In addition, improvements from baseline in HR-QOL and depressive symptoms (assessed by the QOLIE-31 questionnaire and the Montgomery Asberg Depression Rating Scale, respectively) were seen with eslicarbazepine acetate in the open-label extension studies of studies 301 and 302 [17, 18]. Independent of seizure control, eslicarbazepine acetate appeared to improve anger levels, mood and quality of life (assessed by the State-Trait Anger with the Expression Inventory-2, the Hospital Anxiety and Depression Scale and QOLIE-10, respectively), according to a prospective observational study in otherwise healthy epileptic patients (n = 78) [20].
2.1.2 Monotherapy
The efficacy of eslicarbazepine acetate monotherapy was compared with that of carbamazepine controlled release (CR) monotherapy in a randomized, double-blind, noninferiority phase III trial (study 311) in patients aged ≥ 18 years with newly diagnosed epilepsy [21]. Enrolled patients were randomized to receive eslicarbazepine acetate or carbamazepine CR, with treatment initiated at 400 mg once daily and 200 mg once daily, respectively, for 1 week. After the treatment initiation, the study utilized a stepwise design, in which patients had their dosage increased to the first target dose level (eslicarbazepine acetate 800 mg once daily or carbamazepine CR 200 mg twice daily) over a 1-week stabilization period, followed by a 26-week evaluation period. Patients experiencing a seizure during the evaluation period had their eslicarbazepine acetate or carbamazepine CR dosage escalated to the second target dose level (1200 mg once daily or 400 mg twice daily, respectively), with patients escalating to the third target dose level (1600 mg once daily or 600 mg twice daily, respectively) if a further seizure occurred. At any target dose level, patients who remained seizure-free for the entire 26-week evaluation period entered a 6-month maintenance period and a subsequent extension phase. Treatment was discontinued if patients experienced a seizure while receiving the third target dose level during the evaluation period, or at any dose level during the maintenance period or the extension phase [21].
The primary endpoint was the proportion of seizure-free patients for the entire evaluation period at the last evaluated target dose level [21]. Eslicarbazepine acetate was considered noninferior to carbamazepine CR if the lower limit of the one-sided 97.5% confidence interval (CI) for the estimated average risk difference (ARD) of the primary endpoint did not exceed −12%. Noninferiority was tested in the PP set (n = 388 and n = 397 in the eslicarbazepine acetate and carbamazepine CR groups) and if noninferiority was demonstrated, it was subsequently tested in the full-analysis set (FAS; n = 401 and n = 412) [21].
At baseline, patient and disease characteristics were generally similar between the treatment groups [21]. The majority of patients in the eslicarbazepine acetate (70.8%) and carbamazepine CR (74.5%) groups completed the 26-week evaluation period, with 67.6% and 76.9% of patients in the respective groups remaining at the first target dose level [21].
Eslicarbazepine acetate was noninferior to carbamazepine CR in terms of the seizure freedom rates (primary endpoint), assessed in the PP set and subsequently in the FAS [21]. At the end of the 26-week evaluation period, seizure freedom was achieved in 71.1% of eslicarbazepine acetate recipients and 75.6% of carbamazepine CR recipients in the PP set, and 70.8% and 74.0% of patients in the FAS. In the respective population sets, the ARD was − 4.28% (95% CI − 10.30 to 1.74%) and − 3.07% (95% CI − 9.04% to 2.89%). The overall proportion of seizure-free patients between treatment groups was similar regardless of the target dose level and seizure type. At the end of the evaluation period, the probability of treatment failure (i.e. first seizure) was 12% in the eslicarbazepine acetate group and 6% in the carbamazepine CR group, and comparable improvements in HR-QOL (as measured by QOLIE-31 questionnaires) were observed between treatment groups [21].
The noninferior antiepileptic efficacy and comparable improvements in HR-QOL observed during the evaluation period with eslicarbazepine acetate versus carbamazepine CR were maintained over 1 year of treatment [21]. At 1 year, the probability of patients to withdraw from treatment due to AEs or lack of efficacy was 26% with eslicarbazepine acetate and 21% with carbamazepine CR [21].
Following the completion of study 311 [21], patients could enter an open-label extension study (311-EXT) where all patients received eslicarbazepine acetate once daily for ≈ 2 years [22]. Patients treated with eslicarbazepine acetate (n = 109) received treatment at their last evaluated dose level and those switching from carbamazepine CR (n = 97) received the dose of eslicarbazepine acetate that matched the last evaluated dose level of carbamazepine CR in study 311; eslicarbazepine acetate dosage could be adjusted within the range of 800–1600 mg/day, based on clinical response and tolerability. Overall, the majority (89.3%) of patients remained on eslicarbazepine acetate monotherapy and > 80% of patients receiving eslicarbazepine acetate monotherapy (n = 184) were seizure-free during the 2-year extension study. Moreover, all patients and investigators (n = 155 each) rated overall treatment satisfaction as ‘very good’ or ‘good’ at the end of study visit [22].
The efficacy of eslicarbazepine acetate as monotherapy was further supported by two identically designed, double-blind, randomized, historical cohort-controlled phase III trials in adults with uncontrolled focal-onset seizures despite receiving 1–2 ASMs (studies 093-045 [23] and 093-046 [24]). Following an 8-week baseline period, eligible patients were randomized to receive eslicarbazepine acetate 1200 mg (efficacy population, n = 60 in study 093-045; n = 54 in study 093-046) or 1600 mg (n = 118; n = 100) once daily for 18 weeks. The target dosage of eslicarbazepine acetate was achieved during the 2-week titration period and then maintained for 16 weeks, which consisted of a 6-week ASM conversion period and 10-week eslicarbazepine acetate monotherapy period [23, 24]. The primary endpoint was the proportion of patients meeting ≥ 1 predefined exit criteria (signifying worsening seizure control) by day 112 (i.e. at the end of 10-week maintenance period) [23, 24].
In studies 093-045 and -046, eslicarbazepine acetate 1200 or 1600 mg/day was an effective conversion to monotherapy agent, with the Kaplan-Meier estimated exit rate at day 112 being 12.8–44.4%; the upper limits of the 95% CIs for the Kaplan-Meier estimated exit rates for eslicarbazepine acetate 1200 or 1600 mg/day were below the lower limit of the pre-specified exit rate threshold of 65.3% (calculated from the historical controls), indicating statistical superiority [23, 24]. In both trials, 7.4–10% of patients receiving eslicarbazepine acetate 1200 or 1600 mg/day were seizure-free during the 10-week monotherapy period [23, 24].
2.2 In the Paediatric Population
2.2.1 Adjunctive Therapy
The efficacy of eslicarbazepine acetate as an adjunctive therapy for the treatment of focal-onset seizure in children was evaluated in a phase II trial (study 208) [25], which was primarily designed to evaluate the drug’s effects on cognitive function, and a phase III trial (study 305) [26]. Both studies were randomized, double-blind and placebo-controlled [25, 26]. Enrolled children were aged 6–16 years [25] or 2–18 years [26], had refractory focal-onset seizures and were receiving 1–2 ASMs (except oxcarbazepine).
Following a 4-week [25] or 8-week [26] observational baseline period, patients were randomized to receive eslicarbazepine acetate (n = 83 in study 208; n = 134 in study 305) or placebo (n = 40; n = 129) during a double-blind period (part I), which consisted of a 4-week [25] or 6-week [26] titration period and a 8-week [25] or 12-week [26] maintenance period. The target dosage of eslicarbazepine acetate in study 208 (30 mg/kg/day [25]) and 305 (20 mg/kg/day [26]) was achieved during the titration period and administered during the maintenance period; the dosage could be up-or down-titrated to 10–30 mg/kg/day based on tolerability and therapeutic response, with the maximum dosage of 1200 mg/day [25, 26]. Randomization was stratified by age group and patients who completed part I could enter two (part II–III) [25] or four (part II–V) [26, 27] subsequent long-term open-label extension periods (each extension period ≤ 2 years).
Of the randomized patients, 90.4% and 89.6% of eslicarbazepine acetate recipients completed part I of studies 208 and 305 (vs 92.5% and 91.5% of placebo recipients) [25, 26]. In study 208, where efficacy analyses were secondary objectives, adjunctive eslicarbazepine acetate was associated with significant (p ≤ 0.009) benefits over placebo in terms of the LSM change in standardised seizure frequency from baseline to maintenance period (− 34.8% vs − 13.8% [5]) and the responder rate (i.e. patients achieving a ≥ 50% reduction from baseline in seizure frequency; 50.6% vs 25.0%) [25]. In study 305, however, the differences in LSM change from baseline in standardized seizure frequency and the responder rate (co-primary endpoints) between the eslicarbazepine acetate and the placebo groups were not statistically significant (− 18.1% vs − 8.6% and 30.6% vs 31.0%, respectively) [26]. Although the reason for nonsignificance cannot be definitively explained, factors affecting the young age group (e.g. etiological heterogeneity, difficulty in recognizing simple focal-onset seizures, high seizure frequency with risk of imbalance) and the underestimation of the effective target dosage of eslicarbazepine acetate may have played a role [26].
The antiepileptic efficacy of eslicarbazepine acetate in children appeared to be age- and dose-dependent, according to the post-hoc subgroup analysis of study 305 [26]. In patients aged 7–18 years, the LSM change in standardized seizure frequency and the ≥ 50% responder rate were numerically greater in eslicarbazepine acetate than placebo recipients (− 24.4% vs − 10.5%; 35% vs 30.2%). In this age group, the response to eslicarbazepine acetate was generally more prominent in patients receiving 30 mg/kg/day, with the relative reduction of standardized seizure frequency being significantly (p = 0.0478) greater than placebo (− 37.2% vs − 5.2%) [26].
The antiepileptic efficacy of eslicarbazepine acetate was maintained in the open-label extension periods of studies 208 and 305 [5, 25,26,27]. For instance, in the 1-year open-label extension period of study 305 (part II; n = 225), the total median relative change in standardized seizure frequency from open-label baseline period was − 46.7% and the total responder rate was 46.7% [26]. The respective endpoints in patients receiving eslicarbazepine acetate throughout the study were − 40.4% and 41.4% and those in patients who switched from placebo to eslicarbazepine acetate in the extension period were − 51.4% and 51.7% [26]. In the subsequent part III–V extension periods of study 305 (n = 148), the median relative change in standardized seizure frequency from baseline (i.e. the last 4 weeks of part II) was − 22.9%, the overall responder rate was 26.6% and 8.1% of patients achieved seizure freedom [5, 27]. Taking the limitations of open-label uncontrolled data into account, the long-term response to eslicarbazepine acetate treatment observed during open-label extension periods was maintained overall [5, 27].
2.3 In the Real-World Setting
Several large (n > 100) real-world studies of retrospective or prospective design further supported the antiepileptic efficacy of oral eslicarbazepine acetate as adjunctive therapy [28,29,30,31,32,33,34] or monotherapy [28,29,30, 34,35,36,37] in patients with focal-onset epilepsy. Discussion focuses on results from the largest real-world study (Euro-Esli; n = 2058), an exploratory pooled analysis of 14 European real-world studies, including Early-Esli [29], EPOS [32] and ESLIBASE [30], in patients aged 14–88 years with epilepsy who received eslicarbazepine acetate treatment [34].
In Euro-Esli, the median duration of eslicarbazepine acetate treatment was 65 months, with the median dosage being 400 mg/day at baseline and 800 mg/day from month 3 onwards [34]. After 12 months of therapy, 73.4% of patients remained on eslicarbazepine acetate treatment, 75.6% achieved ≥ 50% reduction in seizure frequency and 41.3% were seizure free for ≥ 6 months [34]. Furthermore, eslicarbazepine acetate significantly reduced the median frequencies of total, simple partial, complex partial and secondarily generalized seizures at final follow-up (i.e. last visit) from baseline (p < 0.01 for all) [34].
Responder and seizure freedom rates were generally higher in patients receiving eslicarbazepine acetate as monotherapy than those receiving the drug as adjunctive therapy [38]. In addition, a subgroup descriptive analysis revealed that eslicarbazepine acetate was more effective in patients aged ≥ 65 years, receiving < 2 concomitant ASMs or not receiving other sodium channel blocking ASMs [34], or without intellectual disability [39]; the presence or absence of psychiatric comorbidity or depression had no effect on eslicarbazepine acetate efficacy [39]. Factors such as shorter epilepsy duration, < 2 previously received ASMs, < 5 seizures per month, lower numbers of concomitant ASMs and the absence of seizure at baseline increased the odds of eslicarbazepine acetate treatment retention at 12 months, according to a post-hoc analysis of Euro-Esli [40].
3 Tolerability of Eslicarbazepine Acetate
In the clinical trials and real-world studies discussed in Sect. 2, oral eslicarbazepine acetate as adjunctive therapy or as monotherapy was generally well tolerated in adults with focal-onset epilepsy. The tolerability profile of eslicarbazepine acetate as adjunctive therapy in paediatric patients was similar to that seen in adult patients [25, 26]. The majority of AEs occurred within the first few weeks of eslicarbazepine acetate treatment, with most being of mild or moderate severity [5].
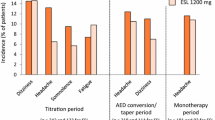
According to a pooled safety analysis of data from studies 301–304 in adults with refractory focal-onset seizures (Sect. 2.1.1), the incidences of treatment-emergent adverse events (TEAEs) in patients receiving adjunctive eslicarbazepine acetate 800 or 1200 mg/day (n = 500 and 490) were 67.0% and 73.1% versus 52.7% with placebo (n = 513) [14, 41]. The most common TEAEs considered possibly related to study drug (> 10% in any treatment group) included dizziness, somnolence and nausea, with the incidence of each TEAE being dose-dependent with eslicarbazepine acetate treatment (Fig. 1). Serious TEAEs occurred in ≤ 5% of patients in any treatment group and TEAEs led to treatment discontinuation in 12.2%, 22.2% and 6.2% of eslicarbazepine acetate 800 mg/day, 1200 mg/day and placebo recipients, respectively; the most common TEAEs leading to treatment discontinuation in the respective groups were dizziness (4.8%, 8.2% and 0.8%), nausea (2.2%, 5.3% and 0%), vomiting (2.0%, 4.3% and 0.2%) and ataxia (1.8%, 3.9% and 0%) [14, 41]. Where reported, adjunctive eslicarbazepine acetate was associated with low incidences of psychiatric and cognitive TEAEs [42], and did not appear to worsen seizure frequency or severity relative to placebo [43]. The tolerability profile of adjunctive eslicarbazepine acetate in elderly patients (aged ≥ 65 years) was consistent to that observed in studies 301–304 [44].
Treatment-emergent adverse events considered possibly related to study drug with > 10% of incidence in any treatment group in a pooled analysis of studies 301, 302, 303 and 304 [14]. ESL eslicarbazepine acetate, PL placebo
In adults with epilepsy, the tolerability profile of eslicarbazepine acetate as monotherapy [21, 23, 24] was consistent to that seen when the drug was administered as adjunctive therapy [9,10,11,12]. For instance, in study 311 in patients with newly diagnosed focal-onset epilepsy (Sect. 2.1.2), the incidences of TEAEs in patients receiving eslicarbazepine acetate (n = 401) or carbamazepine CR (n = 412) monotherapy were 76.3% versus 79.6%, respectively, with the most common TEAEs considered possibly related to study drug including dizziness (7.5% vs 7.0%), headache (6.7% vs 6.8%), somnolence (5.2% vs 7.8%), fatigue (5.0% vs 4.4%) and nausea (4.5% vs 7.3%) [21]. Overall, 14.0% and 18.4% of patients in the respective groups discontinued treatment due to TEAEs [21].
The nature and frequency of TEAEs with adjunctive eslicarbazepine acetate in paediatric patients were also similar to those observed in adult patients, with the safety profile generally consistent across age groups [5, 25, 26]. In placebo-controlled studies in paediatric patients with refractory focal-onset seizures (Sect. 2.2.1), the most frequently reported AEs (> 3% incidence) with adjunctive eslicarbazepine acetate in patients aged 6–11 years were diplopia (9.5%), somnolence (7.4%), dizziness (6.3%), convulsion (6.3%) and nausea (3.2%), whereas somnolence (7.4%), vomiting (4.2%), diplopia (3.2%) and fatigue (3.2%) were most frequently reported in those aged 12–18 years [5].
Long-term safety data from the open-label extension studies of up to 2 years [17, 18, 22, 25,26,27] and post-marketing surveillance of up to 6 years [41] in adult and paediatric patients with focal-onset epilepsy were consistent with the known safety profile of eslicarbazepine acetate and revealed no new safety signals.
3.1 Adverse Events of Special Interest
In general, the effect of eslicarbazepine acetate on clinical laboratory parameters (haematology, blood chemistry, urine and coagulation) appears to be minimal [14, 21, 27]. Cases of clinically relevant hyponatraemia (i.e. Na ≤ 125 mEq/L) have been reported in 1.1–3.3% of adult patients [21, 45] and 0.5–0.8% of paediatric patients [46] treated with eslicarbazepine acetate. Where reported, the incidence of hyponatraemia with eslicarbazepine acetate generally increased in a dose-dependent manner [5]; hyponatraemia-related TEAEs led to treatment discontinuation in ≤ 1.6% patients treated with eslicarbazepine acetate [45]. Of note, the incidence of hyponatraemia in elderly patients was 8.3% and all patients recovered from hyponatraemia without sequelae [44]. Patients with pre-existing renal disease or with symptoms of hyponatraemia, or those concomitantly receiving medications that may cause hyponatraemia (e.g. diuretics, desmopressin, carbamazepine), should be monitored for sodium levels during eslicarbazepine acetate therapy and treatment should be discontinued immediately if clinically relevant hyponatraemia develops [5].
Hypersensitivity reactions (e.g. rash, urticarial, angioedema), serious cutaneous adverse reactions [e.g. Stevens-Johnson Syndrome (SJS), toxic epidermal necrolysis (TEN) and drug reaction with eosinophilia and systemic symptoms (DRESS)] and dose-related PR interval prolongation have been reported with eslicarbazepine acetate in clinical trials or in post-marketing experience [5]. Appropriate monitoring, treatment interruption, modification or discontinuation of eslicarbazepine acetate may be necessary in patients experiencing such AEs; appropriate treatment may also be required. Furthermore, as eslicarbazepine acetate is structurally related to oxcarbazepine, the occurrence of class-related rare adverse reactions, such as bone marrow depression, anaphylactic reactions, systemic lupus erythematosus or serious cardiac arrhythmias after eslicarbazepine acetate treatment cannot be excluded, although they did not occur during the placebo-controlled studies with eslicarbazepine acetate [5].
Suicidal behavior and ideation have been reported with other ASMs and available data does not exclude the possibility of an increase in such risks with eslicarbazepine acetate; patients treated with eslicarbazepine acetate should therefore be monitored for signs of suicidal behaviour or ideation [5].
4 Dosage and Administration of Eslicarbazepine Acetate
Eslicarbazepine acetate is approved in the EU for the treatment of focal-onset seizures, with or without secondary generalisation, as monotherapy in adults with newly diagnosed epilepsy and as adjunctive therapy in adults, adolescents and children > 6 years with epilepsy [5]. Its efficacy and safety have not yet been established in children aged ≤ 6 years. Eslicarbazepine acetate is orally administered and can be taken with or without food. It is available in tablet (200, 400, 600 and 800 mg) and oral suspension (50 mg/mL) formulations and, based on comparability bioavailability data between the formulations (Table 1), patients can freely switch from taking tablets to taking suspensions [5].
The recommended dosage of eslicarbazepine acetate differs between adults and children [5]. For adults, the recommended starting dosage of oral eslicarbazepine acetate is 400 mg once daily, which should be increased to 800 mg once daily after 1–2 weeks. Based on individual response, the dosage may increase up to 1200 mg once daily when given as adjunctive therapy and up to 1600 mg once daily when given as monotherapy (1600 mg not recommended in patients aged ≥ 65 years). The recommend dosage of oral eslicarbazepine acetate in children aged > 6 years with a body weight of ≥ 60 kg is identical as for adults. However, for children with a body weight of < 60 kg, the recommended dosage of oral eslicarbazepine acetate is based on body weight, with the starting dosage being 10 mg/kg/day once daily and increased by 10–30 mg/kg/day weekly or bi-weekly (based on individual response) to the maximum dosage of 1200 mg once daily [5].
Although there are inadequate data on the use of eslicarbazepine acetate in pregnant women, reproductive toxicity has been observed in animal studies after treatment with eslicarbazepine acetate [5]. If women receiving eslicarbazepine acetate become pregnant or are considering pregnancy, the use of eslicarbazepine acetate should be carefully re-evaluated and patients should be advised of the potential increased risk of malformations and offered an antenatal screening. It is also advised to discontinue breastfeeding during eslicarbazepine acetate treatment [5].
Local prescribing information should be consulted for detailed information regarding missed doses, discontinuation, contraindications, drug interactions, use in special patient populations, and warnings and precautions.
5 Place of Eslicarbazepine Acetate in the Management of Focal-Onset Seizures
With a growing number of approved ASMs available, the treatment strategy in patients with focal-onset seizures should be individualized and the choice of an appropriate ASM should be based on various factors, including the seizure type, epilepsy syndrome, the drug efficacy and safety profile, pharmacological properties, patient comorbidities, co-medication, potential drug interactions, drug costs and patient preference [3, 47]. The 2020 updated National Institute and Health and Care Excellence (NICE) guidelines recommend carbamazepine or lamotrigine as first-line therapy for children, adolescents and adults with newly diagnosed focal-onset seizures, and levetiracetam, oxcarbazepine or sodium valproate are recommended if carbamazepine or lamotrigine are not suitable or not tolerated; adjunctive therapy should be considered if a second well-tolerated ASM is ineffective [47]. For patients with refractory focal-onset seizures, the NICE guidelines recommend carbamazepine, clobazam, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, sodium valproate or topiramate as adjunctive therapy; if initial adjunctive therapy is ineffective or not tolerated, other ASMs, including eslicarbazepine acetate, lacosamide, phenobarbital, phenytoin, pregabalin, tiagabine, vigabatrin and zonisamide, may be considered [47]. Recommendations in the 2018 practice guideline update summary from the American Academy of Neurology and the American Epilepsy Society on the use of second- and third-generation ASMs are consistent with NICE guidelines [48, 49].
Eslicarbazepine acetate, a voltage-gated sodium channel blocker, is a newer third-generation ASM belonging to a drug class known as dibenzazepine carboxamide [2, 50]. Eslicarbazepine acetate has been developed to overcome some limitations associated with the use of its chemically related ASMs carbamazepine and oxcarbazepine, thereby providing more favourable antiepileptic efficacy and tolerability profiles [2, 50]. Although direct comparison data are lacking, evidence from the real-world studies indicates that eslicarbazepine acetate is an effective adjunctive therapy when added in patients who have inadequate seizure control with carbamazepine [50]. Moreover, real-world studies suggest that patients experiencing inadequate seizure control and intolerable AEs with oxcarbazepine may quickly switch to eslicarbazepine acetate without clinical neuropsychological or laboratory abnormalities [50, 51]. In addition, eslicarbazepine acetate monotherapy may be effective in patients who have switched from carbamazepine or oxcarbazepine because of their inadequate efficacy or unacceptable AEs [50]. Furthermore, with the convenience of once-daily administration and a potentially improved tolerability profile (e.g. fewer psychiatric/neurological AEs and improvement in lipid profiles), eslicarbazepine acetate is likely to promote treatment adherence [50]. In a retrospective cohort from a large US commercial claims database (n = 983 evaluable), the adherence rate of eslicarbazepine acetate monotherapy was significantly (p = 0.037) higher than that of carbamazepine and numerically higher than that of oxcarbazepine [52].
In clinical trials, oral eslicarbazepine acetate was effective in providing seizure control in adult and paediatric patients with focal-onset epilepsy (Sect. 2). Data from studies 301–304 demonstrated that adjunctive eslicarbazepine acetate was associated with a significant decrease in seizure frequency and an increase in responder rate compared with placebo in adults with refractory focal-onset epilepsy (Sect. 2.1.1). Eslicarbazepine acetate was also an effective monotherapy agent in adults with newly diagnosed focal-onset epilepsy, being noninferior to carbamazepine CR in terms of seizure freedom rates in study 311 (Sect. 2.1.2). In addition, in children with refractory focal-onset seizures in studies 208 and 305, adjunctive eslicarbazepine acetate provided effective seizure control compared with placebo, with the efficacy potentially being age- and dose-dependent (Sect. 2.2.1). Although eslicarbazepine acetate failed to reach statistical significance over placebo in study 305, this may have been due to various factors affecting the young age group and underestimating the effective target dosage of eslicarbazepine acetate. Longer term, the antiepileptic efficacy of eslicarbazepine acetate in adult and paediatric patients was maintained during open-label extension studies, with each extension study period being up to 2 years. Furthermore, results from large real-world studies in adult and paediatric patients with focal-onset epilepsy also supported the antiepileptic efficacy of eslicarbazepine acetate (Sect. 2.3).
Eslicarbazepine acetate was generally well tolerated when administered as adjunctive therapy or monotherapy in adult patients and when administered as adjunctive therapy in paediatric patients, with most AEs being of mild or moderate intensity (Sect. 3). Dizziness, somnolence, nausea, fatigue and diplopia were among the most frequently reported TEAEs with eslicarbazepine acetate in the clinical trials, with no new safety signals identified during longer-term (≤ 6 years) treatment [41]. Of note, although few cases of hyponatraemia (i.e. Na ≤ 125 mEq/L) were reported with eslicarbazepine acetate in the clinical trials (Sect. 3.1), these trials excluded patients with a serum sodium level of < 130 mEq/L (Sect. 2). In a real-world single-centre study of adult inpatients (n = 560), the incidence of eslicarbazepine acetate-induced hyponatraemia was not statistically different to that induced by oxcarbazepine but was higher than that induced by carbamazepine and was more commonly seen in elderly patients [53]. Appropriate monitoring and management of hyponatraemia is recommended before and during the eslicarbazepine acetate treatment (Sect. 3.1) [5].
As with other ASMs, there is a potential teratogenic risk with eslicarbazepine acetate; however, data relating to the use of eslicarbazepine acetate in pregnant women is currently limited. In a review of a global safety database (comprised of clinical trials and 8-year post-marketing surveillance), 79 cases of eslicarbazepine acetate exposure during pregnancy have been identified, of which 10 cases of spontaneous abortions and five cases of congenital anomalies were reported [54]. Of 11 out of 15 cases of spontaneous abortion and/or congenital anomalies, eslicarbazepine acetate was coadministered with other ASMs, such as lacosamide, lamotrigine and levetiracetam, and three cases of congenital anomalies were considered to be possibly related to eslicarbazepine acetate exposure [54]. Further studies and monitoring of eslicarbazepine acetate use in pregnant women would be of interest.
Meta-analyses and indirect comparisons of several newer ASMs as adjunctive therapy in refractory focal-onset epilepsy [4, 55,56,57,58] or as monotherapy in newly diagnosed focal-onset epilepsy [59] are available. Overall, there were no significant differences in antiepileptic efficacy between eslicarbazepine acetate and other third-generation ASMs, including lacosamide, brivaracetam and perampanel, for the treatment of refractory focal-onset epilepsy [55, 57, 58] and eslicarbazepine acetate was considered to be an effective initial monotherapy and a suitable alternative to carbamazepine CR in adult patients with newly diagnosed focal-onset epilepsy [59]. However, given the indirect nature of the comparisons, these findings should be interpreted with caution.
In conclusion, based on extensive evidence from clinical trials and real-world studies, oral eslicarbazepine acetate is an effective and generally well tolerated treatment option for adults, adolescents and children aged > 6 years with focal-onset seizures.
Data Selection Eslicarbazepine acetate: 484 records identified
Duplicates removed | 90 |
Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 211 |
Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 113 |
Cited efficacy/tolerability articles | 40 |
Cited articles not efficacy/tolerability | 30 |
Search Strategy: EMBASE, MEDLINE and PubMed from 2014 to present. Previous Adis Drug Evaluation published in 2014 was hand-searched for relevant data. Clinical trial registries/databases and websites were also searched for relevant data. Key words were eslicarbazepine acetate, Zebinix, Aptiom, Exalief, Stedesa, focal seizures, partial-onset seizures, epilepsy. Records were limited to those in English language. Searches last updated 3 July 2020. | |
References
Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58(4):512–21.
Galiana GL, Gauthier AC, Mattson RH. Eslicarbazepine acetate: a new improvement on a classic drug family for the treatment of partial-onset seizures. Drugs R D. 2017;17(3):329–39.
Moshe SL, Perucca E, Ryvlin P, et al. Epilepsy: new advances. Lancet. 2015;385(9971):884–98.
Slater J, Chung S, Huynh L, et al. Efficacy of antiepileptic drugs in the adjunctive treatment of refractory partial-onset seizures: meta-analysis of pivotal trials. Epilepsy Res. 2018;143:120–9.
European Medicines Agency. Zebinix: summary of product characteristics. 2020. https://www.ema.europa.eu/. Accessed 17 Jun 2020.
US FDA. APTIOM (eslicarbazepine acetate) tablets, for oral use: US prescribing information. 2019. https://www.accessdata.fda.gov/. Accessed 17 Jun 2020.
Keating GM. Eslicarbazepine acetate: a review of its use as adjunctive therapy in refractory partial-onset seizures. CNS Drugs. 2014;28(7):583–600.
Shirley M, Dhillon S. Eslicarbazepine acetate monotherapy: a review in partial-onset seizures. Drugs. 2016;76(6):707–17.
Elger C, Halasz P, Maia J, et al. Efficacy and safety of eslicarbazepine acetate as adjunctive treatment in adults with refractory partial-onset seizures: a randomized, double-blind, placebo-controlled, parallel-group phase III study. Epilepsia. 2009;50(3):454–63.
Ben-Menachem E, Gabbai AA, Hufnagel A, et al. Eslicarbazepine acetate as adjunctive therapy in adult patients with partial epilepsy. Epilepsy Res. 2010;89(2–3):278–85.
Gil-Nagel A, Lopes-Lima J, Almeida L, et al. Efficacy and safety of 800 and 1200 mg eslicarbazepine acetate as adjunctive treatment in adults with refractory partial-onset seizures. Acta Neurol Scand. 2009;120(5):281–7.
Sperling MR, Abou-Khalil B, Harvey J, et al. Eslicarbazepine acetate as adjunctive therapy in patients with uncontrolled partial-onset seizures: results of a phase III, double-blind, randomized, placebo-controlled trial. Epilepsia. 2015;56(2):244–53.
Cramer JA, Velez FF, Anastassopoulos KP, et al. Severity and burden of partial-onset seizures in a phase III trial of eslicarbazepine acetate. Epilepsy Behav. 2015;53:149–53.
Elger C, Koepp M, Trinka E, et al. Pooled efficacy and safety of eslicarbazepine acetate as add-on treatment in patients with focal-onset seizures: data from four double-blind placebo-controlled pivotal phase III clinical studies. CNS Neurosci Ther. 2017;23(12):961–72.
Biton V, Rogin JB, Krauss G, et al. Adjunctive eslicarbazepine acetate: a pooled analysis of three phase III trials. Epilepsy Behav. 2017;72:127–34.
Gil-Nagel A, Elger C, Ben-Menachem E, et al. Efficacy and safety of eslicarbazepine acetate as add-on treatment in patients with focal-onset seizures: integrated analysis of pooled data from double-blind phase III clinical studies. Epilepsia. 2013;54(1):98–107.
Halasz P, Cramer JA, Hodoba D, et al. Long-term efficacy and safety of eslicarbazepine acetate: results of a 1-year open-label extension study in partial-onset seizures in adults with epilepsy. Epilepsia. 2010;51(10):1963–9.
Hufnagel A, Ben-Menachem E, Gabbai AA, et al. Long-term safety and efficacy of eslicarbazepine acetate as adjunctive therapy in the treatment of partial-onset seizures in adults with epilepsy: results of a 1-year open-label extension study. Epilepsy Res. 2013;103(2–3):262–9.
Velez FF, Bond TC, Anastassopoulos KP, et al. Impact of seizure frequency reduction on health-related quality of life among clinical trial subjects with refractory partial-onset seizures: a pooled analysis of phase III clinical trials of eslicarbazepine acetate. Epilepsy Behav. 2017;68:203–7.
Toledo M, Mazuela G, Mauri JA, et al. Levels of anger in epilepsy patients treated with eslicarbazepine acetate. Acta Neurol Scand. 2019;140(1):48–55.
Trinka E, Ben-Menachem E, Kowacs PA, et al. Efficacy and safety of eslicarbazepine acetate versus controlled-release carbamazepine monotherapy in newly diagnosed epilepsy: a phase III double-blind, randomized, parallel-group, multicenter study. Epilepsia. 2018;59(2):479–91.
Trinka E, Pereira A, Moreira J, et al. Long-term efficacy and safety of eslicarbazepine acetate (ESL) monotherapy: results from BIA-2093-311/EXT study –the 2-year open-label extension of the ESL study (BIA-2093-311) [abstract + poster]. In: American Epilepsy Society (AES) 73rd Annual Meeting. 2019.
Sperling MR, Harvey J, Grinnell T, et al. Efficacy and safety of conversion to monotherapy with eslicarbazepine acetate in adults with uncontrolled partial-onset seizures: a randomized historical-control phase III study based in North America. Epilepsia. 2015;56(4):546–55.
Jacobson MP, Pazdera L, Bhatia P, et al. Efficacy and safety of conversion to monotherapy with eslicarbazepine acetate in adults with uncontrolled partial-onset seizures: a historical-control phase III study. BMC Neurol. 2015;15(46):1–13.
Jozwiak S, Veggiotti P, Moreira J, et al. Effects of adjunctive eslicarbazepine acetate on neurocognitive functioning in children with refractory focal-onset seizures. Epilepsy Behav. 2018;81:1–11.
Kirkham F, Auvin S, Moreira J, et al. Efficacy and safety of eslicarbazepine acetate as adjunctive therapy for refractory focal-onset seizures in children: a double-blind, randomized, placebo-controlled, parallel-group, multicenter, phase-III clinical trial. Epilepsy Behav. 2020;105:106962.
European Medicines Agency. Zebinix: assessment report for paediatric studies submitted in accordance with article 46 of regulation (EC) No 1901/2006. 2018. https://www.ema.europa.eu/. Accessed 17 Jun 2020.
Weissinger F, Losch F, Winter Y, et al. Effectiveness of eslicarbazepine acetate in dependency of baseline anticonvulsant therapy: results from a German prospective multicenter clinical practice study. Epilepsy Behav. 2019;101(Pt A):106574.
Villanueva V, Bermejo P, Montoya J, et al. EARLY-ESLI study: long-term experience with eslicarbazepine acetate after first monotherapy failure. Acta Neurol Scand. 2017;136(3):254–64.
Villanueva V, Serratosa JM, Guillamon E, et al. Long-term safety and efficacy of eslicarbazepine acetate in patients with focal seizures: results of the 1-year ESLIBASE retrospective study. Epilepsy Res. 2014;108(7):1243–52.
Lattanzi S, Cagnetti C, Foschi N, et al. Eslicarbazepine acetate as adjunctive treatment in partial-onset epilepsy. Acta Neurol Scand. 2018;137(1):29–32.
Holtkamp M, McMurray R, Bagul M, et al. Real-world data on eslicarbazepine acetate as add-on to antiepileptic monotherapy. Acta Neurol Scand. 2016;134(1):76–82.
Correia FD, Freitas J, Magalhaes R, et al. Two-year follow-up with eslicarbazepine acetate: a consecutive, retrospective, observational study. Epilepsy Res. 2014;108(8):1399–405.
Villanueva V, Holtkamp M, Delanty N, et al. Euro-Esli: a European audit of real-world use of eslicarbazepine acetate as a treatment for partial-onset seizures. J Neurol. 2017;264(11):2232–48.
Giraldez BG, Garamendi-Ruiz I, Zurita J, et al. Clinical outcomes of eslicarbazepine acetate monotherapy for focal-onset seizures: a multicenter audit. Acta Neurol Scand. 2019;140(6):422–8.
Villanueva V, Bermejo P, Montoya J, et al. MONOZEB: long-term observational study of eslicarbazepine acetate monotherapy. Epilepsy Behav. 2019;97:51–9.
Toledano R, Jovel CE, Jimenez-Huete A, et al. Efficacy and safety of eslicarbazepine acetate monotherapy for partial-onset seizures: experience from a multicenter, observational study. Epilepsy Behav. 2017;73:173–9.
Holtkamp M, Delanty N, Sales F, et al. Eslicarbazepine acetate as monotherapy in clinical practice: outcomes from Euro-Esli. Acta Neurol Scand. 2019;139(1):49–63.
Doherty CP, Rheims S, Assenza G, et al. Eslicarbazepine acetate in epilepsy patients with psychiatric comorbidities and intellectual disability: clinical practice findings from the Euro-Esli study. J Neurol Sci. 2019;402:88–99.
Sales F, McMurray R, Loureiro RA, et al. Clinical predictors of 12-month retention in patients treated with eslicarbazepine acetate: real-world evidence from the Euro-Esli study [poster presentation]. In: American Epilepsy Society (AES) 73rd Annual Meeting 2019.
Gama H, Vieira M, Costa R, et al. Safety profile of eslicarbazepine acetate as add-on therapy in adults with refractory focal-onset seizures: from clinical studies to 6 years of post-marketing experience. Drug Saf. 2017;40(12):1231–40.
Andermann E, Biton V, Benbadis SR, et al. Psychiatric and cognitive adverse events: a pooled analysis of three phase III trials of adjunctive eslicarbazepine acetate for partial-onset seizures. Epilepsy Behav. 2018;82:119–27.
Carreno M, Benbadis S, Rocha F, et al. Incidence of seizure exacerbation and seizures reported as adverse events during adjunctive treatment with eslicarbazepine acetate: a pooled analysis of three phase III controlled trials. Epilepsia Open. 2017;2(4):459–66.
Costa R, Steinhoff B, Gama H, et al. Safety, tolerability and efficacy of eslicarbazepine acetate as adjunctive therapy in patients aged >= 65 years with focal seizures. Drugs Aging. 2018;35(12):1109–17.
Wechsler RT, Radtke RA, Smith M, et al. Serum sodium levels and related treatment-emergent adverse events during eslicarbazepine acetate use in adults with epilepsy. Epilepsia. 2019;60(7):1341–52.
Vaisleib I, Duchowny M, Grinnell T, et al. Analysis of sodium levels and hyponatremia events in trials of eslicarbazepine acetate (ESL) in pediatric patients (aged 4-17 years) [abstract no. P5-268]. Neurology. 2018;90(15 Suppl).
National Institute for Health and Clinical Excellence. Epilepsies: diagnosis and management (clinical guideline 137). 2020. https://www.nice.org.nk/. Accessed 3 Jul 2020.
Kanner AM, Ashman E, Gloss D, et al. Practice guideline update summary: efficacy and tolerability of the new antiepileptic drugs II: treatment-resistant epilepsy: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2018;91(2):82–90.
Kanner AM, Ashman E, Gloss D, et al. Practice guideline update summary: efficacy and tolerability of the new antiepileptic drugs I: treatment of new-onset epilepsy: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2018;91(2):74–81.
Lawthom C, Peltola J, McMurray R, et al. Dibenzazepine agents in epilepsy: how does eslicarbazepine acetate differ? Neurol Ther. 2018;7(2):195–206.
Willems LM, Zollner JP, Paule E, et al. Eslicarbazepine acetate in epilepsies with focal and secondary generalised seizures: systematic review of current evidence. Expert Rev Clin Pharmacol. 2018;11(3):309–24.
Mehta D, Lee J, Simeone J, et al. Adherence to AED treatment for patients with focal seizure receiving monotherapy with eslicarbazepine acetate or prior generation generics: evidence from a large us commercial claims database [abstract no. P5.5-003]. Neurology. 2019;92(15 Suppl):5.
Intravooth T, Staack AM, Juerges K, et al. Antiepileptic drugs-induced hyponatremia: review and analysis of 560 hospitalized patients. Epilepsy Res. 2018;143:7–10.
Costa R, Magalhaes LM, Graca J, et al. Eslicarbazepine acetate exposure in pregnant women with epilepsy. Seizure. 2018;58:72–4.
Li-Na Z, Deng C, Hai-Jiao W, et al. Indirect comparison of third-generation antiepileptic drugs as adjunctive treatment for uncontrolled focal epilepsy. Epilepsy Res. 2018;139:60–72.
Hu Q, Zhang F, Teng W, et al. Efficacy and safety of antiepileptic drugs for refractory partial-onset epilepsy: a network meta-analysis. J Neurol. 2018;265(1):1–11.
Brigo F, Trinka E, Bragazzi NL, et al. A common reference-based indirect comparison meta-analysis of eslicarbazepine versus lacosamide as add on treatments for focal epilepsy. Epilepsy Res. 2016;127:12–8.
Brigo F, Bragazzi NL, Nardone R, et al. Efficacy and tolerability of brivaracetam compared to lacosamide, eslicarbazepine acetate, and perampanel as adjunctive treatments in uncontrolled focal epilepsy: results of an indirect comparison meta-analysis of RCTs. Seizure. 2016;42:29–37.
Lattanzi S, Zaccara G, Giovannelli F, et al. Antiepileptic monotherapy in newly diagnosed focal epilepsy. A network meta-analysis. Acta Neurol Scand. 2019;139(1):33–41.
Holtkamp D, Opitz T, Hebeisen S, et al. Effects of eslicarbazepine on slow inactivation processes of sodium channels in dentate gyrus granule cells. Epilepsia. 2018;59(8):1492–506.
Hebeisen S, Pires N, Loureiro AI, et al. Eslicarbazepine and the enhancement of slow inactivation of voltage-gated sodium channels: a comparison with carbamazepine, oxcarbazepine and lacosamide. Neuropharmacology. 2015;89:122–35.
Pellegrino G, Mecarelli O, Pulitano P, et al. Eslicarbazepine acetate modulates EEG activity and connectivity in focal epilepsy. Front Neurol. 2018;9(1054):1–7.
Falcao A, Fuseau E, Nunes T, et al. Pharmacokinetics, drug interactions and exposure-response relationship of eslicarbazepine acetate in adult patients with partial-onset seizures: population pharmacokinetic and pharmacokinetic/pharmacodynamic analyses. CNS Drugs. 2012;26(1):79–91.
Milovan D, Almeida L, Romach MK, et al. Effect of eslicarbazepine acetate and oxcarbazepine on cognition and psychomotor function in healthy volunteers. Epilepsy Behav. 2010;18(4):366–73.
Levy-Cooperman N, Schoedel KA, Chakraborty B, et al. Abuse liability assessment of eslicarbazepine acetate in healthy male and female recreational sedative users: a phase I randomized controlled trial. Epilepsy Behav. 2016;61:63–71.
Vaz-Da-Silva M, Nunes T, Almeida L, et al. Evaluation of eslicarbazepine acetate on cardiac repolarization in a thorough QT/QTc study. J Clin Pharmacol. 2012;52(2):222–33.
Sunkaraneni S, Ludwig E, Fiedler-Kelly J, et al. Modeling and simulations to support dose selection for eslicarbazepine acetate therapy in pediatric patients with partial-onset seizures. J Pharmacokinet Pharmacodyn. 2018;45(4):649–58.
Fontes-Ribeiro C, Nunes T, Falcao A, et al. Eslicarbazepine acetate (BIA 2-093): relative bioavailability and bioequivalence of 50 mg/mL oral suspension and 200mg and 800mg tablet formulations. Drugs R D. 2005;6(5):253–60.
Falcao A, Pinto R, Nunes T, et al. Effect of repeated administration of eslicarbazepine acetate on the pharmacokinetics of simvastatin in healthy subjects. Epilepsy Res. 2013;106(1–2):244–9.
Falcao A, Vaz-da-Silva M, Gama H, et al. Effect of eslicarbazepine acetate on the pharmacokinetics of a combined ethinylestradiol/levonorgestrel oral contraceptive in healthy women. Epilepsy Res. 2013;105(3):368–76.
Acknowledgements
During the peer review process, the manufacturer of eslicarbazepine acetate was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Young-A Heo is a salaried employee of Adis International Ltd/Springer Nature, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: D. Lindhout, Department of Genetics, University Medical Center Utrecht/Stichting Epilepsie Instellingen Nederland, Heemstede, the Netherlands; E. Santamarina, Epilepsy Unit, Neurology Department, Hospital Vall d´Hebron, Barcelona, Spain; P.E. Smith, Welsh Epilepsy Centre, University Hospital of Wales, Cardiff, United Kingdom; A. Strzelczyk, Department of Neurology and Epilepsy Center Frankfurt Rhine-Main, Goethe-University Frankfurt and University Hospital Frankfurt, Frankfurt, Germany.
Enhanced material for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.12486323.
Rights and permissions
About this article
Cite this article
Heo, YA. Eslicarbazepine Acetate: A Review in Focal-Onset Seizures. CNS Drugs 34, 989–1000 (2020). https://doi.org/10.1007/s40263-020-00751-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-020-00751-3