Abstract
Background
Based on reassuring short-term foetal and maternal safety data, there is an increasing trend to administer chemotherapy during the second and third trimesters of pregnancy. The pharmacokinetics (PK) of drugs might change as a result of several physiological changes that occur during pregnancy, potentially affecting the efficacy and safety of chemotherapy.
Objective
With this analysis, we aimed to quantitatively describe the changes in the PK of docetaxel, paclitaxel, doxorubicin and epirubicin in pregnant women compared with non-pregnant women.
Methods
PK data from 9, 20, 22 and 16 pregnant cancer patients from the International Network of Cancer, Infertility and Pregnancy (INCIP) were available for docetaxel, paclitaxel, doxorubicin and epirubicin, respectively. These samples were combined with available PK data from non-pregnant patients. Empirical non-linear mixed-effects models were developed, evaluating fixed pregnancy effects and gestational age as covariates.
Results
Overall, 82, 189, 271, and 227 plasma samples were collected from pregnant patients treated with docetaxel, paclitaxel, doxorubicin and epirubicin, respectively. The plasma PK data were adequately described by the respective models for all cytotoxic drugs. Typical increases in central and peripheral volumes of distribution of pregnant women were identified for docetaxel, paclitaxel, doxorubicin and epirubicin. Additionally, docetaxel, doxorubicin and paclitaxel clearance were increased in pregnant patients, resulting in lower exposure in pregnant women compared with non-pregnant patients.
Conclusion
Given the interpatient variability, the identified pregnancy-induced changes in PK do not directly warrant dose adjustments for the studied drugs. Nevertheless, these results underscore the need to investigate the efficacy of chemotherapy, when administered during pregnancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pregnancy affects the pharmacokinetics of cytotoxic drugs. |
Exposures were decreased in pregnant patients compared with non-pregnant patients. |
Dose adjustments should be considered when maternal outcome is shown to be affected. |
1 Introduction
Given that the pharmacology of drugs is typically unknown in the pregnant population, dosing in pregnancy is based on research in non-pregnant patients. Little research is being performed in order to investigate the effects of pharmacological treatment on the pregnant patient and the foetus. The US Food and Drug Administration (FDA) and the European Medicines Agency have released guidelines on how to cope with this highly vulnerable patient population [1,2,3]. It is advised to make use of the population pharmacokinetics (PK) approach. This method can quantify and explain the PK characteristics and variability in drug concentrations among individuals. In addition, this method enables optimal use of data from a very limited population and may limit the number of required PK assessments. The population PK approach can assess the effects of pregnancy on the PK and hence provide recommendations for dosing adjustments [1, 3].
Cancer complicates one out of 1000–2000 pregnancies [4]. In the treatment of pregnant cancer patients, clinicians were initially reluctant to use chemotherapy, as the short- and long-term effects of exposure of cytotoxic drugs on the foetus were unknown but feared. Recently, the treatment of pregnant women with cytotoxic drugs during pregnancy was shown to be well tolerated in the short and middle-to-long term for both the mother and the foetus, when considering both cancer prognosis and perinatal and selected childhood outcomes. Safety for the foetus has been demonstrated when chemotherapy has been administered after the first trimester [5]. The increased use of chemotherapy has resulted in an increase in livebirths and decrease in prematurity among pregnant cancer patients [4, 5].
With the empirical dosing approach based on non-pregnant patient data, potential gestational effects on the human physiology are ignored. Anecdotal reports suggest less chemotherapy-related toxicity among pregnant women compared with non-pregnant women. Several physiological changes have been described during pregnancy, which may in theory have an effect on the PK of drugs. The most pronounced changes include an increase in body fluids, such as plasma volume, extracellular water and total body water. Additionally, a decrease in plasma protein concentrations, increased glomerular filtration and changes in metabolizing enzyme activity have been reported [6]. It follows that these PK changes might thus result in over- or underexposure, which might have a substantial influence on the efficacy and development of toxicity of the treatment.
We previously reported that the PK of cytotoxic drugs is altered during pregnancy [7, 8]. For all of the drugs that were studied, lower drug concentrations were observed in pregnant patients during the second and third trimesters compared with non-pregnant patients [8]. A decrease in the area under the plasma concentration-time curve (AUC) was observed for docetaxel, paclitaxel, doxorubicin and epirubicin. However, this preliminary analysis was limited by the very small number of pregnant patients who could be included at that time, particularly for patients treated with docetaxel or paclitaxel. This small sample size and large interindividual variability hampered the determination of a continuous gestational effect on the PK of all four studied drugs. Despite the fact that the magnitude of the effect of pregnancy on PK is anticipated to be largest in the third trimester, a continuous relationship with gestational age (GA) is expected given the time-dependent change and interplay of the various physiological parameters. Considering the limitations of the previous preliminary analysis, the inclusion of patients in this patient cohort was continued and we report the updated results herein.
2 Methods
2.1 Patients and Sampling
PK datasets from previously reported analyses were extended with PK data for docetaxel, doxorubicin and epirubicin from newly included pregnant patients. Patients were recruited from the International Network of Cancer, Infertility and Pregnancy (INCIP) registry (NCT00330447). This study was approved by Institutional Review Boards and Independent Ethics Committees at participating institutions and was carried out in accordance with the ethical principles of the Declaration of Helsinki.
PK samples were centrifuged and stored at – 80 °C after withdrawal. Subsequently, PK samples were transported to a central facility where drug concentrations were measured using validated bioanalytical methods [9, 10]. Previously published clinical trial PK data from non-pregnant patients were included and served as the control data (Table 1) [8]. For paclitaxel, the dataset from a recently published PK model was used and was enriched with our PK data from pregnant patients [11]. Due to the limited data, PK data collected postpartum were considered as being from non-pregnant patients in the current analysis.
2.2 Model Development
2.2.1 Structural Model
The structural model was developed using the concentration-time data collected from both pregnant and non-pregnant patients. For the description of docetaxel, doxorubucin and epirubicin concentrations, one- to three-compartment models with linear elimination were first evaluated. For paclitaxel, a linear three-compartment model was first tested. However, a recently published PK model for paclitaxel performed better in terms of parameter precision and goodness-of-fit (GOF) plots [11]. This model consisted of a three-compartment model with first-order distribution to the first peripheral compartment (V2), saturable distribution to the second peripheral compartment (V3), and saturable elimination from the central compartment (V1).
2.2.2 Stochastic Model
Between-subject variability (BSV) was evaluated for all parameters, using an exponential error model (Eq. 1):
where Pi represents the individual parameter estimate for individual i, Ppop represents the typical population parameter estimate as defined by the structural model, and ηi represents the BSV effect for subject individual i, which was assumed to be normally distributed following N (0, ω2). Residual unexplained variability (RUV) was described by a proportional error model for all four drugs (Eq. 2):
where Cobs,ij represents the observed concentration for individual i and observation j, Cpred,ij represents the individual predicted concentration, and εp,ij represents the proportional error distributed following N (0, σ2). Separate residual variabilities were estimated for the data from pregnant and non-pregnant patients, as data were available from different clinical studies and different bioanalytical methods were used.
2.2.3 Covariate Analysis
In order to identify relationships between covariates and the structural model parameters, plots of \(\eta\)-values of the parameter of interest versus the covariate were visually assessed for trends. If available, total body weight (WT) at time of PK sampling was first tested in the PK model that included non-pregnant patients only (Eq. 3).
where P represents the WT-adjusted population parameter. All clearance (CL) and volume parameters (i.e. V1, V2 and V3) were scaled to the median WT in the dataset. The exponents (COV) were fixed to 0.75 for CL parameters and 1 for volumes of distribution according to allometric principles [12]. WT at time of PK sampling was available for all patients. Similarly, gender was tested as a covariate for the control patients, for the docetaxel, doxorubicin and epirubicin models.
Next, relationships between GA and CL and volume parameters were univariately tested in the model. Following visual inspection of the covariate plots, continuous models using GA or discrete gestational effects were considered. Covariate effects that were already present in the original paclitaxel PK model were retained in our model, with the exception of bilirubin since this covariate was not prospectively collected for all pregnant patients. In addition, no further covariate testing was performed for docetaxel, doxorubicin and epirubicin, in addition to WT and gender, because of unavailability in the pregnant or non-pregnant populations.
2.3 Model Selection and Evaluation
Discrimination between models was guided by physiological plausibility, GOF plots, precision of parameter estimates and change in objective function value (dOFV). A drop of ≥ 3.84 points, corresponding to a p value < 0.05 (Chi-square distribution with 1 degree of freedom [df]), was considered a significant improvement of the fit for hierarchical nested models. The adequacy of the models was assessed by GOF plots and visual predictive checks (VPCs) [13]. Parameter precision was assessed using the sampling importance resampling (SIR) procedure [14].
2.4 Simulations
Stochastic simulations using the final PK models were performed to evaluate the effect of pregnancy on the exposure of the respective drugs. Individual concentration-time curves were simulated for patients treated at the dose regimen at which most patients in the pregnancy study were treated (i.e. 100 mg/m2 for docetaxel, 175 mg/m2 for paclitaxel, 60 mg/m2 doxorubicin, and 100 mg/m2 for epirubicin, using a standardized body surface area [BSA] of 1.8 m2). Interindividual variability was taken into account in these simulations by using random sampling from a covariance matrix from the final PK model. For each simulation dataset, 2000 patients were simulated (1000 pregnant patients and 1000 non-pregnant patients). To determine the AUC from time zero to 48 h (AUC48) and maximum plasma concentration (Cmax) of each drug, a dense sampling schedule was used in the simulations. The AUC48 was determined by accumulation of the concentration in the central compartment over 48 h. The Cmax was the highest simulated concentration in the same interval. The time interval of 48 h was a standardized time frame based on the time points for the observations in the datasets.
2.5 Software
Non-linear mixed-effects modelling was performed using NONMEM® (version 7.3; ICON Development Solutions, Ellicott City, MD, USA) and Perl-speaks-NONMEM (PsN; version 4.4.8), with first-order conditional estimation with interaction (FOCE-I) as the estimation method. Piraña (version 2.9.7; Certara, Princeton, NJ, USA) was used as the graphical user interface for NONMEM [15,16,17], and R (version 3.4.3; The R Foundation for Statistical Computing, Vienna, Austria) was used for data management and graphical diagnostics [18].
3 Results
3.1 Docetaxel
A total of 533 plasma samples from 44 patients (9 pregnant and 35 non-pregnant patients) were available for docetaxel. In addition, PK samples were collected postpartum from one patient (Table 1). With the exception of one treatment cycle, all cycles in which PK samples were collected in pregnant patients took place during the third trimester. Median GA was 31.8 weeks (range 26.1–35.0).
The data were best described by a linear three-compartment model with first-order elimination. WT was not available for the control patients and was therefore not tested as a covariate. The inclusion of gender did not result in a better description of the control data compared with the model without this covariate and was therefore not retained. After graphical exploration, it could be concluded that a continuous gestational effect could not be identified. A discrete pregnancy effect was however estimated for CL, V1 and V2, and an overprediction of the higher concentration was observed in pregnant patients (Electronic Supplementary Figs. S1 and S3). For pregnant patients, fold changes of 1.08 (95% confidence interval [CI] 0.92–1.24), 1.19 (95% CI 0.90–1.50) and 1.12 (95% CI 0.82–1.50) were estimated for CL, V1 and V2, respectively. After inclusion of the pregnancy effect, GOF plots and VPCs showed an improved fit (Figs. 1 and 2); however, OFV did not decrease significantly (dOFV = − 1.6 points, p = 0.665, df = 4) and BSV and RUV did not show a decrease. A trend for pregnancy-induced changes was thus observed, albeit it could not be formally identified as a covariate. Final model parameters are depicted in Table 2. Simulations showed that docetaxel exposure in pregnant patients was lower in terms of both AUC48 (mean ± standard deviation [SD] 3648 ± 892.3 ng × h/mL vs. 3916 ± 950.7 ng × h/mL) and Cmax (mean ± SD 2759 ± 518.5 ng/mL vs. 2938 ± 548.9 ng/mL) compared with non-pregnant patients. Relative change in pregnant patients compared with non-pregnant patients is depicted in Fig. 2.
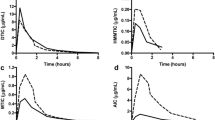
Visual predictive checks of the final pharmacokinetic models for docetaxel, paclitaxel, doxorubicin and epirubicin, stratified by population. Solid lines and darker blue areas represent the median observed values and simulated 80% confidence interval around the median. Dashed lines and light blue areas represent the 10% and 90% percentiles of the observed values and 80% prediction intervals of the simulated percentiles (n = 1000)
Relative change in AUC48 and Cmax for docetaxel, paclitaxel, doxorubicin and epirubicin in pregnant patients compared with non-pregnant patients using the final pharmacokinetic models (n = 1000; relative change (%) = ((pregnant – non-pregnant)/non-pregnant) × 100%). AUC48 area under the plasma concentration-time curve from time zero to 48 h, Cmax maximum plasma concentration
3.2 Paclitaxel
A total of 6145 plasma samples from 708 patients (20 pregnant and 688 non-pregnant patients) were available for paclitaxel. PK samples from pregnant patients were obtained in the second (n = 11) and third trimesters (n = 14); median GA was 31.0 weeks (range 13.7–35.7). A previously reported PK model was used as a starting point [11]. In this model, BSA, gender, bilirubin and age were included as covariates on the parameter describing the maximal elimination rate (VMEL). Since bilirubin levels were not measured for all pregnant patients at the time of PK sampling, bilirubin as a covariate was removed from the base model. This model was updated with the concentration-time curves of the pregnant patients, and the covariate effects for BSA, gender, bilirubin and age were retained for all patients. Continuous covariate relationships between GA and structural PK parameters could not be identified (see Electronic Supplementary Fig. S1). The inclusion of discrete covariate effects on V1, V3 and KMEL resulted in a decrease in OFV of 30 points (model without pregnancy effect vs. the base model; p < 0.00001, df = 3). Additionally, GOF (Fig. 1), covariate plots (Electronic Supplementary Fig. S1) and VPCs (Fig. 1) indicated an improved model fit, and both BSV and RUV were reduced. The typical fold change in V1, V3 and KMEL for pregnant patients was 2.27 (95% CI 1.57–3.10), 2.28 (95% CI 1.54–3.63), and 1.32 (95% CI 1.03–1.69), respectively. Simulations showed lower AUC48 (mean ± SD 14.8 ± 3.48 μmol × h/L vs. 16.9 ± 4.40 μmol × h/L) and Cmax (mean ± SD 3.69 ± 0.906 μmol/L vs. 4.85 ± 1.50 μmol/L) in pregnant patients compared with non-pregnant patients.
3.3 Doxorubicin
A total of 580 samples from 87 patients (26 pregnant and 61 non-pregnant patients) were available for doxorubicin. Data from four pregnant patients were excluded because of sampling errors; for these patients, infusion and sample withdrawal times were unavailable. PK samples from pregnant patients were collected during the second (n = 14) and third trimesters (n = 13); median GA was 28.7 weeks (range 15.0–36.3).
A linear three-compartment model with first-order elimination best described the doxorubicin data. The inclusion of WT as a covariate resulted in an improved fit of the non-pregnant data (dOFV = − 65.3 points; p < 0.00001, df = 4). Thus, all CL and volume parameters were scaled to a standard WT of 70 kg for both pregnant and non-pregnant patients. The inclusion of gender did not result in a better description of the control data compared with the model without this covariate and was therefore not retained. Graphical exploration indicated higher CL and V1 in pregnant patients compared with non-pregnant patients; however, there was no clear trend of a continuous gestational effect (electronic supplementary Fig. S1). Moreover, the GOF plots showed an overprediction of the concentrations for the pregnant patients (electronic supplementary Fig. S3). For pregnant patients, fold changes of 1.13 (95% CI 1.00–1.28) and 1.08 (95% CI 0.85–1.35) were estimated for CL and V1. The addition of these pregnancy effects resulted in improvements in GOF (electronic supplementary Fig. S2) and VPCs, and both BSV in CL and the RUV decreased. Nevertheless, the VPCs of the final model still indicated that the variability in the observed concentrations was lower in pregnant versus non-pregnant patients, which was not captured by the simulated percentiles (Fig. 1).
The inclusion of discrete covariate effects on CL and V1 resulted in a decrease in OFV of 5.7 points (model without pregnancy effect vs. the base model, p = 0.059, df = 2). Simulations showed that the doxorubicin exposure in pregnant patients was lower in terms of both AUC48 (mean ± SD 2090 ± 736.1 ng × h/mL vs. 2324 ± 812.4 ng × h/mL) and Cmax (mean ± SD 2223 ± 393.5 ng/mL vs. 2371 ± 396.1 ng/mL) compared with non-pregnant patients.
3.4 Epirubicin
A total of 976 plasma samples from 80 patients (16 pregnant and 64 non-pregnant patients) were available for epirubicin. PK curves from pregnant patients were obtained both in the second (n = 13) and third trimesters (n = 9); median GA was 26.8 weeks (range 19.0–34.0).
The data were best described by a linear three-compartment model with first-order elimination. The inclusion of WT and gender did not improve the model and were thus not included. Continuous covariate relationships between GA and structural PK parameters could not be identified (see Electronic Supplementary Fig. S1). However, the inclusion of discrete covariate effects on V1 and V2 resulted in a decrease in OFV of 60 points (p < 0.0001, df = 2); however, GOF, VPCs and covariate plots (electronic supplementary Fig. S1, and Fig. 1) showed an improved model fit, and BSV and RUV decreased. Similar to doxorubicin, the variability in the observed concentrations was overpredicted for the pregnant population. The typical fold change in V1 and V2 of pregnant patients was 1.79 (95% CI 1.49–2.12) and 2.94 (95% CI 1.91–4.29), respectively. Simulations showed slightly lower AUC48 (mean ± SD 1992 ± 508.5 ng × h/mL vs. 1997 ± 512.3 ng × h/mL) and Cmax (mean ± SD 2108 ± 355.4 ng/mL vs. 2233 ± 411.5 ng/mL) in pregnant patients compared with non-pregnant patients.
4 Discussion
With this analysis, we report on the updated population analysis of the effect of pregnancy on the PK of docetaxel, paclitaxel, doxorubicin and epirubicin. Previously reported models were extended with newly collected data. With this addition, the datasets changed substantially. The PK models were therefore re-estimated and covariate effects were re-assessed. Additionally, we successfully determined the effect of pregnancy on the saturable PK of paclitaxel.
For all four drugs, typical increases in the central volume of distribution of pregnant patients were observed. Increases were estimated for docetaxel and epirubicin V2 and paclitaxel V3 in pregnant patients. Additionally, increases in CL were observed for docetaxel and doxorubicin, and in KMEL for paclitaxel. For docetaxel, only modest increases in typical parameters were observed and the inclusion of these pregnancy effects in the model did not result in a statistically significant improvement of the model, meaning that the PK model for non-pregnant patients, which is characterized by large BSV, also accounts for the small changes in docetaxel PK during pregnancy. However, GOF plots and VPCs showed an improved description of both the pregnant and non-pregnant data. The VPCs for doxorubicin and epirubicin did however show a slight overprediction of the variability in the pregnant population. This might be explained by the large BSV within the included non-pregnant population, while this variability may be lower in pregnant patients since this is a much more homogeneous population (all relatively young, non-pretreated females without much comorbidity).
All drugs in this analysis are mainly eliminated by hepatic CL. Docetaxel, paclitaxel, doxorubicin and epirubicin are all metabolized by cytochrome P450 (CYP) 3A4, whereas paclitaxel is also metabolized by CYP2C8 and epirubicin is also metabolized by UGTB7. A modest increase in CYP3A4 activity during pregnancy has been described, therefore a change in the CL of drugs metabolized by CYP3A4 is expected. For CYP2C8 and UGTB7, gestational changes have not yet been described [6]. Despite the known pregnancy-related increase in CYP3A4 activity, an effect of pregnancy could not be identified for the CL of epirubicin. Docetaxel and paclitaxel are largely distributed to the peripheral tissues and are highly protein bound, while doxorubicin and epirubicin are both distributed to the peripheral tissues and show moderate protein binding to albumin. As the albumin and α1-glycoportein concentrations decline during pregnancy and body fluids increase, an increase in the central and peripheral volumes of distribution was expected.
We were not able to identify continuous gestational effects on the PK of either docetaxel, paclitaxel, doxorubicin or epirubicin. Quantitative analyses have shown that the largest changes in glomerular filtration rate, body fluids, metabolizing enzyme activity and plasma protein concentrations occur gradually in the first 12 weeks following conception. Thereafter, changes remain relatively small until the end of the third trimester [6]. Contradictory results have been reported regarding the foetal safety of chemotherapy during the first weeks after conception. The extent of distribution of cytotoxic drugs across the placenta varies per drug and the safety of administration of cytotoxic drugs during organogenesis is still not fully unravelled [19]. For this reason, patients were treated with chemotherapy in the second and third trimesters only. The absence of PK data obtained during the first trimester can most likely explain the unidentifiability of a continuous gestational effect in our analysis. The effect of WT on doxorubicin PK and the effect of BSA, gender and age on paclitaxel PK were included in the respective PK models. Although several covariates have been reported to have an influence on the PK of the investigated cytotoxic drugs, no additional covariates could be investigated due to a lack of available data [20,21,22,23]. Additionally, the effect of bilirubin on the PK of paclitaxel was removed from the PK model because bilirubin levels were not measured at the time of PK sampling in the pregnant population. Several methods for handling missing covariates have been proposed in the past [24, 25]. As the change in bilirubin levels during pregnancy has not been described quantitatively, using such methods could potentially lead to the introduction of bias in the bilirubin covariate effect [6, 26]. Nevertheless, as covariates could have explained part of the interindividual variability in PK and differences between pregnant and non-pregnant patients, our results from non-pregnant patients are similar to the previously reported PK models.
Using a population PK approach, we were able to specify the gestational effects on the PK of the aforementioned drugs, despite the limited number of patients available. In addition, the population PK approach enabled the use of all available data from non-pregnant patients as control data. Previously identified covariates, such as gender, WT and age, were included in the models, thereby accounting for potential differences between the pregnant and non-pregnant patient populations. To our knowledge, this PK analysis included the largest number of pregnant patients receiving chemotherapy thus far. PK data from non-pregnant and pregnant patients were combined, allowing for an adequate estimation of structural models, and, most importantly, the gestational effects.
The results of our analysis provide a good approximation of the pregnancy-related changes in the PK of docetaxel, paclitaxel, doxorubicin and epirubicin in patients who will potentially be treated with these drugs. For all four drugs, decreased systemic drug exposures were observed in pregnant patients, although the effects were relatively small compared with the observed interpatient variability. The decrease in exposure suggests that, potentially, a higher dose should be administered to pregnant patients. However, an exposure–response relationship has not been described for the four cytotoxic drugs described in this study, in neither non-pregnant nor pregnant patients. Despite being the largest dataset with PK in pregnant cancer patients, the current dataset is too small to assess the effect of lower exposure on toxicity and efficacy; therefore, the magnitude of impact of the typical underexposure in pregnant patients on the efficacy of these drugs remains unknown. In breast cancer patients and patients with Hodgkin lymphoma, no significant differences in long-term overall survival were observed between pregnant and non-pregnant patients; however, significant numbers of patients would be needed to find an effect on long-term survival, if possible at all [27,28,29]. Long-term efficacy results from pregnant patients, derived from the INCIP registry (www.cancerinpregnancy.org), continue to be collected to address this question. As the effects found on PK are relatively small compared with the overall interpatient variability, it is advised to administer paclitaxel, docetaxel, epirubicin and doxorubicin similarly in pregnant and non-pregnant patients.
5 Conclusion
From this analysis, it can be concluded that the plasma concentrations of four widely used cytotoxic drugs, i.e. docetaxel, paclitaxel, doxorubicin and epirubicin, are decreased in pregnant women. The population PK models adequately described the effect of pregnancy on the PK of these four drugs, despite the limited number of pregnant patients. These data underscore the need to further investigate maternal prognosis when chemotherapy is used during pregnancy. Only when this maternal outcome is affected do alternative drugs (characterized by a smaller influence of gestational changes) or dose adjustments based on efficacy and toxicity of these drugs in pregnant patients need to be considered. The PK models developed in this study can be used to guide any future dose adjustments.
References
FDA Guidance for Industry – Pharmaockinetics in Pregnancy – Study Design, Data Analysis, and Impact on Dosing and Labeling. FDA Guidance. US FDA; 2004.
FDA Guidance for Industry – Establishing Pregnancy Exposure Registries. FDA Guidance. US FDA; 2002.
European Medicines Agency (EMA). Guideline on the exposure to medicinal products during pregnancy: need for post-authorization data. London: European Medicines Agency; 2006.
de Haan J, Verheecke M, Van Calsteren K, Van CB, Shmakov RG, Gziri MM, et al. Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: a 20-year international cohort study of 1170 patients. Lancet Oncol. 2018;19:337–46.
Amant F, Vandenbroucke T, Verheecke M, Fumagalli M, Halaska MJ, Boere I, et al. Pediatric outcome after maternal cancer diagnosed during pregnancy. N Engl J Med. 2015;373:1824–34.
Abduljalil K, Furness P, Johnson TN, Rostami-Hodjegan A, Soltani H. Anatomical, physiological and metabolic changes with gestational age during normal pregnancy. Clin Pharmacokinet. 2012;51:365–96.
Van Calsteren K, Verbesselt R, Ottevanger N, Halaska M, Heyns L, Van Bree R, et al. Pharmacokinetics of chemotherapeutic agents in pregnancy: a preclinical and clinical study. Acta Obstet Gynecol Scand. 2010;89:1338–45.
van Hasselt JGC, Van Calsteren K, Heyns L, Han S, Mhallem Gziri M, Schellens JHM, et al. Optimizing anticancer drug treatment in pregnant cancer patients: pharmacokinetic analysis of gestation-induced changes for doxorubicin, epirubicin, docetaxel and paclitaxel. Ann Oncol. 2014;25:2059–65.
Hendrikx JJMA, Hillebrand MJX, Thijssen B, Rosing H, Schinkel AH, Schellens JHM, et al. A sensitive combined assay for the quantification of paclitaxel, docetaxel and ritonavir in human plasma using liquid chromatography coupled with tandem mass spectrometry. J Chromatogr B. 2011;879:2984–90.
Beijnen JH, Meenhorst PL, Van Gijn R, Fromme M, Rosing H, Underberg WJM. HPLC determination of doxorubicin, doxorubicinol and four aglycone metabolites in plasma of AIDS patients. J Pharm Biomed Anal. 1991;9:995–1002.
Crombag M-RBS, de Vries Schultink AHM, Koolen SLW, Wijngaard S, Joerger M, Schellens JHM, et al. Impact of older age on the exposure of paclitaxel: a population pharmacokinetic study. Pharm Res. 2019;36:33.
West GB, Brown JH, Enquist BJ. A general model for the origin of allometric scaling laws in biology. Science. 1997;276:122–6.
Nguyen T, Mouksassi M-S, Holford N, Al-Huniti N, Freedman I, Hooker A, et al. Model evaluation of continuous data pharmacometric models: metrics and graphics. CPT Pharmacomet Syst Pharmacol. 2017;6:87–109.
Dosne A-G, Bergstrand M, Karlsson MO. An automated sampling importance resampling procedure for estimating parameter uncertainty. J Pharmacokinet Pharmacodyn. 2017;44:509–20.
Lindbom L, Ribbing J, Jonsson EN. Perl-speaks-NONMEM (PsN)—a Perl module for NONMEM related programming. Comput Methods Programs Biomed. 2004;75:85–94.
Keizer RJ, van Benten M, Beijnen JH, Schellens JHM, Huitema ADR. Pirana and PCluster: a modeling environment and cluster infrastructure for NONMEM. Comput Methods Programs Biomed. 2011;101:72–9.
Beal S, Boeckmann A, Sheiner L. NONMEM user guides. San Francisco: University of California; 1988.
RC Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2009.
Vandenbroucke T, Verheecke M, Fumagalli M, Lok C, Amant F. Effects of cancer treatment during pregnancy on fetal and child development. Lancet Child Adolesc Health. 2017;1:302–10.
Joerger M, Huitema ADR, Richel DJ, Dittrich C, Pavlidis N, Briasoulis E, et al. Population pharmacokinetics and pharmacodynamics of doxorubicin and cyclophosphamide in breast cancer patients: a study by the EORTC-PAMM-NDDG. Clin Pharmacokinet. 2007;46:1051–68.
Kontny NE, Würthwein G, Joachim B, Boddy AV, Krischke M, Fuhr U, et al. Population pharmacokinetics of doxorubicin: establishment of a NONMEM model for adults and children older than 3 years. Cancer Chemother Pharmacol. 2013;71:749–63.
Bruno R, Vivier N, Veyrat-Follet C, Montay G, Rhodes GR. Population pharmacokinetics and pharmacokinetic-pharmacodynamic relationships for docetaxel. Invest New Drugs. 2001;19:163–9.
Sandstrom M, Lindman H, Nygren P, Johansson M, Bergh J, Karlsson M. Population analysis of the pharmacokinetics and the haematological toxicity of the fluorouracil-epirubicin-cyclophosphamide regimen in breast cancer patients. Cancer Chemother Pharmacol. 2006;58:143–56.
Graham JW. Missing data analysis: making it work in the real world. Annu Rev Psychol. 2009;60:549–76.
Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7:147–77.
Ince DI, Eissing T, Hempel G. gestation-specific changes in the anatomy and physiology of healthy pregnant women : an extended repository of model parameters for physiologically based pharmacokinetic modeling in pregnancy. Clin Pharmacokinet. 2017;56:1303–30.
Amant F, Von Minckwitz G, Han SN, Bontenbal M, Ring AE, Giermek J, et al. Prognosis of women with primary breast cancer diagnosed during pregnancy: results from an international collaborative study. J Clin Oncol. 2013;31:2532–9.
Ploquin A, Pistilli B, Tresch E, Frenel JS, Lerebours F, Lesur A, et al. 5-year overall survival after early breast cancer diagnosed during pregnancy: a retrospective case-control multicentre French study. Eur J Cancer. 2018;95:30–7.
Maggen C, Dierickx D, Lugtenburg P, Laenen A, Cardonick E, Smakov R, et al. Obstetric and maternal outcomes in patients diagnosed with Hodgkin lymphoma during pregnancy: a multicentre, retrospective, cohort study. Lancet Haematol. 2019;6:e551–61.
Koolen SLW, Oostendorp RL, Beijnen JH, Schellens JHM, Huitema ADR. Population pharmacokinetics of intravenously and orally administered docetaxel with or without co-administration of ritonavir in patients with advanced cancer. Br J Clin Pharmacol. 2010;69:465–74.
Acknowledgements
The authors thank Liesbeth Leemans and Katrien Van Tornout for sample collections, and the Research HPC Facility of the Netherlands Cancer Institute for support in the use of computational resources.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Funding for the INCIP registry, sample bioanalysis and sample logistics was provided by the European Research Council under the European Union’s Horizon 2020 research and innovation programme (grant agreement number 647047), the Research Foundation-Flanders and the Belgian Cancer Plan, Ministry of Health, Belgium.
Conflicts of Interest
Frédéric Amant is a senior clinical investigator for the Research Fund-Flanders; Kristel Van Calsteren received a clinical research fund from the University Hospitals Leuven; Thomas Dorlo was personally supported by a Dutch Research Council (NWO)/ZonMw Veni grant; Michael J. Halaska was supported by the Charles University research project Progres Q28 and Q34; and Jos Beijnen is a part-time employee, patent holder (partly) and stock holder (indirectly) of Modra Pharmaceuticals BV, a spin-out company developing oral taxane formulations and therapies, which is not related to the submitted work. Julie M. Janssen, Robert Fruscio, Petronella Ottevanger, Carolien P. Schröder, Ingrid Boere, Petronella O. Witteveen, Rebecca C. Painter, Ruud Bekkers, Vit Drochytek and Alwin D.R. Huitema have no conflicts of interest to declare.
Ethics approval
The data used in this study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by institutional review boards and independent ethics committees at participating 137 institutions.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
All individual participants signed informed consent regarding publishing their data.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Author contributions
JJ, TD and AH performed the analysis, data interpretation and writing of the manuscript. KVC and FA designed the study, included patients, contributed to the writing of the manuscript, and provided financial support. MH, RF, PO, CS, IB, PW, RP, RB and VD included patients and contributed to the writing of the manuscript. JB contributed to drug analysis and writing of the manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Janssen, J.M., Van Calsteren, K., Dorlo, T.P.C. et al. Population Pharmacokinetics of Docetaxel, Paclitaxel, Doxorubicin and Epirubicin in Pregnant Women with Cancer: A Study from the International Network of Cancer, Infertility and Pregnancy (INCIP). Clin Pharmacokinet 60, 775–784 (2021). https://doi.org/10.1007/s40262-020-00961-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-020-00961-4