Abstract
Background
Compassionate drug use (CDU) provides early access to not yet authorised medicines and is funded by pharmaceutical companies. The observational retrospective study Compass-O monitored the CDU of onco-haematological drugs, managed by seven Italian units for cytotoxic drug preparations (Unità Farmaci Antiblastici [UFA]), between 1 January, 2016 and 31 December, 2021.
Objective
We aimed to evaluate the CDU of onco-haematological drugs managed by seven Italian UFA, between 2016 and 2021.
Methods
The seven UFA provided anonymised data concerning CDU approved in the study period. The early access and potential cost savings for the National Health System (Servizio Sanitario Nazionale [SSN]) were analysed for CDU concerning drug-therapeutic indication combinations with complete data and reimbursed by SSN up to December 2023 (date of study execution), according to the executive decision of the Italian Medicines Agency (Agenzia Italiana del Farmaco [AIFA]). Both analyses distinguished solid/liquid tumours and categorised the combinations as innovative (fully/conditionally) or non-innovative based on AIFA assessments.
Results
Compass-O collected 783 CDU authorisations, with 572 (73.1%) analysable in terms of early access and cost savings. On average, early access amounted to 514 days and the total cost savings was €376,115,801. Compassionate drug use approvals involved mainly solid tumours (93.7% vs 6.3% for liquid tumours), and the combination of trastuzumab emtansine-breast cancer was the most dispensed (n = 73; early access = 426 days; potential cost savings: €610,388). Out of 572 CDU approvals, 200 (35%) were innovative drug-therapeutic indication combinations, with 598 days of early access and a total potential saving of €113,124,069.
Conclusions
The study Compass-O showed a significant economic burden of CDU and a relevant need for early access, particularly for innovative drugs. However, there is currently no structured monitoring of CDU in Italy, suggesting the need for a national observatory, of which Compass-O can be the pilot phase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Compassionate drug use (CDU) is a widespread treatment option, providing early access to not yet authorised medicines for patients with life-threatening diseases. In Italy, there is currently no structured monitoring of CDU, suggesting the need for a national observatory. |
Compass-O is an observational retrospective study planned to evaluate the CDU of onco-haematological drugs managed by seven Italian units for cytotoxic drug preparations between 2016 and 2021. |
Compass-O collected 783 CDU authorisations, the early access amounted to 514 days and the total potential cost saving was €376,115,801, thus showing a significant economic burden of CDU and a relevant need for early access, particularly for innovative drugs. |
1 Introduction
Compassionate drug use (CDU), according to the definition by the European Medicines Agency (EMA), is “a treatment option that allows the use of an unauthorised medicine outside a clinical study under strictly controlled conditions” [1]. This helps to make medicines under development available to patients with life-threatening, long-lasting or seriously debilitating diseases, with no valid therapeutic alternatives, and who cannot enter clinical trials [1]. In fact, a CDU is guided by ethical reasons, differing fundamentally from clinical trials, where drugs are investigated to demonstrate their efficacy and safety [2]. The access to CDU can be provided on a named basis, for individual use, or enrolling patients into specific programmes, either way, the cost of treatment is the responsibility of the pharmaceutical company upon a physician’s request [3]. However, in Italy and a few other countries, such as the USA and Spain, the request for a CDU must be further evaluated and authorised by an ethics committee [4]. In a work regarding CDU requests, the authors illustrated the ethical and clinical issues emerging from the decision-making process of an Italian ethics committee, as the balance between a treatment’s efficacy/safety and quality of life, the importance of a clear realistic adequate communication, and the right to hope and simultaneous palliative care [5].
The EMA provides recommendations and the legal framework for CDU in the European Union (EU Regulation No. 726/2004), upon which every member state has formulated its own rules and legislations [6]. Of 28 European states, 18 (64%) have nationalised regulations and procedures for the CDU [6]. In Italy, it is in force via the Decree of 7 September, 2017, issued by the Italian Ministry of Health, and aligning with the above EU regulations [7]. As shown by Pilunni et al. [8], in Italy, the majority of CDU requests concern, rather than unauthorised treatments, drugs with at least one indication approved by the EMA but waiting for a price negotiation by the Italian Medicines Agency (Agenzia Italiana del Farmaco [AIFA]), and are actually unavailable on the Italian market. Thus, the CDU is actually a practice to accelerate the access of patients to innovative treatments with proven efficacy, offsetting the price and reimbursement procedures (taking, on average, from 287 to 340 days) [9,10,11]. This is particularly significant for rare diseases, as highlighted in a recently published scoping review [12]. A noteworthy finding is that most (71.7%) of the drugs used in compassionate use programmes subsequently receive marketing approval, often within 5 years of the programme being in place. The literature on the economic impact of CDU, with specific reference to Italy, is rather limited. The study by Pilunni et al. analysed the cost of drugs subject to individual requests for CDU, if used at the reimbursed price [8]. In a subsequent paper, the same authors examined the regional spread of CDUs but did not assess their economic impact [11]. Two studies by Jommi et al. collected evidence on the economic impact of CDUs, excluding and including certain collateral aspects, such as avoided hospitalisation costs [3, 13].
The study Compass-O arises in this context, and it is aimed to investigate the spreading of the CDU practice in Italy for onco-haematological therapies, quantifying two main aspects: the ability to ensure access to novel treatments, and the economic impact from the perspective of the National Health Service (Servizio Sanitario Nazionale [SSN]). Compass-O was conducted and coordinated by Fondazione Ricerca e Salute (ReS) and the University of Campania “Luigi Vanvitelli”, with the collaboration of the Italian Society of Pharmacology (Società Italiana Farmacologia) and the Italian Society of Hospital Pharmacy (Società Italiana di Farmacia Ospedaliera).
2 Methods
2.1 Study Design
Compass-O is a retrospective observational study aimed at analysing data on CDU of onco-haematological drugs that occurred from 1 January, 2016 to 31 December, 2021. This nationwide and multicentre study involved the units for cytotoxic drug preparations (Unità Farmaci Antiblastici [UFA]) of seven Italian centres, affiliated with the following public healthcare institutions spread across the national territory: Ospedale Dei Colli (Naples), Fondazione Policlinico Universitario Agostino Gemelli-IRCCS (Rome), Istituto Romagnolo per lo Studio dei Tumori “Dino Amadori” - IRCCS (Meldola), Aziende Socio Sanitarie Territoriali Valle Olona (Busto Arsizio), S.M. Goretti Hospital (Latina), Santa Maria della Misericordia Hospital (Perugia) and Ospedale Belcolle (Viterbo). The seven centres were first chosen based on their availability to participate in the study. However, attempts were made to include centres (i) equally located across the country and (ii) affiliated with both small and large hospitals. The study was firstly approved by the Ethics Committee of the coordinating centre L. Vanvitelli University of Campania, Naples, Italy (Protocol No. 0037627 of 14 December, 2022), and subsequently by the ethics committee of each UFA. This study, given its observational and retrospective nature, did not interfere with normal clinical practice.
2.2 Data Source
Data were collected separately in each UFA through a common data model, specifically designed for the Compass-O study. This model facilitated data harmonisation and enabled their later sharing and dissemination in an aggregated anonymised format within a centralised database used for analysis by the Fondazione ReS. The UFA were asked to provide the following information concerning CDUs that occurred in the study period:
-
I.
drugs (active ingredient, Anatomical Therapeutic Chemical code [14] and therapeutic indication [TI] (International Classification of Diseases, Ninth Revision codes));
-
II.
number of patients treated with each “drug-TI” combination;
-
III.
average dosage used, start/end date of treatment in CDU and start date of treatment under reimbursement regimes by the SSN.
2.3 Statistical Analyses
The data provided from the seven UFA through the common data model were analysed using descriptive statistics to depict the CDU of onco-haematological drugs, according to two main aspects: the early access (EA) and the potential cost savings (CS) for the SSN. Both EA and CS were analysed by the “drug-TI” combination, and separately by drug, and by TI. The 31 December, 2023 was set as the cutoff date.
The specific TIs, when appropriate and useful for the analysis, were grouped according to the tumour site, for example “lung cancer” (including, i.e. non-small cell lung cancer, lung cancer, small cell lung cancer, lung microcytoma), “breast cancer” (including, i.e. metastatic triple-negative breast cancer, HER2+/HR− breast cancer, HER2− breast cancer) and “skin cancer” (including, i.e. squamous cell carcinoma, basal cell carcinoma). The TIs were also divided into “solid” and “liquid” tumours.
2.4 Early Access Evaluation
The analysis of EA was conducted only for CDUs concerning “drug-TI” combinations with complete data and reimbursed by the SSN up to the cutoff date, according to resolutions of AIFA. The EA of a “drug-TI” combination was defined as the duration, measured in days, from the start of CDU to the initiation of the reimbursability status. The start date of the CDU was obtained by the common data model, whereas the start date of reimbursement was sourced from AIFA resolutions published in the Official Journal of the Italian Republic (Gazzetta Ufficiale) [15]. When the data collected from the centres were not sufficiently detailed to confidently match the indication approved for reimbursement with that used in CDUs, further clarification was requested from the UFA.
2.5 Potential Cost Savings Evaluation
The analysis of CS, in a similar way to the previous analysis for EA, was conducted only for the “drug-TI” combinations that, as of the cutoff date, had been reimbursed by the SSN. As the CDU can be activated on an individual basis when no valid therapeutic alternatives are available, evaluating the actual CS for a drug without a comparator is challenging. This evaluation would need to account for the social and hospital costs of the patient, which is beyond the scope of this work that was focussed on costs of therapies.
Thus, the CS was intended as avoided costs if the therapy employed in CDUs was charged on the SSN, and it was calculated for each approval as “mg/day*therapy length*price/mg”. The mg/day and therapy length (i.e. the days of therapy in CDU, calculated as the period between the start and end dates of therapy in CDU) were retrieved from the common data model, therefore only approvals with complete information were analysed for the CS. The price/mg was retrieved by the public price published in the AIFA resolutions for the reimbursement net of the compulsory discounts (5%+5%) for all non-innovative “drug-TI” combinations. In the case of drugs available in multiple packaging formats with different costs, we selected the ex-factory price of the package most closely matching the dosage described in the Summary of Product Characteristics [16].
2.6 Early Access and Potential Cost Savings According to Innovative Status
The EA and the CS were analysed according to the “innovative status” established by AIFA [17]. Therefore “drug-TI” combinations were classified as “innovative” when AIFA reported a “full” or “conditional” therapeutic innovation, otherwise as “non-innovative”. In the few cases where it was not possible to precisely associate the innovative status because of a lack of information on the specific indication, the “drug-TI” combination was considered “innovative”.
3 Results
3.1 EA and Potential CS for the SSN for Compassionate Use of Onco-Haematological Drugs
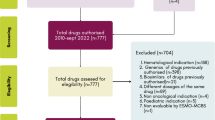
During the observational period, Compass-O collected 783 CDUs. The number of CDUs increased noticeably from 50 (6.4%) in 2017 to 229 (29.2%) in 2021 (Fig. 1). Out of the 176 CDUs in 2016, 128 (72.7%) were initiated by patients that year, while 48 (27.3%) began prior to 2016 (approved previously), and continued into the following years, falling within our study period.
Out of the 783 CDUs, 572 (73.1%) were deemed suitable for analyses in terms of EA and potential CS (Fig. 2). The remaining 26.9% were excluded from analysis because of the following reasons:
-
drug reimbursement not yet established by AIFA up to the study cutoff date (n = 87);
-
incomplete data (n = 51);
-
patient death without starting CDU, despite its approval (n = 37);
-
date of AIFA reimbursement prior to the end date of therapy in CDU (n = 36). This situation occurred because patients may continue to receive the treatment until the depletion of the reserve of the drug paid by the pharmaceutical company, or because of the length between the AIFA decision and the inclusion of the drug in the Regional Hospital Therapeutic Handbook.
Out of 572 CDU, 536 (93.7%) were referred to solid tumours and 36 (6.3%) to liquid tumours. Overall, the average EA for treatment amounted to 514 days, with an average potential CS for the SSN of €657.545 (Table 1).
Regarding the solid tumours, the combinations “drug-TI” most dispensed were: trastuzumab emtansine-breast cancer (73 CDUs; EA: 426 days; potential CS: €610,388), nivolumab-pulmonary carcinoma (63; 351 days; €624.049) and nivolumab-renal carcinoma (49; 560 days; €2.652.021) (Table 2).
Overall, nivolumab was the most used drug (146; 434 days; €1.299,749), and lung cancer was the therapeutic indication most frequently subject to CDU (186; 578 days; €426,598) [Table 3].
For liquid tumours, the most represented “drug-TI” combinations were (Table 2): blinatumomab-acute lymphoblastic leukaemia (nine CDUs), carfilzomib-multiple myeloma (six CDUs) and belantamab mafodotin-multiple myeloma (six CDUs). The analyses revealed an average EA to treatment of 1018, 181 and 376 days, respectively, and an average potential CS for the SSN of €21,162, €319,772 and €1,589,189. Blinatumomab was the most used drug (nine CDUs; EA: 1081 days; potential CS: €21,162), and multiple myeloma was the most frequent TI subject to CDU (13; 272 days; €902.829) (Table 3). Data of EA and CS for all the combinations, drugs and TI gathered through the study Compass-O are reported in Tables 1 and 3 of the ESM (solid tumours), and in Tables 2 and 4 (liquid tumours).
3.2 EA and Potential CS According to Innovative Status of Onco-Haematological Drugs
Based on the categorisation according to the innovative status, out of the 572 CDUs, 372 (65%) concerned non-innovative “drug-TI” combinations, and 200 (35%) involved innovative “drug-TI” combinations (Table 4). The latter allowed 598 days of EA and a total CS of €113,124,069.
The innovative “drug-TI” combination resulting in the most significant potential savings for the SSN is trastuzumab emtansine for breast cancer (Table 5). Throughout the study period, there were 73 CDUs, allowing for an EA of 426 days and yielding the greatest CS of €610,388 per treatment. Following this, the combinations trastuzumab deruxtecan for breast cancer (n = 33; EA = 772 days; potential CS: €1,031,858) and brigatinib for lung cancer (25; 1,101 days; €72,776) were observed.
4 Discussion
The Compass-O study provided a descriptive overview of the utilisation of CDU of onco-haematological drugs in seven Italian centres, from 2016 to 2021. Thanks to the collaboration with the UFA, data regarding 783 CDU approvals were collected: they concerned 69 different drugs, 35 different TIs and 93 drug-TI combinations, and mainly the treatment of solid tumours (90.5% vs 9.5% for liquid tumours).
The Compass-O study deepened two different aspects of the CDU: the EA and the potential CS for the SSN. Regarding the EA, we observed that, by means of the CDU, onco-haematological patients could access various drugs on average 514 days earlier than their authorisation date for reimbursement in Italy. This means creating significant therapeutic opportunities, especially in the case of life-saving and innovative medicines. According to the latest national report on medicine use in Italy (year 2022), the number of innovative medicines marketed from 2014 to 2022 increased from 9 (4% of new therapeutic entities marketed in that year) to 49 (17%) [18]. This trend could partly explain the noticeable increase in CDU approvals observed during our study period (2016–21).
The drug trastuzumab deruxtecan can be seen as a clear example of these benefits. In 2023, it was authorised in Italy for the treatment of both HER2-positive [19] and HER2-low breast cancer [20], following the encouraging results of DESTINY-Breast03 [21] and DESTINY-Breast04 [22] studies. For the period 2016–21, we collected 33 CDUs for such a combination, resulting in 772 days of EA prior to the AIFA resolutions [19, 20].
In contrast, in some cases, the CDU generated the EA to therapies subsequently showing an unfavourable risk-benefit profile. In December 2023, the combination belantamab mafodotin, for the treatment of multiple myeloma, did not obtain renewal for marketing authorisation from EMA because of a lack of efficacy, as shown in the DREAMM-3 study [15, 16]. Here, we observed this combination as the second most frequently dispensed among CDUs for liquid tumours, with the involvement of six patients and an EA of 376 days prior to the AIFA resolution [23]. The analysis based on the innovative status established by AIFA underlined that the EA for a drug considered as innovative was important (598 days), corroborating the social and health value of the CDU.
The Compass-O study analysed also the potential CS associated with the CDU, whose costs are covered by the pharmaceutical companies. For the analysable 572 CDUs, we estimated a total potential CS, for the SSN, of over €376 million. Economic analyses of CDU in Italy are also reported in a few other works, previously published by Jommi et al. [3] [13] and Pilunni et al. [11] [ however, a direct comparison with the present study is not feasible owing to methodological differences. In the first paper by Jommi et al., the authors assessed the avoided costs if patients were treated with the standard of care (when existing and reimbursed) as an alternative to CDU, resulting in a net economic benefit that ranged from €26.5 million to €50.6 million [3]. In the second work, the evidence on the economic impact of CDU was updated and integrated with a cost analysis from the perspective of the SSN, resulting in a major economic benefit of €47 million to €75 million [13]. The authors considered several other economic benefits, including the costs avoided by not using alternative treatments, the costs of side effects attributable to the drug used in CDU as well as those potentially avoided by not using alternative therapies, the cost of the drugs (and their side effects) administered in combination with those in the CDU and the expenses related to diagnostic services for determining treatment eligibility (both of which are not covered from industry). The analysis of Pilunni et al. [11] concerned CDU requests not all related to onco-haematological drugs.
4.1 Strength and Limitations
Despite the seven UFA involved in Compass-O varying in size and being evenly distributed throughout Italy, the results obtained through their data cannot be considered representative of the entire national population, and they are difficult to generalise to the national level. Furthermore, the localisation of these UFA within hospitals or facilities specialised in treating specific tumours (i.e. lung tumours) might have influenced the nature of the data collected (numerous compassionate use approvals for lung carcinomas). It is also noteworthy that some major Italian oncological centres, such as the National Cancer Institute (Istituto Nazionale dei Tumori), the European Institute of Oncology (Istituto Europeo di Oncologia) and the Veneto Institute of Oncology (Istituto Oncologico Veneto), were not included in Compass-O. This might have led to an underestimation of the CDU during the study period in Italy. Instead, the potential savings for the SSN were likely overestimated because our methodological approach is based on ex-factory prices, and does not take in account the confidential discounts applied in tendering procedures or re-negotiation for incoming indications. Additionally, our analysis did not account for the extra costs to the SSN arising from diagnostic procedures and serious adverse events associated with drugs used in CDUs, and for the long-term cost impacts, as reported in other studies [3, 13]. Consequently, our analysis, which considers the avoided costs of drugs, offers an alternative perspective for evaluating the economic impact of CDU.
It is important to point out that the present study focused exclusively on onco-haematological drugs. While these drugs constitute the majority of those involved in compassionate use programmes, they are not the only drugs. According to the latest updated list from AIFA (7 June, 2024), there are currently CDUs in Italy for the treatment of other conditions, such as HIV, Dravet syndrome, Lennox–Gastaut syndrome, sickle cell disease and Waldenstrom macroglobulinemia [24].
We recognise that it would be more accurate to separate drugs not yet authorised by the EMA from those approved but still under price negotiation with AIFA, when the negotiation provides a certain number of free therapeutic cycles as a form of discount. However, this distinction should take into account that a drug might not be authorised by the EMA at the beginning of a patient’s CDU but might undergo authorisation and negotiation during the treatment period. Therefore, maintaining this distinction for each patient and each treatment time could be challenging and could generate a misinterpretation of results.
In addition to shedding light on the CDU in Italy, the Compass-O study demonstrated the strategic role of the UFA for data collection and drug research. They could play a pivotal role within the regional oncological networks, not only to prepare oncologic drugs but also to generate evidence and research on these drugs.
5 Conclusions
The Italian and multicentric study Compass-O revealed that CDU of onco-haematological drugs is a large-scale phenomenon, and in the last few years, it benefited many patients with limited therapeutic options. In recent years, the literature reporting results of Italian CDU has notably increased, suggesting a greater diffusion and interest in this practice. Nevertheless, comprehensive data for Italy are currently unavailable because centralised monitoring of ongoing CDU in our country by health institutions is lacking. The establishment of such a national observatory, at first for onco-haematological drugs, would be facilitated by an informatics platform for data entry, to be created starting from the common data model used in the present study.
It is worth underlying that the Decree of 7 September, 2017, in Article 4 (paragraphs 3, 4 and 5), establishes that all requests for CDU evaluated by the ethics committees must be sent to AIFA for further evaluation. A practice example of this situation can be found in the article by Montanaro et al., which reports on the activity of the Ethics Committee of the University Hospital of Bologna from 2010 to 2016 [25]. Hence, because an interaction between these two authorities is already foreseen, it could be easy to move further toward the establishment of this observatory. To this end, we believe that the National Report OsMed, which annually provides an analytical description of medicine use in both national and regional contexts, could be a valuable starting point to establish this observatory [18]. In the latest report, a list of compassionate use programmes for rare diseases activated in 2022 can be found; however, a dedicated and extensive section on this topic could be added, taking advantage of this initiative, promoted by AIFA and active since 2001. Such an observatory could also monitor other EA programmes active in Italy, such as the 648 List [26] and the 5% Fund [27], being relevant to the SSN reform proposal aimed at unifying these programmes into a single programme [28]. In conclusion, Compass-O can be considered a significant pilot project, and the inclusion of more Italian UFA is strongly encouraged to provide a better understanding and description of the phenomenon of CDU.
References
European Medicines Agency. Compassionate use. Available from: https://ema.europa.eu/en/human-regulatory-overview/research-development/compassionate-use. Accessed 9 Apr 2024.
Watson T. A global perspective on compassionate use and expanded access. Ther Innov Regul Sci. 2017;51(2):143–5.
Jommi C, Pantellini F, Stagi L, Verykiou M, Cavazza M. The economic impact of compassionate use of medicines. BMC Health Serv Res. 2021;21(1):1303.
Borysowski J, Ehni HJ, Górski A. Ethics review in compassionate use. BMC Med. 2017;15(1):136.
De Panfilis L, Satolli R, Costantini M. Compassionate use programs in Italy: ethical guidelines. BMC Med Ethics. 2018;19(1):22.
Balasubramanian G, Morampudi S, Chhabra P, Gowda A, Zomorodi B. An overview of compassionate use programs in the European Union member states. Intractable Rare Dis Res. 2016;5(4):244–54.
Ministero della Salute. Decreto Ministeriale del 7 settembre 2017. Disciplina dell’uso terapeutico di medicinale sottoposto a sperimentazione clinica. GU Serie Generale n.256 del 02-11-20172017.
Pilunni D, Daloisio V, Campopiano R, Pani M, Navarra P. Compassionate drug uses and saving for the national health system: the case study of Fondazione Policlinico Gemelli. Eur Rev Med Pharmacol Sci. 2021;25(20):6365–77.
Raimondo P, Casilli G, Isernia M, Lidonnici D, Ravasio R, Ronco V, et al. Le tempistiche autorizzative di AIFA: un confronto tra le due commissioni consultive e tecnico-scientifiche succedutesi nel periodo 2015–2020. Glob Reg Health Technol Assess. 2020;7:109–14.
Agenzia Italiana del Farmaco (AIFA). Rapporto sulle tempistiche di autorizzazione delle procedure di prezzi e rimborso dei farmaci nel triennio 2018-2020: risultati preliminari area strategia ed economia del farmaco settore HTA ed economia del farmaco: Settembre 2021. 2021. Available from: https://www.aifa.gov.it/documents/20142/1307543/2021.09.06_Rapporto_procedure_prezzi_rimborso_farmaci_triennio_2018-2020.PDF. Accessed 9 Apr 2024.
Pilunni D, Navarra P. Compassionate drug uses in Italy: analysis of regional and local diffusion. Ann Ist Super Sanita. 2023;59(1):43–50.
Wu J, Yang Y, Yu J, Qiao L, Zuo WA-O, Zhang B. Efficacy and safety of compassionate use for rare diseases: a scoping review from 1991 to 2022. Orphanet J Rare Dis. 2023;18(1):368. https://doi.org/10.1186/s13023-023-02978-x.
Jommi CA-O, Pantellini F, Giuliani GA-OX, Cavazza MA-O. Impatto economico di 14 programmi di uso compassionevole dei farmaci in Italia, nella prospettiva del Servizio Sanitario Nazionale. Glob Reg Health Technol Assess. 2024;11:115–23. Italian. https://doi.org/10.33393/grhta.2024.2735.
Gatti L. L’Italia prova a inserire i Pdta digitali per governare l’innovazione. 2024. Available from: https://www.aboutpharma.com/digital-health/litalia-prova-a-inserire-i-pdta-digitali-per-governare-linnovazione/. Accessed 10 Apr 2024.
Dimopoulos MA, Hungria VTM, Radinoff A, Delimpasi S, Mikala G, Masszi T, et al. Efficacy and safety of single-agent belantamab mafodotin versus pomalidomide plus low-dose dexamethasone in patients with relapsed or refractory multiple myeloma (DREAMM-3): a phase 3, open-label, randomised study. Lancet Haematol. 2023;10(10):e801–12.
European Medicines Agency. EMA confirms recommendation for non-renewal of authorisation of multiple myeloma medicine Blenrep. 15 December 2023. Available from: https://www.ema.europa.eu/en/news/ema-confirms-recommendation-non-renewal-authorisation-multiple-myeloma-medicine-blenrep. Accessed 9 Apr 2024.
Agenzia Italiana del Farmaco (AIFA). Innovative medicinal products. Available from: https://www.aifa.gov.it/en/farmaci-innovativi. Accessed 9 Apr 2024.
The Medicines Utilisation Monitoring Centre. National report on medicines use in Italy. 2022. Rome: Italian Medicines Agency; 2023.
Agenzia Italiana del Farmaco (AIFA). Determina 26 giugno 2023. Riclassificazione del medicinale per uso umano «Enhertu», ai sensi dell’articolo 8, comma 10, della legge 24 dicembre 1993, n. 537. (Determina n. 452/2023). GU Serie Generale n.153 del 03-07-2023; 2023.
Agenzia Italiana del Farmaco (AIFA). Determina 15 dicembre 2023 Regime di rimborsabilita' e prezzo a seguito di nuove indicazioni terapeutiche del medicinale per uso umano «Enhertu». (Determina n. 760/2023). GU Serie Generale n.296 del 20-12-2023; 2023.
Cortés J, Kim SB, Chung WP, Im SA, Park YH, Hegg R, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386(12):1143–54.
Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9–20.
Agenzia Italiana del Farmaco (AIFA). Determina 24 novembre 2021 Riclassificazione del medicinale per uso umano «Blenrep», ai sensi dell'articolo 8, comma 10, della legge 24 dicembre 1993, n. 537. (Determina n. DG/1388/2021). GU Serie Generale n.291 del 07-12-20212021.
Agenzia Italiana del Farmaco (AIFA). Farmaci a uso compassionevole (compassione use programs). 7 June 2024. Available from: https://www.aifa.gov.it/en/farmaci-a-uso-compassionevole. Accessed 26 Jun 2024.
Montanaro NA-O, Melis M, Proni S, Chiabrando G, Motola D. Six-year activity on approval of compassionate use of medicines by the Ethics Committee of the University Hospital of Bologna (Italy): time to update rules and recommendations. Eur J Clin Pharmacol. 2017;73(4):479–85. https://doi.org/10.1007/s00228-016-2186-y. Epub 2016 Dec 29.
Agenzia Italiana del Farmaco (AIFA). Legge 23 dicembre 1996, n. 648 Conversione in legge del decreto-legge 21 ottobre 1996, n. 536, recante misure per il contenimento della spesa farmaceutica e la rideterminazione del tetto di spesa per l’anno 1996. GU Serie Generale n.300 del 23-12-1996; 1996.
Agenzia Italiana del Farmaco (AIFA). LEGGE 24 novembre 2003, n. 326. Conversione in legge, con modificazioni, del decreto-legge 30 settembre 2003, n. 269, recante disposizioni urgenti per favorire lo sviluppo e per la correzione dell'andamento dei conti pubblici. 2003. https://www.normattiva.it/uri-res/N2Ls?urn:nir:stato:legge:2003-11-24;326!vig=. Accessed 12 Jul 2024.
Popoli PA-O, Giuliani GA-OX, Cavaliere AA-OX, Jommi CA-O. Accesso precoce ai farmaci: una proposta di riforma per il Servizio Sanitario Nazionale. Glob Reg Health Technol Assess. 2024;11:148–53. Italian. https://doi.org/10.33393/grhta.2024.3098.
Acknowledgements
The authors thank all other members of the Compass-O Study group: Dondi L, Calabria S, Ronconi G, Addesi A, Maggioni AP, (Fondazione ReS); Scavone C (Dipartimento di Medicina Sperimentale - Università Degli Studi della Campania “Luigi Vanvitelli” and Centro di Farmacovigilanza e Farmacoepidemiologia della Regione Campania, Naples, Italy); Giordano M, (P.O. Monaldi, A.O. Ospedali Dei Colli, Naples, Italy); Lagana’ G, Ballerio A, (ASST Valle Olona, Busto Arsizio, Italy); Celenza R (A.O. di Perugia, Ospedale Santa Maria della Misericordia, Perugia, Italy); Vergati A (P.O. Belcolle, ASL Viterbo, Viterbo, Italy).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Italian Society of Pharmacology (Società Italiana Farmacologia).
Conflicts of Interest
The authors have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
The study was approved by the Ethics Committee of the coordinating centre L. Vanvitelli University of Campania, Naples, Italy (Protocol No. 0037627 of 14 December, 2022).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
The data that support the findings of this study are not openly available to maintain the privacy of study participants. For more information, please contact the corresponding author. All other data relevant to the study were obtained from the published literature or other publicly available sources and have been presented in the article and the supplementaryiInformation.
Code Availability
Not applicable.
Authors’ Contributions
ID, CP: conceptualisation, methodology, validation, formal analysis, investigation, resources, data curation, writing - original draft, writing - review and editing, visualisation; LD: methodology, software, validation, formal analysis; IE, NM, AC, MP, AC, AD: conceptualisation, supervision, project administration, funding acquisition; AM: writing - original draft, writing - review and editing; AC: conceptualisation, supervision, funding acquisition; GdM, AC, DT, CM, CD, ER, AS, GB, GB, TG, RM: resources, writing - review and editing. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dell’Anno, I., Dondi, L., Esposito, I. et al. The Early Access and the Potential Cost Savings by the Compassionate Use of Onco-haematological Drugs: Results from the Italian Study Compass-O. Clin Drug Investig 44, 577–586 (2024). https://doi.org/10.1007/s40261-024-01381-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-024-01381-z