Abstract
Background and Objectives
Globalagliatin, a glucokinase activator, plays a vital role in glucose homeostasis. The aim of this study was to assess the safety, pharmacokinetics, and pharmacodynamics of globalagliatin in Chinese patients with type 2 diabetes.
Methods
In this dose-titration study, 24 patients were randomized (3:1 ratio) to receive globalagliatin or placebo. The 28-day titration was divided into two stages, each comprising 12 subjects. In stage I (low-dose), globalagliatin or placebo was administered at ascending doses of 20, 40, 80, and 120 mg once daily, increased at weekly intervals. As the treatment was well tolerated, stage II (high-dose) was initiated, with ascending doses of 80, 160, 240, and 320 mg. Safety, pharmacokinetic and pharmacodynamic analysis were conducted.
Results
Following once-daily titration with ascending doses of globalagliatin of 20–120 mg (stage I) and 80–320 mg (stage II) for 7 days, globalagliatin caused mildly high incidences of hypoglycemia and hypertriglyceridemia. The mean maximum plasma concentration (Cmax) of globalagliatin increased from 7.76 to 138.13 ng/mL (stage I), and 29.36 to 471.50 ng/mL (stage II), which occurred at 3–5 h post-dose. A steady state was achieved after 7 days of once-daily dosing in stage I and stage II, respectively. Mean area under the plasma-concentration curve for steady-state 24-h interval (AUC0-24) increased from 106.13 to 2461.95 ng·h/mL (stage I) and 369.71 to 9218.38 ng·h/mL (stage II). Fasting plasma glucose (FPG) decreased continuously during the titration period. Compared with the placebo, high-dose globalagliatin significantly increased the reductions in FPG, the area under the curve of 24-h glucose levels, and glycated albumin, with least-squares mean changes (relative to baseline) of − 4.08 mmol/L (95% CI − 5.05 to − 3.12) (P < 0.01), − 103.93 mmol/L (95% CI − 135.80 to − 72.06) (P < 0.01), and − 4.71% (95% CI − 6.91 to − 2.51) (P < 0.01)), respectively. High-dose globalagliatin significantly increased the Matsuda index, indicating improved insulin resistance.
Conclusions
Globalagliatin was well tolerated and showed favorable pharmacokinetic profiles in Chinese patients with type 2 diabetes. High-dose globalagliatin reduced plasma glucose, and improved insulin resistance.
Trial Registration
Clinicaltrials.gov indentifier, NCT03414892.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Globalagliatin was well tolerated and showed favorable pharmacokinetic profiles in Chinese patients with type 2 diabetes. |
High-dose globalagliatin reduced plasma glucose, and improved insulin resistance. |
1 Introduction
The number of people with type 2 diabetes mellitus worldwide has quadrupled in the past 30 years, and is predicted to reach 642 million by 2040, with particularly rapid increases in Asia, including China [1]. Besides dietary and lifestyle changes, medications are often required to control blood glucose. However, current treatments are insufficient to meet demand, and new therapeutic approaches, including identifying drugs with novel mechanisms of action, are urgently needed [2].
Glucokinase (GK) is a unique hexokinase expressed mainly in pancreatic β-cells and hepatocytes [3] that catalyzes the first step of glucose metabolism. It acts as a “glucose sensor” in pancreatic β-cells to control glucose-stimulated insulin secretion and plays a vital role in increasing hepatic glucose uptake and glycogen synthesis [4, 5]. Activation of GK improves insulin secretion and decreases hepatic glucose output, targeting two major defects in type 2 diabetes [5].
The GK activators (GKAs) are currently a major focus of research to identify new antidiabetic agents. Although several GKAs were discontinued after early-stage clinical trials because of adverse effects or limited efficacy [6,7,8], at least six are currently under investigation and have improved glycemic control or promoted relative improvements in insulin secretion [9, 10]. One such activator (dorzagliatin) is currently being evaluated in a phase III clinical trial.
Globalagliatin is an oral, selective GKA that targets both pancreatic β-cells and hepatocytes [the chemical structure and physical characteristics of globalagliatin are provided in the Online Supplementary Material (OSM), Fig. 1]. In animal experiments, globalagliatin improved glycemic control, insulin resistance (IR), and islet function (OSM, Fig. 2 and OSM, Table 1). In five completed phase I studies, including one in healthy Chinese participants, 2–120 mg and 20–400 mg globalagliatin presented favorable tolerability and safety for healthy subjects and type 2 diabetes patients, respectively, with an obvious hypoglycemic effect. The pharmacokinetic data from these trials support once-daily drug administration (OSM, Table 2).
The safety and efficacy of globalagliatin are not well explored in patients with type 2 diabetes. Here, we report the safety, pharmacokinetics, and pharmacodynamics of once-daily globalagliatin administration to Chinese patients with type 2 diabetes in a 28-day dose-ascending study.
2 Methods
This was a phase Ib, single-blind, fixed-sequence, dose-titration study with multiple oral doses of globalagliatin or placebo administered to patients with type 2 diabetes. All subjects enrolled in the study were recruited from the First Affiliated Hospital with Nanjing Medical University, Nanjing, China (NCT03414892, ClinicalTrials.gov). The study was conducted in accordance with the principles of good clinical practice and the Declaration of Helsinki, and was approved by the ethics committee of the hospital. All participants provided written, informed consent before the start of the study.
2.1 Study Patients
Patients with type 2 diabetes (aged 18–70 years with disease duration > 3 months) were eligible to participate if they were either drug naïve (defined as not having received any antidiabetic drug in the 3 months before enrolment), or used only metformin in the previous 3 months. The stable dose for metformin was ≥ 1500 mg/day in the previous 2 months (if the patient could not tolerate 1500 mg/day, the stable dose could have been the maximum tolerated dose, but needed to be at least ≥ 1000 mg/day). The patients had a body mass index (BMI) of 18–35 kg/m2, glycated hemoglobin (HbA1c) of 7–11% (53–97 mmol/mol), and fasting plasma glucose (FPG) values of 126–240 mg/dL (7–13.3 mmol/L). The key exclusion criteria were triglyceride (TG) levels > 500 mg/dL (5.7 mmol/L) and fasting plasma C-peptide levels < 1 ng/mL (333 pmol/L).
2.2 Study Design
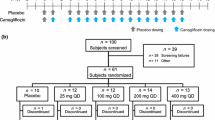
The trial involved a screening period (from days − 14 to − 5), a baseline period (from days − 4 to − 1), a titration period (from days 1 to 28), and a follow-up point (day 42). A total of 24 patients with type 2 diabetes were randomized (in a 3:1 ratio) to receive globalagliatin (n = 18) or placebo (n = 6). In both groups, a stable dose of metformin was maintained for the patients already taking metformin. Patients were admitted to the phase I clinical trial ward during the baseline period. All subjects remained as inpatients of the ward for baseline and titration period, and were served standardized meals. On day 42, the patients underwent follow-up data collection either face-to-face or via telephone.
The 28-day titration period was divided into two stages. In stage I, 12 subjects received globalagliatin (n = 9) or placebo (n = 3) at a starting dose of 20 mg once daily; if well tolerated [e.g., patients presented no hypoglycemia (hypoglycemia was defined as blood glucose ≤ 3.9 mmol/L) and FPG > 6.1 mmol/L], the dose was titrated up to 40, 80, and 120 mg once daily, increased at weekly intervals. Subjects in the placebo group underwent a simulated titration to maintain blinding. If the subjects showed favorable tolerance to a dose of 120 mg, stage II was initiated. This titration stage involved the 12 remaining subjects (nine in the globalagliatin group and three in the placebo group) and doses of 80, 160, 240, and 320 mg during weeks 1, 2, 3, and 4, respectively, using a similar approach. Patients with FPG ≥ 15 mmol/L for two consecutive days were withdrawn from the study.
During both stages, the decision of whether to increase the dose was determined based on the patient’s blood glucose level. In the event of clinically significant hypoglycemia, the dose was discontinued (when using the minimum dose) or reduced to a well-tolerated level (half the sum of the last dose and the current dose). If the dose escalation was stopped (e.g., if increasing the dose would have increased the risk of hypoglycemia, and the dose could have been maintained if the patient’s glucose was well controlled) before the maximum dose was reached, no further dose increases were permitted. The dose could have been lowered only once for each subject, and subjects were withdrawn from the study if they still could not have tolerated the lower dose. After reaching the maximum or maximum tolerated dose, patients continued to take this stable dose until day 28. We defined the globalagliatin-treated subjects in stages I and II as low-dose and high-dose groups, respectively (Fig. 1).
2.3 Safety Assessments
The primary endpoint of this study was safety. The secondary research purpose was to evaluate the pharmacokinetic and pharmacodynamic characteristics of globalagliatin, including glucose-lowering efficacy and effects on islet function and insulin sensitivity.
Safety assessments included physical examinations, vital signs, 12-lead electrocardiograms, and clinical laboratory tests, including hematology, serum biochemistry, and urinalysis tests at weekly intervals and on day 42. If the follow-up was conducted by telephone, patients were asked about the outcomes of their adverse events (AEs) and locally conducted test results. AEs were monitored throughout the study. A continuous glucose-monitoring device (FreeStyle Libre H, Abbott, Alameda, CA, USA) was employed for safety monitoring of interstitial blood glucose concentrations.
2.4 Pharmacokinetic Assessments
Pharmacokinetic samples were collected weekly on days 1, 7, 14, 21, and 28. Blood samples of approximately 2 mL were collected according to the scheduled time points of pre-dose, 1, 2, 3, 4, 5, 6, 8, 12, and 24 h post-dose (the 24-h samples were to be taken pre-dose on days 2, 8, 15, 22, and 29). The sampling tubes were slowly inverted four to five times immediately after blood collection to mix with anticoagulant, then centrifuged at 4 °C and 1700 g for 10 min. The supernatant plasma samples were transferred and divided into two storage tubes for detection and backup, respectively, then stored at or below − 60 °C until analysis.
2.4.1 Determination of Globalagliatin
Plasma globalagliatin concentrations were measured using a validated liquid chromatography–tandem mass spectrometry (LC–MS/MS) method.
The chromatographic separation was performed on a Shimadzu (LC-20AD) HPLC system using an Agela Venusil XBP C18 (3 µm, 100 Å, 2.1 × 50 mm) column. The column temperature was maintained at 40 °C and chromatographic separation was achieved with mobile phase of water containing 5 mM ammonium acetate and 1.0% formic acid, and acetonitrile in a designated gradient (0.50 min, B = 40%; 2.00 min, B = 50%; 2.10 min, B = 90%; 3.10 min, B = 90%; 3.20 min, B = 40%; 4.20 min, stop) at a flow rate of 0.4 mL/min. Quantitation was achieved by MS/MS detection in positive ion mode, using an Applied Biosystems (AB) Sciex TRIPLE QUAD 6500 mass spectrometer equipped via an electrospray ionization (ESI) source by multiple-reaction monitoring (MRM) scanning.
Plasma samples were processed by protein precipitation with acetonitrile. The lower limit of quantification (LLOQ) was 0.20 ng/mL, and the upper limit of quantification (ULOQ) was 200 ng/mL. Samples above the ULOQ were diluted and reanalyzed to yield results within the calibrated range. Calibration curves and quality control samples (QCs) were included in all analytical runs. Total QCs should number at least 5% of the total samples analyzed, or be at least six in number (low-, mid-, and high-QCs, in duplicate).
2.4.2 Pharmacokinetic Analysis
Pharmacokinetic parameters were assessed with a noncompartmental model using the WinNonlin software (version 7.0, Pharsight Corporation, Mountain View, CA, USA). The following key pharmacokinetic variables were calculated for each patient at each dose using the actual sampling time relative to medication administration on day 1 (first dose) and on days 7, 14, 21, and 28 (multiple doses):
Day 1 (first dose): Tmax (time to maximum plasma concentration), Cmax (maximum plasma concentration), AUC0-24 (area under the plasma concentration–time curve from time 0 to 24 h);
Days 7, 14, 21, and 28 (multiple doses): Tmax, Cmax, AUC0–tau (area under the concentration versus time curve during one dosage interval (24 h) at steady-state), accumulation ratio Rac,Cmax [calculated as Cmax (only day 7)/Cmax (day 1)] and Rac,AUC [AUC0-tau (only day 7)/AUC0-24(day 1)], etc.
In this study, terminal half-life could not be calculated due to limited sampling time points at terminal elimination (only to 24 h post-dose).
2.5 Pharmacodynamic Assessments
Hemoglobin (HbA1c) was assessed during the screening period and glycated albumin (GA) was assessed on day − 1; both were again assessed on day 28 after the treatment was completed. Blood samples were taken on days − 1, 7, 14, 21, and 28 to measure the area under the curve of 24-h glucose levels (AUC0–24,glucose) at the following time points: 0, 0.5, 1, 2, 3, 4, 5, 6, 8, 10, 11, 12, 14, 18, and 24 h. Only FPG was measured throughout the entire titration treatment period, at about 8:00 am before breakfast and taking medicine.
Participants underwent a mixed meal tolerance test (MMTT) on days − 1 and 28. Blood samples were taken at 0, 0.5, 1, 1.5, 2, and 3 h, with consumption of a standard meal at 0 h. Two-hour glucose level during MMTT was called 2-h postprandial glucose (2hPG). These samples facilitated the analysis of the following indicators: homeostasis model assessment-IR (HOMA-IR) = (I0 × G0)/22.5; HOMA-β = (20 × I0)/(G0 − 3.5); Matsuda’s insulin sensitivity index (Matsuda index) = 1000/((I0[µIU/ml] × G0[mg/dl])^(1/2) × (mean MMTT G[mg/dl] × mean MMTT I[µIU/ml])); ΔI30/ΔG30 = (I30 − I0)/(G30 − G0); ΔC30/ΔG30 = (C30 − C0)/(G30 − G0); AUC0–180,insulin/AUC0–180,glucose = (0.25 × I0 + 0.5 × I30 + 0.5 × I60 + 0.5 × I90 + 0.75 × I120 + 0.5 × I180)/(0.25 × G0 + 0.5 × G30 + 0.5 × G60 + 0.5 × G90 + 0.75 × G120 + 0.5 × G180), and AUC0–180,C-peptide/AUC0–180,glucose = (0.25 × C0 + 0.5 × C30 + 0.5 × C60 + 0.5 × C90 + 0.75 × C120 + 0.5 × C180)/(0.25 × G0 + 0.5 × G30 + 0.5 × G60 + 0.5 × G90 + 0.75 × G120 + 0.5 × G180) (the subscript numbers indicate the time in minutes, I represents insulin (µIU/mL), G represents glucose (mmol/L), C represents C-peptide (ng/mL), and AUC represents the area under the curve). Insulin and C-peptide levels were analyzed using electrochemiluminescence (Roche Diagnostics GmbH, Mannheim, Germany).
2.6 Statistics
All statistical analyses were performed using the SAS software (version 9.4, SAS Institute, Cary, NC, USA). The Kruskal–Wallis H test was performed to compare the baseline data. FPG, AUC0–24,glucose, GA, HbA1c, Matsuda index, and AUC0–180,C-peptide/AUC0–180,glucose, which were suitable to use the analysis of covariance (ANOVA), were adjusted for corresponding baseline values and whether to use metformin per group. Least-squares (LS) means and 95% confidence intervals (CIs) were calculated for each group. A two-sided significance level of 0.05 was used. HOMA-IR, HOMA-β, ΔI30/ΔG30, ΔC30/ΔG30, and AUC0–180,insulin/AUC0–180,glucose were expressed as median (interquartile range).
3 Results
3.1 Study Subjects
Between January and November 2018, 63 individuals were assessed for eligibility. Finally, 24 participants were randomized to receive globalagliatin or placebo at a 3:1 ratio. In stage I (20, 40, 80, and 120 mg), 12 subjects were enrolled (n = 9 in the globalagliatin group; n = 3 in the placebo group). Two participants in the globalagliatin group were withdrawn from the study on days 4 and 7 owing to high FPG levels (OSM, Fig. 3). Among the seven remaining participants in the globalagliatin group, six reached the 120 mg dose, while one remained at 20 mg. Stage II (doses of 80, 160, 240, and 320 mg) commenced if no hypoglycemic episodes occurred. Six subjects tolerated the highest dose of 320 mg, while the three remaining individuals in the globalagliatin group tolerated maximum doses of 120, 160, and 200 mg, respectively. The patients’ baseline characteristics are presented in Table 1. No significant differences were recorded in baseline indicators among the groups.
3.2 Safety Results
Treatment-emergent adverse events (TEAEs) were reported in 7/9 (77.8%) and 9/9 (100%) patients in the low-dose and high-dose globalagliatin groups, respectively. The occurrence of TEAEs was 100% (6/6) in the placebo group. No serious adverse effects (SAEs), severe hypoglycemia, or nocturnal hypoglycemia were observed. The incidence of AEs overall and TEAEs was moderately higher in the high-dose group relative to the placebo group, owing to the increased incidence of hypertriglyceridemia and hypoglycemia.
The rates of hypertriglyceridemia were 22.2% (2/9), 33.3% (3/9), and 0% (0/6) in the low-dose, high-dose, and placebo groups, respectively, with mean (SD) changes of TG on day 28 (relative to baseline) of − 0.07 (0.23) mmol/L, 0.24 (0.65)mmol/L, and 1.27 (1.52) mmol/L. Hypertriglyceridemia in all five patients returned to normal during the titration period or at follow-up, and the highest level of TG was 4.72 mmol/L (normal reference ranges 0–2.25 mmol/L). Two patients already had hypertriglyceridemia at baseline and required lipid-lowering agents after titration because of the continued increase in TG levels. Hypoglycemia was not observed in the placebo or low-dose groups. However, 3/9 (33.3%) subjects in the high-dose group had a total of 18 episodes of hypoglycemia at 120–320 mg, all before daytime meals, and 3/18 (16.7%) showed hypoglycemia with the administration of glucose < 3.0 mmol/L. These cases of hypoglycemia were all asymptomatic, and glucose levels returned to normal either without intervention or by eating food such as candy. Two participants in the high-dose group had their globalagliatin dose reduced (from 160 to 120 mg, and 240 to 200 mg) because of hypoglycemia.
A female patient in the low-dose group had an extended QT interval corrected by Bazett’s formula (QTcB; mean value of three measurements), which occurred four times (within 1 h before drug administration in the morning at doses of 40, 80, 80, and 120 mg). All the QTcB values were < 470 ms and returned to normal within 3 h after the administration of globalagliatin without any further intervention. The QTcB value remained at normal levels on day 42.
Myasthenia was observed in a male participant in the low-dose group on day 24. The patient felt a little weak in the upper limbs, and head magnetic resonance imaging indicated sinusitis, which resolved without intervention. The myasthenia showed improvement 1 month after globalagliatin discontinuation. Other AEs were transient, including sinus bradycardia, sinus arrhythmia, prolonged PR interval, hypochloremia, hyponatremia, increased blood urea, and gastrointestinal infection (Table 2).
3.3 Pharmacokinetic Analysis
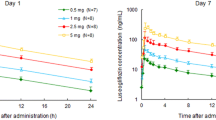
Following once-daily titration with ascending doses of globalagliatin of 20–120 mg (stage I) and 80–320 mg (stage II) for 7 days, the mean Cmax of globalagliatin increased from 7.76 to 138.13 ng/mL (stage I), and 29.36 to 471.50 ng/mL (stage II); AUC0-24 increased from 106.13 to 2461.95 ng·h/mL (stage I) and 369.71 to 9218.38 ng·h/mL (stage II). The mean plasma concentration–time profiles of gobalagliatin were depicted in Fig. 2. The description of pharmacokinetic parameters was summarized in Table 3.
Mean plasma concentration of globalagliatin versus nominal time profiles by day. Plasma concentration (mean ± SD) of globalagliatin: a (linear scale, stage I), b (semi-logarithmic scale, stage I), c (linear scale, stage II), and d (semi-logarithmic scale, stage II). The scheduled time points were up to 24 h post-dose on days 1 and 7 (20 mg in stage I and 80 mg in stage II), 14 (40 mg in stage I and 160 mg in stage II), 21 (80 mg in stage I and 240 mg in stage II), and 28 (120 mg in stage I and 320 mg in stage II)
After oral administration, globalagliatin was gradually absorbed, reaching a mean Cmax at a median time of approximately 3–5 h, and was then slowly eliminated.
There was no significant difference in trough plasma concentration between days 6 and 7 (Fig. 3), indicating that steady-state conditions were achieved after 7 days of daily dosing with 20, 40, 80, and 120 mg (stage I) and 80, 160, 240, and 320 mg (stage II). Exposure, based on Cmax and AUC0–tau during a dosing interval at steady state, increased in a more than proportionate manner with increasing doses over the dose ranges of 20–120, 80–320, and 20–320 mg.
Mean pre-dose plasma concentration of globalagliatin versus day plots by up-titration of the dose every 7 days and 28 days of once-daily dosing. Pre-dose plasma concentration (mean ± SD) of globalagliatin: a (linear scale, stage I), b (semi-logarithmic scale, stage I), c (linear scale, stage II), and d (semi-logarithmic scale, stage II). The scheduled time points were pre-dose on days 1, 7, 14, 21, 28, and 24 h post-dose (the 24-h samples were to be taken pre-dose on days 2, 8, 15, 22, and 29). Stage I: 20 mg (n = 9), 40 mg (n = 6), 80 mg (n = 6) and 120 mg (n = 6); stage II: 80 mg (n = 9), 160 mg (n = 8), 240 mg (n = 6) and 320 mg (n = 6)
The mean Rac,Cmax and Rac,AUC were 2.02 and 2.43, respectively, after 7 days of once-daily dosing with 20 mg during stage I, and 2.39 and 2.67, respectively, after 7 days of once-daily dosing with 80 mg during stage II (Table 3). Based on these accumulation ratio values > 2, moderate drug accumulation was evident following once-daily dosing for 7 days.
3.4 Pharmacodynamics
The FPG showed a continuous decline during the titration period, and its reduction from baseline gradually became greater with increasing drug administration time and dose, and the same trend was shown in AUC0–24,glucose, but with less monitoring points (Fig. 4a, b).
Compared with the placebo, low-dose globalagliatin nonsignificantly increased the reductions in FPG, AUC0–24,glucose, and GA on day 28 (relative to baseline); high-dose globalagliatin significantly increased the reductions in FPG, AUC0–24,glucose and GA, with LS mean changes (relative to baseline) of − 4.08 mmol/L (95% CI − 5.05 to − 3.12) (P < 0.01), − 103.93 mmol/L (95% CI − 135.80 to − 72.06) (P < 0.01), and − 4.71% (− 6.91 to − 2.51) (P < 0.01), respectively. No significant differences were observed in HbA1c among any of the groups (Table 4).
Both IR and β-cell function were assessed by the MMTT at baseline and on day 28. Compared with the placebo and low-dose group, high-dose globalagliatin significantly increased the Matsuda index (P < 0.01), indicating improved insulin sensitivity. AUC0–180,C-peptide/AUC0–180,glucose increased after the administration of globalagliatin, but was nonsignificant (Table 4). Decreased HOMA-IR and 2hPG, and improved several β-cell function indicators, such as AUC0-180,insulin/AUC0–180,glucose, ΔI30/ΔG30 and ΔC30/ΔG30, were calculated after the administration of high-dose globalagliatin, but were not suitable for using ANOVA (OSM, Table 3).
4 Discussion
The study design, which involved stepwise dose increases until glycemic control was achieved, was in accordance with the expected future use of globalagliatin. All subjects remained hospitalized and received standardized meals for the duration of the 28-day study treatment. This facilitated the observation of drug effects and reduced the effects of several other issues, such as differences in diet and lifestyle. We recruited participants with fasting plasma C-peptide levels ≥ 1 ng/mL to exclude patients who might have required basal insulin therapy.
Hypoglycemic episodes occurred only in the high-dose group, without severe, nocturnal, or symptomatic hypoglycemia. An increase in TG levels associated with the administration of globalagliatin was identified, which has also been observed with other GKAs [6,7,8]. These findings suggest that GKAs increase hepatic GK activity. The high incidence of hypertriglyceridemia in the high-dose group requires further investigation. One patient in the low-dose group had QT prolongation with all the QTcB values < 470 ms, and returned to normal within 3 h after the administration of globalagliatin without intervention. The effect of GKAs on the QTc interval was also shown with piragliatin treatment in a dose-dependent manner [11]. One subject in the low-dose group had mild myasthenia, which has not been reported for other GKAs, and seems to have no relationship to the mechanism of action for globalagliatin. The case of myasthenia improved 1 month after globalagliatin discontinuation. These adverse effects need to be monitored in the phase II study. Another common adverse effect of GKAs, elevated liver enzymes, did not occur.
Following once-daily titration with ascending doses of globalagliatin of 20–120 mg (stage I) and 80–320 mg (stage II for 7 days, globalagliatin was absorbed with peak plasma concentrations at approximately 3.00–5.00 h, and the Cmax and AUC0–24 increased with ascending dose levels from 20 to 320 mg. The inter-individual variability in Cmax and AUC0–24 values was considerable. The steady-state conditions were achieved after 7 days of daily dosing for each dose level, which was in line with the previous phase Ib studied in Caucasian, African, and mixed patients with type 2 diabetes and phase Ia studyed in Chinese healthy subjects (Data not published). In both stages, slight accumulation of globalagliatin was observed with a mean Rac of 2–3 based on Cmax and AUC0–24. Therefore the pharmacokinetic results, combined with previous phase I study conclusions, supported the practice of dose escalation every 7 days and once-daily oral administration of globalagliatin, which is a convenient regimen.
During the dose-titration period, FPG reduction (relative to baseline) was greater in the globalagliatin groups than in the placebo group, showing both time and dose dependence. Because this was a 28-day dose-titration study, we focused on GA, which reflects the mean blood glucose level in the previous 2–3 weeks, rather than on HbA1c, to evaluate therapeutic efficacy. On day 28, the reductions in GA, FPG, and AUC0–24,glucose (relative to baseline) were significantly higher in the high-dose group than in the placebo group, indicating a favorable hypoglycemic effect of globalagliatin. A nonsignificant decline in HbA1c was noted in the high-dose group relative to the placebo group, which could be attributed to the ascending-dose design and short treatment period.
Insulin resistance is a major pathogenesis of type 2 diabetes [12]. The risk of IR is difficult to estimate. With an estimated prevalence of 33% in America by 2050, IR is a leading cause of mortality [13]. It is associated with many diabetes-related diseases, such as hypertension, dyslipidemia, and cardiovascular disease (including coronary heart disease) [14, 15]. Therefore, improving IR and glycemic control are major targets of type 2 diabetes treatment, particularly during the early stages of the disease [16].
In the present study, globalagliatin reduced HOMA-IR in the high-dose group, which was consistent with the results of previous research in dorzagliatin [10]. HOMA-IR primarily reflects hepatic IR [17]; however, IR includes hepatic and peripheral IR, which can be reflected by the Matsuda index derived from the MMTT results. The high-dose group exhibited a significantly increased Matsuda index (compared with both the low-dose globalagliatin and placebo groups), and, to our knowledge, this is the first time this has been achieved in clinical trials involving GKAs. Thus, we speculate that an adequate dose of globalagliatin may improve IR, which suggests a potentially novel clinical indication for globalagliatin.
This study had several limitations. The first is the relatively small sample size and inclusion of only Chinese participants. Second, the 28-day treatment period was too short. Studies of several GKAs, such as AZD1656 and MK-0941, have reported that glycemic improvements are not sustained with continuing therapy [7, 8], while dorzagliatin and PF-04937319 have still shown good glycemic control at week 12 [9, 10]. Therefore, further research is needed to verify the long-term safety and efficacy of globalagliatin.
5 Conclusion
In conclusion, our study showed that globalagliatin was well tolerated and showed favorable pharmacokinetic profiles in Chinese patients with type 2 diabetes. High daily doses of globalagliatin reduced plasma glucose and improved IR. Although globalagliatin was related to a higher incidence of hypoglycemia and hypertriglyceridemia, these adverse effects were mild and have been reported for other GKAs. Based on our results, the phase II study will use 80 mg daily as the starting dose, while the safety and efficacy of globalagliatin will be assessed in a larger cohort and over a longer period.
References
Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88–98.
Upadhyay J, Polyzos SA, Perakakis N, et al. Pharmacotherapy of type 2 diabetes: an update. Metabolism. 2018;78:13–42.
Sternisha SM, Miller BG. Molecular and cellular regulation of human glucokinase. Arch Biochem Biophys. 2019;663:199–213.
Matschinsky FM, Zelent B, Doliba N, et al. Glucokinase activators for diabetes therapy: May 2010 status report. Diabetes Care. 2011;34(Suppl 2):S236–43.
Scheen AJ. New hope for glucokinase activators in type 2 diabetes? Lancet Diabetes Endocrinol. 2018;6:591–3.
Katz L, Manamley N, Snyder WJ, et al. AMG 151 (ARRY-403), a novel glucokinase activator, decreases fasting and postprandial glycaemia in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18:191–5.
Kiyosue A, Hayashi N, Komori H, et al. Dose-ranging study with the glucokinase activator AZD1656 as monotherapy in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15:923–30.
Meininger GE, Scott R, Alba M, et al. Effects of MK-0941, a novel glucokinase activator, on glycemic control in insulin-treated patients with type 2 diabetes. Diabetes Care. 2011;34:2560–6.
Amin NB, Aggarwal N, Pall D, et al. Two dose-ranging studies with PF-04937319, a systemic partial activator of glucokinase, as add-on therapy to metformin in adults with type 2 diabetes. Diabetes Obes Metab. 2015;17:751–9.
Zhu D, Gan S, Liu Y, et al. Dorzagliatin monotherapy in Chinese patients with type 2 diabetes: a dose-ranging, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Diabetes Endocrinol. 2018;6:627–36.
Zhi J, Zhai S, Boldrin M. Dose-dependent effect of piragliatin, a glucokinase activator, on the QT interval following short-term multiple doses in patients with type 2 diabetes mellitus. Clin Pharmacol Drug Dev. 2017;6:258–65.
DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88:787–835.
Boyle JP, Thompson TJ, Gregg EW. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29.
DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–94.
Adeva-Andany MM, Martinez-Rodriguez J, Gonzalez-Lucan M, et al. Insulin resistance is a cardiovascular risk factor in humans. Diabetes Metab Syndr. 2019;13:1449–55.
Tahrani AA, Piya MK, Kennedy A, Barnett AH. Glycaemic control in type 2 diabetes: targets and new therapies. Pharmacol Ther. 2010;125:328–61.
Tahrani AA, Barnett AH, Bailey CJ. Pharmacology and therapeutic implications of current drugs for type 2 diabetes mellitus. Nat Rev Endocrinol. 2016;12:566–92.
Acknowledgements
The authors thank all patients who participated in the study, as well as the site investigators (from the Endocrinology Department and Phase I Clinical Trial Center of the First Affiliated Hospital of Nanjing Medical University) and the coordinators (project participants from Yabao, clinical research coordinator and associate).
Author information
Authors and Affiliations
Contributions
SZ, FS, CXS, XQZ, and TY: contributed to the study design, conduct of the study, and data analysis; SZ, YD, SJD, LJX, JC, SFZ, HWZ, YC, ZZF, and HWZ: contributed to patient recruitment and data collection; QF and JZ: contributed to the data analysis. All authors were involved in the writing of the manuscript and approved the final version of the manuscript; XQZ and TY: are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding authors
Ethics declarations
Funding
This study was funded by Yabao Pharmaceutical R&D Co., Ltd.
Conflict of interest
Globalagliatin was originally developed by Eli Lilly and Company and later licensed out to Yabao for clinical development. There are no other potential conflicts of interest relevant to this study.
Ethics approval
All procedures in this study were in accordance with the 1964 Helsinki Declaration (and its amendments), and approved by the appropriate institutional review boards and regulatory agencies.
Informed consent
All participants provided written informed consent prior to inclusion.
Availability of data and material
The datasets obtained and/or analyzed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zheng, S., Shao, F., Ding, Y. et al. Safety, Pharmacokinetics, and Pharmacodynamics of Globalagliatin, a Glucokinase Activator, in Chinese Patients with Type 2 Diabetes Mellitus: A Randomized, Phase Ib, 28-day Ascending Dose Study. Clin Drug Investig 40, 1155–1166 (2020). https://doi.org/10.1007/s40261-020-00971-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-020-00971-x