Abstract
Background and Objective
Chronic gastritis frequently progresses into precancerous intestinal metaplasia and intraepithelial neoplasia lesions. Rebamipide is a free radical scavenger and we assessed its efficacy on clinical symptoms, gastric mucosal lesions, pathologic grade, and immunohistochemistry in chronic gastritis patients.
Methods
178 eligible patients were randomized into treatment and control groups. Both groups followed an optimized lifestyle for 26 weeks, but the treatment group was additionally medicated with rebamipide 0.1 g three times per day. Upper gastrointestinal endoscopy was performed in all patients to evaluate the severity of gastritis by the Modified Lanza Scoring (MLS) and histological changes were evaluated by the Updated Sydney System Score (USSS). Gastric mucosa immunohistochemistry in the treatment group was performed using the intestinal metaplasia markers caudal type homeobox transcription factor 2 (CDX2) and trefoil factor 3 (TFF3) detection.
Results
There were significant outcome differences between the treatment and control groups regarding the clinical symptom scores (2.62 ± 1.86 vs. 1.55 ± 1.61, P = 0.0001), gastric mucosal lesion scores (0.57 ± 1.05 vs. 0.16 ± 0.90, P = 0.002), and inflammation (P < 0.05). Only in the treated patients were the rates of intestinal metaplasia (P = 0.017 vs. P = 0.123) and low-grade intraepithelial neoplasia (P = 0.005 vs. P = 0.226) significantly reduced after 26 weeks. The percentages of CDX2 (31.5 vs. 15.7 %, P = 0.021) and TFF3 (44.9 vs. 25.8 %, P = 0.012) expressing gastric mucosa cells were significantly lower after rebamipide medication than pre-treatment values.
Conclusions
Rebamipide improved the clinical symptoms, gastric mucosal lesions, and pathological grades of chronic gastritis patients and decreased the expression rates of CDX2 and TFF3 in gastric cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Chronic gastritis can progress through the precancerous gastric lesions intestinal metaplasia and gastric intraepithelial neoplasia, resulting in gastric cancer. |
Besides clinical symptom and gastric mucosal lesion scores, the markers for intestinal metaplasia caudal homeobox transcription factor 2 (CDX2) and trefoil factor 3 (TFF3) can be used to evaluate the progression of chronic gastritis. |
Treatment with rebamipide 0.1 g three times per day for 26 weeks significantly reduced clinical symptom and gastric mucosal lesion scores as well as inflammation, intestinal metaplasia, and low-grade intraepithelial neoplasia. The percentages of CDX2 and TFF3 expressing gastric mucosa cells were significantly lower after rebamipide medication than pre-treatment values. |
1 Introduction
Chronic gastritis is prevalent in 80–90 % of patients undergoing upper gastrointestinal endoscopy. A major cause of chronic gastritis and the subsequent development of stomach cancers is Helicobacter pylori infections, which have been reported to induce active inflammation as well as the production of oxygen free radicals that produce DNA damage [1, 2]. In an earlier 5-year observation study, it was noted that successful eradication of H. pylori infections decreased the grades of gastric corpus and antrum atrophies as well as the levels of metaplasia [3]. Subsequently, it was proposed that the most appropriate intervention prior to the appearance of precancerous lesions is H. pylori infection medication [4]. Gastric carcinogenesis originates in the normal mucosa as superficial gastritis and progresses through atrophic gastritis, intestinal metaplasia, and gastric intestinal dysplasia, resulting in gastric cancer. Recently, the term gastric intestinal dysplasia was replaced by intraepithelial neoplasia and defined together with intestinal metaplasia as gastric precancerous lesions [5, 6]. There are several markers for aberrant cell development of the stomach. Ectopic caudal type homeobox transcription factor 2 (CDX2) protein is only expressed in gastric mucosa with intestinal metaplasia [7–9] and, since intestinal metaplasia has been proposed as gastric precancerous lesion, CDX2 overexpression was noted as a potential marker for gastric cancer [10–12]. Another marker for gastric cancer is serum trefoil factor (TFF) 3 [13, 14]. As a mucosa protective agent, rebamipide inhibits the activation of white blood cells [15], scavenges free radicals [16], and increases gastric mucus secretion [17] and prostaglandin levels [18].
In this randomized controlled study, we evaluated the efficiency of rebamipide for medication of chronic gastritis and focused on improvements of symptoms, gastric mucosal lesions, and changes of CDX2 and TFF3 expression.
2 Patients and Methods
2.1 Patients
Participants eligible for the study were patients with diagnosed chronic gastritis by endoscopy within 1 week who had least two of the following symptoms: symptoms of upper abdominal pain; distension; acid reflux; belching; loss of appetite; and nausea and vomiting [19]. Exclusion criteria were as follows: other digestive diseases, such as peptic ulcer or malignant diseases (including high-grade intraepithelial neoplasia); patients with severe cardiac, hepatic, or renal dysfunction and a history of neoplastic diseases; patients treated with drugs such as proton pump inhibitors (PPIs), histamine H2 receptor antagonists, mucosal protective agents, or antibiotics 2 weeks prior to the initiation of the study; patients administered aminosalicylic acid (aspirin), non-steroidal anti-inflammatory drugs (NSAIDs), or anticoagulants; patients with mental illnesses or alcohol and drug addiction as well as allergies or a medical history of hypersensitivity to the investigational drugs; and, in case of women, pregnancy or lactation.
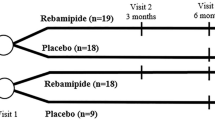
A total of 180 patients were initially enrolled and 178 patients were included in our randomized controlled study. H. pylori infections were diagnosed in 110 participants, and in 108 patients the infections were successfully eradicated. These participants were randomized into treatment group 1 (n = 54) and control group 1 (n = 54). The other 70 participants without H. pylori infections were randomized into treatment group 2 (n = 35) and control group 2, but one patient in control group 2 was lost during the study period (n = 34) (Fig. 1).
2.2 Detection and Treatment of Helicobacter pylori Infection
Helicobacter pylori status was determined using the 13C-urea breath test [20]. Patients with H. pylori infections were treated with a combination of esomeprazole 20 mg, colloidal bismuth pectin 200 mg, clarithromycin 500 mg, and amoxicillin 1000 mg twice daily for 14 days. Patients in whom H. pylori infections were not eradicated after the first treatment period received an additional 2-week course of medication consisting of levofloxacin 500 mg, furazolidone 100 mg, a standard dose of a PPI, and bismuth.
2.3 Rebamipide Treatment
Patients in the treatment groups received an open-labeled treatment of rebamipide 100 mg (Zhejiang Otsuka Pharmaceutical Co. Ltd., Hangzhou, Zhejiang, China) orally three times per day for 26 weeks. Both the treatment and control groups were advised to avoid alcohol, tobacco, and spicy foods during the treatment period, which was confirmed verbally during the visits.
2.4 Symptom Evaluation
A questionnaire was used to investigate symptoms such as upper abdominal pain, distension, acid reflux, belching, loss of appetite, and nausea and vomiting before and after treatment. All of these symptoms were rated by patients on a visual analog scale from 0 for none to 3 for worst [21].
2.5 Endoscopic Evaluation
The endoscopic appearance of the mucosa was observed and evaluated according to the modified Lanza Scoring (MLS) [22] from grade 0 to 5: grade 0 = no erosion or hemorrhage; grade 1 = erosion and hemorrhage localized in one area, <2 lesions; grade 2 = erosion and hemorrhage localized in one area, 3–5 lesions; grade 3 = erosion and hemorrhage in two areas, with >6 lesions in one area and <10 lesions in the whole stomach; grade 4 = erosion and hemorrhage appeared in three or more areas; and grade 5 = gastric ulcer.
2.6 Histological Evaluation
Biopsy specimens were taken from the most severe affected site (the same site the biopsy specimen was taken from at enrollment) after 26 weeks of therapy. After the paraffin-embedded tissues were sliced into 4 µm slides, they were dewaxed, dehydrated, and subsequently stained with hematoxylin and eosin (H&E). All specimens were evaluated and graded for gastritis according to the Updated Sydney System Score (USSS) for chronic mononuclear inflammatory cells, neutrophil activity, and intestinal metaplasia: 0 = normal, 1 = mild, 2 = moderate, and 3 = severe [23]. The scores were used to assess the improvement in clinical symptoms. If low-grade intraepithelial neoplasia turned into non-intraepithelial neoplasia, the treatment was considered efficacious. Between-group efficacy differences were used to assess the improvement in low-grade intraepithelial neoplasia.
2.7 Immunohistochemistry Evaluation
Samples harvested from the patients in the treatment groups before and after medication were treated with a standard procedure. Before immunohistochemistry, deparaffinized sections were placed in 0.01 M of citrate buffer (95 °C) for 15–20 min and washed in phosphate buffered saline (PBS) several times after natural cooling. The sections were then placed in the blocking solution (10 % normal goat serum in PBS) for 10 min and then incubated with rabbit polyclonal anti-CDX2 1:400 dilution (Abcam, Shanghai, China) or rabbit polyclonal anti-TFF3 1:200 dilution (Abcam, Shanghai, China) antibodies overnight at 4–8 °C. The streptavidin–peroxidase (S-P) method was used to detect the expression of CDX2 and TFF3. Positive cells were characterized by yellow or dark-brown signals in the nucleus (CDX2) or in the plasma (TFF3). The positive rate was calculated according to the number of positive cells at magnification (400×): 0 points for ≤5 %; 1 point for 5–25 %; 2 points for 25–50 %; 3 points for 50–75 %; and 4 points for >75 %. The coloring depth of the positive cells was calculated as follows: 0 points for basically non-stained; 1 point for yellow staining; 2 points for brown staining; and 3 points for dark-brown staining. The two points were then multiplied and if the product was 0, it indicated a negative (−) result; if the product was between 1 and 4 points, it indicated a weakly positive result (+); if the product was between 5 and 8 points, it indicated a moderately positive result (++); and if the product was between 9 and 12 points, it indicated a strongly positive result (+++).
2.8 Statistical Analysis
SPSS® (SPSS Inc., Chicago, IL, USA) version 17.0 for Windows software was used for all statistical calculations. Student’s t test was used for analysis of normally distributed data. The Wilcoxon test was used for non-normally distributed data. All values were expressed as the mean ± standard deviation. Statistical significance was defined at the P < 0.05 level.
3 Results
3.1 Characteristics of the Patients
A comparison of demographic characteristics showed that there were no significant differences between the four groups in terms of age, sex, or clinical and pathologic features (Table 1).
3.2 Effects of Rebamipide on Clinical Symptoms
Pre- and post-treatment differences in clinical symptoms between treatment group 1 and control group 1 in the successful H. pylori eradication therapy group were 2.48 ± 1.84 and 1.52 ± 1.81 (P = 0.004), respectively. Pre- and post-treatment differences in clinical symptoms between treatment group 2 and control group 2 in the H. pylori-negative group were 2.83 ± 1.90 and 1.59 ± 1.81 (P = 0.001), respectively. Differences in clinical symptoms between the treatment and control groups were 2.62 ± 1.86 and 1.55 ± 1.61 (P = 0.0001), respectively (Tables 2, 3).
3.3 Comparison of Gastric Mucosa Improvements
All patients were graded for endoscopic MLS before and after treatment. Compared with the control groups, a significant improvement in endoscopic scores was observed in the treatment groups after 26 weeks both in the H. pylori eradication and the H. pylori-negative groups (0.70 ± 1.12 vs. 0.28 ± 0.90, P = 0.036 and 0.37 ± 0.91 vs. −0.03 ± 0.87, P = 0.015, respectively) (Table 4). Comparing all treatment patients with all control patients, the gastric mucosal lesion scores improved significantly in the treatment groups (P = 0.002) (Table 5).
3.4 Changes in Histology
Patients with chronic gastritis always presented with varying degrees of neutrophil and mononuclear cell infiltration. At the end of the study, significantly higher reductions in neutrophil cell infiltration were achieved in treatment groups 1 and 2 (0.44 ± 1.08 vs. 0.04 ± 0.88, P = 0.037; 0.40 ± 0.77 vs. –0.04 ± 0.36, P = 0.006, respectively) than in control groups 1 and 2. Apparent reductions were observed in control group 1, but the effect did not reach statistical significance, whereas no reduction was observed in control group 2. Besides inflammation status, intestinal metaplasia before and after treatment were also evaluated, and in the treatment groups apparent reductions were observed but without statistical significance (Table 6).
Next, we investigated the pre- and post-treatment pathologically positive rates of intestinal metaplasia and low-grade intraepithelial neoplasia, which were both greatly reduced in the rebamipide group compared with the control group (P = 0.017 and P = 0.005, respectively) (Table 7).
3.5 Changes in Expression of CDX2 and TFF3
To investigate the effect of rebamipide on epithelial cells of the gastric mucosa with intestinal metaplasia further, the expression of CDX2 and TFF3 were determined in samples harvested from 89 patients in the rebamipide group before and after treatment. The immunohistochemistry results showed that the CDX2 and TFF3 positive cell ratio was significantly changed after 26 weeks of therapy (Fisher’s exact test: 31.5 vs. 15.7 %, P = 0.021 for CDX2 and 44.9 vs. 25.8 %, P = 0.012 for TFF3) (Figs. 2, 3, 4, 5; Table 8).
4 Discussion
In our patients, 61 % had H. pylorus infections, indicating that these bacteria are a major contributor to chronic gastritis. Gastric carcinoma has been attributed to H. pylorus infections [24, 25], but other authors have suggested that H. pylori may only produce an environment conducive to carcinogenesis and interact with other lifestyle and environmental exposures [26, 27]. Since eradication of H. pylori infections became a common measure to reduce the incidence of gastric cancer, the question arose whether intestinal metaplasia of the stomach can be reversed [28]. A previous study indicated that eradication alone does not completely prevent gastric cancer, and that this approach may be useful only in patients without atrophic gastritis or intestinal metaplasia [29]. Therefore, drugs that can restore normal gastric cell architecture after chronic gastritis are needed.
Rebamipide is an amino acid derivative of 2-quinolinone and has been shown to act via several mechanisms. Besides inhibition of reactive oxygen species, it stimulates prostaglandin and the prostaglandin EP4 receptor, leading to reduced gastric acid and enhanced mucus glycoprotein synthesis. Furthermore, rebamipide has anti-inflammatory properties that act by reducing inflammatory cytokines and chemokines in addition to inhibition of neutrophil cell activation [30]. Notably, rebamipide increased endothelial growth factor (EGF) and EGF receptor (EGF-R) expression in gastric mucosa of rats, thereby facilitating cell proliferation and re-epithelialization [31], and restored sonic hedgehog activity, which has implied an action in reversing gastric atrophy [32]. Other effects of rebamipide were reported to be normalization of the oxidative state and tyrosine nitration of ERK in portal hypertensive gastric mucosa, thereby reversing the impaired mucosal healing [33] and triggering vascularization via induction of proangiogenic genes [34]. In our study, CDX2 and TFF3 were selected as the immunohistochemical indicators to analyze the degree of intestinal metaplasia. Progressive loss of TFF1 and TFF2, together with the induction of TFF3, has been proposed as being involved in the early stages of the multi-step gastric carcinogenesis pathway [35]. CDX2 is a marker for intestinal metaplasia [7–9] and both markers were significantly reduced in the rebamipide treatment groups (Table 8), which indicates a reduction of intestinal metaplasia and low-grade intraepithelial neoplasia cells. Also, inflammation was reduced significantly more in the rebamipide treatment groups, as evidenced by less visible neutrophil cell infiltration (Table 6).
5 Conclusion
Helicobacter pylori eradication therapy and gastric mucosa recovery treatment, with regular follow-ups and periodic upper gastrointestinal endoscopy, are effective procedures in patients with chronic gastritis. Rebamipide is an efficacious medication for mucosa recovery of chronic gastritis patients.
References
Chang CS, Chen WN, Lin HH, Wu CC, Wang CJ. Increased oxidative DNA damage, inducible nitric oxide synthase, nuclear factor kappaB expression and enhanced antiapoptosis-related proteins in Helicobacter pylori-infected non-cardiac gastric adenocarcinoma. World J Gastroenterol. 2004;10:2232–40.
Farinati F, Cardin R, Degan P, Rugge M, Mario FD, Bonvicini P, et al. Oxidative DNA damage accumulation in gastric carcinogenesis. Gut. 1998;42:351–6.
Ito M, Haruma K, Kamada T, Mihara M, Kim S, Kitadai Y, et al. Helicobacter pylori eradication therapy improves atrophic gastritis and intestinal metaplasia: a 5-year prospective study of patients with atrophic gastritis. Aliment Pharmacol Ther. 2002;16:1449–56.
Malfertheiner P, Megraud F, O’Morain C, Bazzoli F, El-Omar E, Graham D, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772–81.
Rugge M, Capelle LG, Cappellesso R, Nitti D, Kuipers EJ. Precancerous lesions in the stomach: from biology to clinical patient management. Best Pract Res Clin Gastroenterol. 2013;27:205–23.
Yakirevich E, Resnick MB. Pathology of gastric cancer and its precursor lesions. Gastroenterol Clin N Am. 2013;42:261–84.
Almeida R, Silva E, Santos-Silva F, Silberg DG, Wang J, De Bolos C, et al. Expression of intestine-specific transcription factors, CDX1 and CDX2, in intestinal metaplasia and gastric carcinomas. J Pathol. 2003;199:36–40.
Bai YQ, Yamamoto H, Akiyama Y, Tanaka H, Takizawa T, Koike M, et al. Ectopic expression of homeodomain protein CDX2 in intestinal metaplasia and carcinomas of the stomach. Cancer Lett. 2002;176:47–55.
Bornschein J, Wex T, Peitz U, Kuester D, Roessner A, Malfertheiner P. The combined presence of H. pylori infection and gastro-oesophageal reflux disease leads to an up-regulation of CDX2 gene expression in antrum and cardia. J Clin Pathol. 2009;62:254–9.
Xiao ZY, Ru Y, Sun JT, Gao SG, Wang YF, Wang LD, et al. Expression of CDX2 and villin in gastric cardiac intestinal metaplasia and the relation with gastric cardiac carcinogenesis. Asian Pac J Cancer Prev. 2012;13:247–50.
Shiotani A, Iishi H, Uedo N, Ishihara R, Ishiguro S, Tatsuta M, et al. Helicobacter pylori-induced atrophic gastritis progressing to gastric cancer exhibits sonic hedgehog loss and aberrant CDX2 expression. Aliment Pharmacol Ther. 2006;24(Suppl 4):71–80.
Mutoh H, Sakurai S, Satoh K, Tamada K, Kita H, Osawa H, et al. Development of gastric carcinoma from intestinal metaplasia in Cdx2-transgenic mice. Cancer Res. 2004;64:7740–7.
Huang Z, Zhang X, Lu H, Wu L, Wang D, Zhang Q, et al. Serum trefoil factor 3 is a promising non-invasive biomarker for gastric cancer screening: a monocentric cohort study in China. BMC Gastroenterol. 2014;14:74.
Aikou S, Ohmoto Y, Gunji T, Matsuhashi N, Ohtsu H, Miura H, et al. Tests for serum levels of trefoil factor family proteins can improve gastric cancer screening. Gastroenterology. 2011;141(837–45):e1–7.
Kim JS, Kim JM, Jung HC, Song IS. Expression of cyclooxygenase-2 in human neutrophils activated by Helicobacter pylori water-soluble proteins: possible involvement of NF-kappaB and MAP kinase signaling pathway. Dig Dis Sci. 2001;46:2277–84.
Sakurai K, Sasabe H, Koga T, Konishi T. Mechanism of hydroxyl radical scavenging by rebamipide: identification of mono-hydroxylated rebamipide as a major reaction product. Free Radic Res. 2004;38:487–94.
Iijima K, Ichikawa T, Okada S, Ogawa M, Koike T, Ohara S, et al. Rebamipide, a cytoprotective drug, increases gastric mucus secretion in human: evaluations with endoscopic gastrin test. Dig Dis Sci. 2009;54:1500–7.
Qi Z, Jie L, Haixia C, Xiaoying Z. Effect of rebamipide on quality of peptic ulcer healing in rat. Dig Dis Sci. 2009;54:1876–83.
Du Y, Bai Y, Xie P, Fang J, Wang X, Hou X, et al. Chronic gastritis in China: a national multi-center survey. BMC Gastroenterol. 2014;14:21.
Di Rienzo TA, D’Angelo G, Ojetti V, Campanale MC, Tortora A, Cesario V, et al. 13C-Urea breath test for the diagnosis of Helicobacter pylori infection. Eur Rev Med Pharmacol Sci. 2013;17(Suppl 2):51–8.
Naito Y, Yoshikawa T, Iinuma S, Yagi N, Matsuyama K, Boku Y, et al. Rebamipide protects against indomethacin-induced gastric mucosal injury in healthy volunteers in a double-blind, placebo-controlled study. Dig Dis Sci. 1998;43:83S–9S.
Lanza FL, Royer GL Jr, Nelson RS. Endoscopic evaluation of the effects of aspirin, buffered aspirin, and enteric-coated aspirin on gastric and duodenal mucosa. N Engl J Med. 1980;303:136–8.
Chitapanarux T, Praisontarangkul OA, Lertprasertsuke N. An open-labeled study of rebamipide treatment in chronic gastritis patients with dyspeptic symptoms refractory to proton pump inhibitors. Dig Dis Sci. 2008;53:2896–903.
Sakaki N, Kozawa H, Egawa N, Tu Y, Sanaka M. Ten-year prospective follow-up study on the relationship between Helicobacter pylori infection and progression of atrophic gastritis, particularly assessed by endoscopic findings. Aliment Pharmacol Ther. 2002;16(Suppl 2):198–203.
Asaka M, Sugiyama T, Nobuta A, Kato M, Takeda H, Graham DY. Atrophic gastritis and intestinal metaplasia in Japan: results of a large multicenter study. Helicobacter. 2001;6:294–9.
Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–62.
Peek RM Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37.
Walker MM. Is intestinal metaplasia of the stomach reversible? Gut. 2003;52:1–4.
Cheung TK, Xia HH, Wong BC. Helicobacter pylori eradication for gastric cancer prevention. J Gastroenterol. 2007;42(Suppl 17):10–5.
Arakawa T, Higuchi K, Fujiwara Y, Watanabe T, Tominaga K, Sasaki E, et al. 15th anniversary of rebamipide: looking ahead to the new mechanisms and new applications. Dig Dis Sci. 2005;50(Suppl 1):S3–11.
Tarnawski A, Arakawa T, Kobayashi K. Rebamipide treatment activates epidermal growth factor and its receptor expression in normal and ulcerated gastric mucosa in rats: one mechanism for its ulcer healing action? Dig Dis Sci. 1998;43:90S–8S.
Nishizawa T, Suzuki H, Nakagawa I, Minegishi Y, Masaoka T, Iwasaki E, et al. Rebamipide-promoted restoration of gastric mucosal sonic hedgehog expression after early Helicobacter pylori eradication. Digestion. 2009;79:259–62.
Kinjo N, Kawanaka H, Akahoshi T, Yamaguchi S, Yoshida D, Anegawa G, et al. Significance of ERK nitration in portal hypertensive gastropathy and its therapeutic implications. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1016–24.
Tarnawski AS, Chai J, Pai R, Chiou SK. Rebamipide activates genes encoding angiogenic growth factors and Cox2 and stimulates angiogenesis: a key to its ulcer healing action? Dig Dis Sci. 2004;49:202–9.
Leung WK, Yu J, Chan FK, To KF, Chan MW, Ebert MP, et al. Expression of trefoil peptides (TFF1, TFF2, and TFF3) in gastric carcinomas, intestinal metaplasia, and non-neoplastic gastric tissues. J Pathol. 2002;197:582–8.
Author contributions
Xue Han carried out the experiments and drafted the manuscript. Lu Zhou, Xin Chen, and Shu Li participated in the design and conceived the study. Kui Jiang and Bangmao Wang collected data and participated in the study design and coordination. All authors approved the final manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors have no conflicts of interest.
Ethical approval
All procedures in this study were performed in accordance with the 1964 Helsinki declaration and its amendments. The study protocol was approved by the ethics review board of the General Hospital of Tianjin Medical University (Tianjin, China).
Informed consent
Written informed consent was obtained from all patients enrolled.
Rights and permissions
About this article
Cite this article
Han, X., Jiang, K., Wang, B. et al. Effect of Rebamipide on the Premalignant Progression of Chronic Gastritis: A Randomized Controlled Study. Clin Drug Investig 35, 665–673 (2015). https://doi.org/10.1007/s40261-015-0329-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-015-0329-z