Abstract
Background
Biosimilars have been adopted by clinicians more slowly than anticipated in the post-marketing phase.
Objectives
We aimed to reveal the perceptions and attitudes of pediatric rheumatologists towards biosimilars and the obstacles to biosimilar therapy.
Methods
A web-based survey designed to determine the knowledge, experience, and perceptions of pediatric rheumatologists about biosimilars was electronically mailed to the participants between April and August 2021. Responses were collected anonymously and subsequently analyzed.
Results
A total of 114 pediatric rheumatologists including fellows (32.4%), specialists (29.8%), and seniors (37.7%) responded to the questionnaire. According to the data, 75 (65.8%) physicians had already prescribed at least one biosimilar. The vast majority of participants were aware of the potential cost savings of biosimilars (84, 73.3%). Participants who felt insufficiently informed were 41.8%, 67.6%, and 83.7% among seniors, specialists, and fellows, respectively. In pediatric rheumatology, the scarcity of clinical trials and real-life data (64%) and inadequate information about tolerance to the biosimilars and related side effects in children (49.1%) were the most common barriers expressed by prescribers. Nearly half (45%) of the pediatric rheumatologists preferred to prescribe biosimilars in the treatment of biologic-naive cases. However, most (93%) were reluctant to switch a reference molecule to a biosimilar while the patient was doing well under the originator medicine.
Conclusions
This survey provided insights into the concerns about prescribing biosimilars among pediatric rheumatologists. In the field of pediatric rheumatology, further education about biosimilars and real-life experiences is required to better inform about treatment options in children.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Pediatric rheumatologists understand and embrace biosimilars insufficiently. |
Pediatric rheumatologists are hesitant to switch patients while under a reference biologic treatment to a biosimilar. |
The major concerns regarding biosimilar prescription among pediatric rheumatologists are lack of available real-life data and insufficient information about the tolerance and adverse events in pediatric patients. |
1 Introduction
Biotechnologic drugs (also known as biologics) are pharmaceutical products that are either derived from living microorganisms or contain living cell fragments [1, 2]. In recent years, the emergence of biologic therapeutics has revolutionized the treatment of severe, debilitating, and life-threatening diseases, including oncologic, rheumatologic, gastroenterologic, immunologic, neurologic, and several rare diseases. Likewise, the incorporation of biologics into healthcare systems has led to significant patient improvement in pediatric rheumatology over the past 15 years [3,4,5].
As targeted therapeutics, biologics differ from the drugs that are manufactured chemically by using conventional methods. In this regard, complex biotechnology techniques such as recombinant DNA render them expensive, so much so that five biologics were among the top ten budgets allocated to drugs envisioned for 2021 worldwide [6]. In addition, according to data reported by the pharmaceutical industry for 2020, a total of ten biologic therapeutics were designated as “blockbusters”; this is a monetary definition that means more than $1billion in annual sales [7].
As a result, complex and costly production protocols of biologics have resulted in the development of biosimilars, which are approved by agencies such as the US Food and Drug Administration, European Medicines Agency, and the World Health Organization as alternatives to biologics. Biosimilars offer the potential to mitigate rising drug costs and increase patient satisfaction and access to critical biologic treatments. The Food and Drug Administration defines a biosimilar as a product that is highly similar, but not entirely identical, to the reference medicinal product (also called a bio-original) in terms of quality, safety, and efficacy [8].
Biosimilars, which may have significant cost-saving advantages over biologic products, were expected to shake up the pharmaceutical market. However, market access has been slower than foreseen [9]. This incoherence has been attributed to various factors including regulatory challenges, legal issues, payer policy disparities, and hesitancy of clinicians [10].
As more biosimilars enter the market, understanding the awareness of and current clinician attitudes towards biosimilars is crucial to identifying the educational need for biosimilars, promoting their use, and ultimately reducing the costs of biologic drugs. Several studies have been reported among physicians and pharmacists that highlight the reluctance to accept and use biosimilars on an equal basis with the reference product.[11,12,13,14,15,16]. A new systematic review of biosimilar surveys between 2014 and 2018 that evaluated physicians’ knowledge and beliefs about biosimilarity announced that healthcare providers in the USA and Europe were cautious about prescribing these agents and they showed limited familiarity with biosimilars [17]. This review did not include data on the opinions of pediatric rheumatologists. It is well known that the safety and efficacy of drugs in pediatric patients requires even greater precision than in adults. In addition, rational drug use is a particularly important issue for children as they may face drug exposure throughout their lives. The process of obtaining approval and licensing of drugs in pediatric patients is often carried out on the basis of studies conducted in adult patients. A lack of long-term safety and effectiveness data for new drugs in children can lead to delays in clinicians adopting them for use in daily practice. Biosimilars have not yet been embraced among physicians for their use in children. Thus, the treatment of pediatric rheumatologic conditions with biosimilars comes to the fore as a delicate subject that needs to be revealed. However, there are limited current data on the perceptions and attitudes of pediatric rheumatologists towards biosimilars in the literature [18, 19]. Here, we aimed to reveal these perceptions among pediatric rheumatologists with web-based international research.
2 Methods
2.1 Survey Design
As part of the PReS (European Society for Pediatric Rheumatology)-EMERGE (Emerging Rheumatologists and Researchers) group, we designed a 30-question online questionnaire in English and Turkish using the SurveyMonkey online software. Expert review based on the reviewed literature ensured that the questionnaire questions achieved maximal authenticity. The questionnaire included multiple choice, rating scale, dropdown, demographic, open-ended, and slider question types. The answers were collected under five main headings in the following manner: (1) the first six questions collected demographic data (age, sex, origin, affiliation, academic degree, and year in pediatric rheumatology) of the participants; (2) the following four questions were about the source and route of knowledge on biosimilars; (3) nine questions were about the participant’s experiences with biosimilars; (4) nine questions were regarding perceptions and attitudes of clinicians in the case of biosimilar use; and (5) two questions were associated with the nocebo effect (see Electronic Supplementary Material [ESM]).
2.2 Participants and Invitation Method
Clinicians consisting of fellows, senior physicians, and general practitioners who were in direct contact with patients in their daily practice and dealing with chronic pediatric rheumatic diseases were invited to participate in the survey. The qualifications of these participants were as follows: pediatric rheumatology fellows attending the pediatric rheumatology fellowship training program, senior physicians with an academic degree at a university or a training hospital, and general experts who completed a pediatric rheumatology fellowship training program and dealing with pediatric rheumatic diseases. They were invited cross-sectionally to complete an online self-administered survey via e-mail. During April 2021, the questionnaire was e-mailed to the members of PReS and announced on the PReS website. It was also sent electronically to the members of the Pediatric Rheumatology Bulletin Board, a worldwide electronic mail list and to the Turkish national e-mail group consisting of pediatric rheumatologists. Finally, it was included in the Pediatric Global Musculoskeletal Taskforce newsletter, which is sent monthly to a large group of pediatric rheumatologists, pediatric orthopedic surgeons, and allied health professionals from all over the world.
Each of the respondents filled out the questionnaire voluntarily and anonymously, containing no individual identifiers to maintain confidentiality, in approximately 10 min in one step. The survey was open over 15 weeks, from April 26 to August 7, 2021. During the collection of responses, to encourage the participation of physicians, an e-mail with a reminder message was sent to the participants three times at intervals. Each participant could participate at most once through the link provided to them.
2.3 Data Analysis and Ethics Approval
To avoid missing answers leading to erroneous or misleading results, only the responses of participants who completed more than 80% of the questionnaire were included in the analysis. Descriptive statistics and basic comparisons were obtained from the Basic Statistics feature of Survey Monkey. For further analysis, SPSS software version 22.0 was used. Numbers, percentages, mean, median, maximum, minimum, and standard deviation were used to present the data. The data are expressed as the mean ± standard deviation for continuous variables and as the frequency for nominal variables. The Chi-square test was applied to determine any correlation between the two selected variables. The Mann–Whitney U test was employed for variables with a distribution that was not normal. The Kruskal–Wallis test was performed for assessing the differences among three groups on a single, non‐normally distributed continuous variable. Continuous variables were compared using a t test.
The present survey was performed in line with the principles of the Declaration of Helsinki. It was reviewed and approved by the Ethics Committee of Istanbul University (number 21.05.2021-204228). We did not collect any personal or confidential data and we have followed the Consensus-Based Checklist for Reporting of Survey Studies (CROSS) [see ESM] [20].
3 Results
3.1 Demographics of Respondents
The survey drew the participation of 114 pediatric rheumatologists globally and all of them completed more than 80% of the questionnaire. They were aged between 30 and 81 years, with a median age of 39.5 years. Among the respondents, 82 (71.9%) were women. Turkey and the USA came in first and second, respectively, as having the greatest participation rates among the 22 countries. The origin of the respondents per country is shown in Fig. 1.
Among all respondents, 107 (93.8%) were hospital-based rheumatologists, four (3.5%) were office/private hospital based, and three (2.6%) were both hospital and office/private hospital based. Most of the participants (61, 53.5%) reported more than 5 years of work experience in pediatric rheumatology. Table 1 outlines the academic status, case, and prescription volume of respondents.
3.2 Sources of Information About Biosimilars and Feelings Associated with the Existing Knowledge
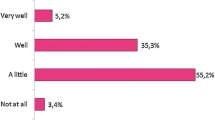
Slightly more than half of the senior physicians (23, 53.5%) felt well informed about biosimilars whereas four (10.8%) fellows reported that they were sufficiently knowledgeable about biosimilars. Two (4.7%) of seniors and one (2.9%%) of the specialists were extremely well informed and the proportions of those who were inadequately or partially informed were 41.9%, 67.6%, and 83.8% among seniors, specialists, and fellows, respectively (Fig. 2). Regarding knowledge level, which was evaluated with a scale from 0 to 100, the fellows had a significantly lower level of knowledge (median 30, interquartile range 32, 95% confidence interval 28.3–43.6) compared with the seniors (median 71, interquartile range 42, 95% confidence interval 55.5–72.5; p < 0.01) and a non-significantly lower level than the specialists (median 50, interquartile range 36, 95% confidence interval 44–60; p = 0.02); however, there was no significant difference between specialists and seniors (p = 0.23).
When countries were compared by clustering, European clinicians felt more informed about biosimilars than their American (p = 0.021) and Turkish (p = 0.034) counterparts. However, there was no significant difference between pediatric rheumatologists in Turkey and the USA in this regard (p = 0.37).
Concerning the diversity of information resources, the most common routes were congresses, symposia, self-research, and scientific journals. A significant portion of the respondents stated that they wished to be informed about biosimilar medicines in the future through congresses/symposia (86, 75.4%) and webinars (70, 61.4%) (see ESM). Two seniors noted that they had obtained information through anecdotal experiences or colleagues and two specialists quoted through a hospital pharmacy. We also compared the knowledge levels of physicians who prescribed and did not prescribe a biosimilar. Pediatric rheumatologists who prescribed previously had significantly higher knowledge levels (p < 0.01) and they were more likely to agree that if the prescription of biosimilar medicines becomes more frequent, it will enable cost savings and reduce healthcare expenses (p = 0.01).
3.3 Experiences of Biosimilar Products
At the time of investigation, 75 (65.8%) pediatric rheumatologists had already prescribed at least one biosimilar. Nearly half of the fellows (18, 48.6%) had not yet prescribed any biosimilar, and a quarter (11, 25.5%) of the seniors who had been dealing with pediatric rheumatic disorders for at least 10 years had never prescribed a biosimilar. The majority of respondents (71, 62.3%) stated that they have been using biosimilars in clinical practice for fewer than 5 years. Figure 3 outlines the indications and product names for biosimilars prescribed.
Of those physicians who had prescribed biosimilars before, 10 (12.2%) prescribed them every day, 20 (24.4%) prescribed them about once a week, and 20 (24.4%) a few times a month. According to the question investigating the adverse event(s) experienced by clinicians when treating patients with a biosimilar, 35 (46.6%) participants did not report any adverse events. Local reactions at the injection site (29, 25.4%) were the most frequently marked among the options (Table 2). In clinical practice, 25 (21.9%) pediatric rheumatologists considered switching to the original biologic when treating their patients with the biosimilar, while 36 (31.6%) considered switching to a biosimilar when treating their patients with the reference drug.
3.4 Perceptions, Incentive Issues, and Obstacles to Adoption and Prevalent Prescription of Biosimilars
Nearly half of the clinicians agreed that “a biosimilar product has the same dosage and route of administration compared to the reference branded product”, “has no significant difference from the reference molecule in terms of safety and efficacy”, and they pertinently endorsed biosimilars as chemically identical to the original branded drug. However, 16 (14%) participants considered a biosimilar as a unique biologic drug. Seven (6.1%) clinicians held the view that a biosimilar is a counterfeit copy of an original biologic medicine and four (3.5%) respondents predicted that biosimilars will not carve out a niche in contemporary healthcare systems.
The majority of pediatric rheumatologists (92, 80.7%) agree that the cost savings of biosimilars for national health systems encourage physicians to choose biosimilars when prescribing. Among respondents, 15% considered prescribing biosimilars as critical for expanding the general use of biologics and implementing innovation in biopharmaceutical manufacturing. Three-quarters of the participants (86, 75.4%) believed that they were sufficiently informed about when to prescribe a biosimilar in terms of indications, and nearly half of them (41.2%, 54.4%, and 58.8%) thought they are well informed about the quality, efficacy, and dosage of biosimilars. Ninety-five (83.3%) respondents cited “healthcare cost savings” as a positive factor while talking about their motivation to prescribe biosimilars. Approximately one-fourth of the participants (24.6%) preferred biosimilars as they are the sole biologic drug options in the centers where they work. They also agreed that “biosimilars are more appropriate forms (dosage or route of administration) for children than reference products.”
Among 109 responses to the question of barriers to prescribing biosimilars, it was noteworthy that 64% of the responses reported were “lack of clinical trial and real-life experience data in pediatric rheumatology”. The potential obstacles for selecting biosimilars are outlined in Fig. 4.
When respondents were asked whether they agreed with various assertions about biosimilars (108, 94.7% answered); 39 (34.2%) thought that biologic therapy could be switched from a reference to a biosimilar product as biosimilars were equally safe and effective for children, but few participants (15, 13.2%) currently considered switching to a biosimilar in children insufficiently treated with an original biologic.
The majority of pediatric rheumatologist participants worldwide (84, 73.3%) were aware of the cost-saving potential of biosimilars. The percentage of participants who preferred to prescribe biosimilars in the treatment of biologic-naive cases was 45%. However, nearly one-third (34, 29.8%) were still hesitant to initiate biosimilars in biologic-naïve children or to switch a reference product to a biosimilar. Furthermore, most (93%) were reluctant to switch a reference molecule to a biosimilar while the patient was doing well under the originator medicine.
When compared in terms of propensity to prescribe biosimilars, one-third of seniors, specialists, and fellows indicated that they would prefer biosimilars as the initial biologic therapy for patients (Fig. 5). The nocebo effect was rated as a barrier to starting or switching to a biosimilar by 41% of respondents, and 12% stated that they had observed the nocebo effect of a biosimilar.
4 Discussion
In our study, a significant portion of senior physicians, specialists, and fellows stated that they felt inadequate or partially knowledgeable about biosimilars. Regarding biosimilar awareness, there was similarity between our results and surveys from Europe and the USA [17]. In 2016, Pasina et al. investigated hospital specialists’ views about biosimilars. Only 49 (22.9%) doctors from the group stated that they had full or sufficient knowledge about the scientific principles of biosimilars [21]. Likewise, Cassar et al. pointed out that only 6% of Maltese clinicians were familiar with biosimilarity, 35% had a basic understanding, and 59% could not define biosimilars or had never heard of them [22]. Our study reflects similar results to the Maltese clinicians. Furthermore, in a recent survey among French rheumatologists, only six (5.2%) physicians felt very well informed about biosimilars [14]. We can also cite a survey of 575 biologic prescribers on biosimilars (2019) in six Western European countries (France, Germany, Italy, Spain, Switzerland, and the UK) [15]. This periodically applied survey focused on exploring biosimilar prescribing habits and insights from clinicians specialized in one of ten practice areas: dermatology, endocrinology, gastroenterology, hematology oncology, immunology, nephrology, neurology, oncology, ophthalmology, and rheumatology. The percentage of physicians who rated themselves as “familiar” or “very familiar” with biosimilars increased from 76% in 2013 to 90% in 2019. Consequently, gaps in prescriber knowledge and insufficient familiarity remain valid challenges for the adoption of biosimilars. It is clear that prescribers dealing with pediatric rheumatic diseases need additional education and encouragement on biosimilars both by congresses, symposia, self-research, and scientific journals.
A pooled literature review of 90 studies (in 2018) indicated that switching originator drugs to biosimilars did not make a significant difference in clinical efficacy and safety in the majority of patients [23]. However, clinicians are still skeptical of switching or prescribing these products, with their hesitation centered on the safety and efficacy of biosimilars. A systematic review by Leonard et al. considers that healthcare providers are still cautiously approaching biosimilars and citing safety and efficacy concerns as key deterrents to biosimilar use [17]. Greater than 60% of physicians in distinct studies highlighted the lack of high-level evidence of safety for biosimilars and were also concerned about biosimilar immunogenicity. The main concerns were related to the tendency of biosimilars to elicit an immune response by themselves or their excipients or pharmaceutical ingredients [24, 25]. The underlying safety (particularly immunogenicity) and efficacy concerns deterred the majority of clinicians from switching patients who had already tolerated the original product to the biosimilar [11, 13, 21, 25,26,27,28]. In particular, both US and European clinicians did not seem well aware of the existence of biosimilars as alternative safe and effective treatment options for their patients. “Physicians were generally reluctant to use biosimilars as a first-line option for patients requiring biologic therapy. For this reason, it was stated that they perceived it more as second or third-line treatment options. Some doctors, on the other hand, shunned changes in the original biologic drug users and limited biosimilars only to biologic-naïve patients.” [11, 12, 21, 28, 29]. A recent international survey of rheumatologists has identified concerns that biosimilars may not be reliable copies of bio-originals and that long-term efficacy and safety data are insufficient [30]. Although we did not question about immunogenicity, most of the respondents stated that main barriers to the prescription of biosimilars are ‘‘Lack of clinical trials and evidence acquired from real-life data in pediatric rheumatology’’. Similarly, in a Belgian survey of rheumatologists and patients with rheumatoid arthritis, the two most frequently stated reasons for not prescribing biosimilars were “less studied than principle” and “no clinical studies have been conducted in a particular indication” [31]. The nocebo effect is defined as a situation where a patient develops side effects or symptoms that can occur with a drug or other therapy just because the patient believes they may occur. The nocebo effect has reemerged with the widespread adoption of biosimilars [32]. It was also rated as a barrier to starting or switching to a biosimilar product by 41% of respondents in our survey, and 12% of the respondents stated that they had observed the nocebo effect of a biosimilar. The nocebo effect reduces the quality of life of patients and adversely affects treatment compliance rates in patients receiving biosimilars. Healthcare providers responsible for patients treated with biosimilars should be aware of the nocebo effect and adopt strategies to minimize it [33]. Consequently, pediatric rheumatologists, like other specialties, need conclusive and comprehensive evidence of safety and efficacy to safely prescribe biosimilars.
Despite the aforementioned doubts, clinicians dealing with chronic rheumatic diseases in children are aware of the potential cost savings of prescribing biosimilars. To the question exploring the motivations to prescribe biosimilars, 95 (83.3%) respondents quoted “healthcare cost savings” as a positive influencer. This knowledge of the cost-saving potential is somewhat reassuring for prescribing biosimilars in certain patients, particularly in biologic-naïve cases or those facing treatment failure with other biologics. Consistent with the above issues, in the study investigating the perceptions of French rheumatologists, cost savings, freeing up resources allowing the treatment of additional patients, patients’ access to innovative drugs, and incentives by health policy makers were cited as positive factors that could encourage the prescribing of biosimilars [14]. Despite this acceptance, it is noteworthy that nearly half of the fellows (48.6%) and a quarter of the senior physicians who have been dealing with pediatric rheumatic diseases for at least 10 years (25.5%) in the current study have not prescribed a biosimilar before. These ratios show that the adaptation of biosimilars to pediatric rheumatology has not been completed yet.
We found it worth presenting evidence about the views of pediatric rheumatologists on biosimilars and, when compared to previous studies, our study included the participation of 114 clinicians from different working environments, with different academic status, on the international platform [12, 14, 16]. It provides up-to-date information from seniors, specialists, and fellows on perceptions about biosimilars and, to our knowledge, no biosimilar research has ever focused on such a large number of pediatric rheumatologists worldwide.
The study results demonstrate that pediatric rheumatologists are still hesitant to adopt and embrace biosimilars and require more education to decide to prescribe biosimilars. More comprehensive research is needed on switching from an originator to a biosimilar and the long-term effect of biosimilar prescribing, as well as educational initiatives targeting pediatric rheumatologists, including those of all qualifications.
5 Limitations
Several limitations need to be discussed. First, for reasons beyond our control, the method we used to send e-mail invitations to complete the survey did not allow receipt tracking and therefore we were unable to calculate a response rate. Second, because clinicians working in centers worldwide could not be contacted directly, a homogeneous participation could not be achieved. As the majority of respondents were from Turkey and the USA, the results may not be representative of all pediatric rheumatologists. The number of participants from most other countries was low, despite the efforts of the authors, PReS and PRINTO authorities, and the repeated reminder messages that were sent. PReS and PRINTO are the main meeting points of pediatric rheumatologists worldwide aiming to produce international projects in this field, and the low participation from countries other than Turkey indicates that further improvements could be made in the international collaboration and communication among pediatric rheumatologists. Third, the survey could have included more detailed questions about switches or factors considered by physicians when prescribing a biosimilar. However, we felt 30 questions to be sufficient for an online survey, as overly long surveys can decrease the participation or completion rate [34, 35].
6 Conclusions
Based on the current research, pediatric rheumatologists worldwide are still somewhat hesitant to use biosimilars in their clinical practice. However, this study may facilitate the understanding of obstacles to the widespread use of biosimilars among the pediatric rheumatology community. Targeted communication efforts, educational approaches in various platforms, and growing evidence that biosimilars are safe and effective in children can help overcome misconceptions about biosimilars among pediatric rheumatologists and encourage them to prescribe biosimilars when required.
References
Lahaye C, Tatar Z, Dubost JJ, Soubrier M. Overview of biologic treatments in the elderly. Jt Bone Spine. 2015;82(3):154–60.
Strand V, Goncalves J, Isaacs JD. Immunogenicity of biologic agents in rheumatology. Nat Rev Rheumatol. 2021;17(2):81–97.
Cimaz R, Maioli G, Calabrese G. Current and emerging biologics for the treatment of juvenile idiopathic arthritis. Expert Opin Biol Ther. 2020;20(7):725–40.
Cabrera N, Lega JC, Kassai B, Wouters C, Kondi A, Cannizzaro E, et al. Safety of biological agents in paediatric rheumatic diseases: a real-life multicenter retrospective study using the JIRcohorte database. Jt Bone Spine. 2019;86(3):343–50.
Kuemmerle-Deschner JB, Gautam R, George AT, Raza S, Lomax KG, Hur P. A systematic literature review of efficacy, effectiveness and safety of biologic therapies for treatment of familial Mediterranean fever. Rheumatology (Oxf). 2020;59(10):2711–24.
Top selling biologics market, 2021–2030: focus on product landscape assessment, ongoing clinical trials, promotional content analysis, other life cycle management strategies, competition from biosimilars, annual treatment cost comparison, sales evolution and future opportunity. 2021. https://www.businesswire.com/news/home/20210803005814/en/Global-Top-Selling-Biologics-Market-Report-2021-2030---ResearchAndMarkets.com.
Alfaleh MA, Alsaab HO, Mahmoud AB, Alkayyal AA, Jones ML, Mahler SM, et al. Phage display derived monoclonal antibodies: from bench to bedside. Front Immunol. 2020;11:1986.
US Food and Drug Administration. Guidances (drugs): biosimilars. https://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/guidances/ucm290967.htm. Accessed 28 Nov 2019.
Moorkens E, Godman B, Huys I, Hoxha I, Malaj A, Keuerleber S, et al. The expiry of Humira® market exclusivity and the entry of adalimumab biosimilars in Europe: an overview of pricing and national policy measures. Front Pharmacol. 2020;11:591134.
Leonard E. Factors affecting healthcare provider knowledge and acceptance of biosimilar medicines: a systematic review. J Manag Care Spec Pharm. 2019;25(1):102–12. https://doi.org/10.17615/cky7-df88.
O’Callaghan J, Bermingham M, Leonard M, Hallinan F, Morris JM, Moore U, et al. Assessing awareness and attitudes of healthcare professionals on the use of biosimilar medicines: a survey of physicians and pharmacists in Ireland. Regul Toxicol Pharmacol. 2017;88:252–61.
Gibofsky A, McCabe D. US rheumatologists’ beliefs and knowledge about biosimilars: a survey. Rheumatology (Oxf). 2021;60(2):896–901.
Cohen H, Beydoun D, Chien D, Lessor T, McCabe D, Muenzberg M, et al. Awareness, knowledge, and perceptions of biosimilars among specialty physicians. Adv Ther. 2017;33(12):2160–72.
Beck M, Michel B, Rybarczyk-Vigouret MC, Levêque D, Sordet C, Sibilia J, et al. Rheumatologists’ perceptions of biosimilar medicines prescription: findings from a French web-based survey. BioDrugs. 2016;30(6):585–92.
Reilly MS. Biosimilar substitution: European prescriber perspectives. Ann Oncol. 2019;30. https://www.annalsofoncology.org/article/S0923-7534(19)59837-4/pdf.
Ismailov RM, Khasanova ZD. Biosimilar knowledge among oncology/hematology team members in Colorado, USA: an educational initiative and follow-up survey. BioDrugs. 2018;32(5):499–506.
Leonard E, Wascovich M, Oskouei S, Gurz P, Carpenter D. Factors affecting health care provider knowledge and acceptance of biosimilar medicines: a systematic review. J Manag Care Spec Pharm. 2019;25(1):102–12.
Maccora I, Lombardi N, Crescioli G, Bettiol A, Bonaiuti R, Pagnini I, et al. OBSIDIAn: real world evidence of Originator to BioSImilar Drug switch in juvenile idiopathic arthritis. Rheumatology (Oxf). 2021. https://doi.org/10.1093/rheumatology/keab572.
Demirkan FG, Ulu K, Öztürk K, Karadağ ŞG, Özdel S, Sönmez HE, et al. Toward the integration of biosimilars into pediatric rheumatology: adalimumab ABP 501 experience of PeRA research group. Expert Opin Biol Ther. 2022;22(2):197–202.
Sharma A, Minh Duc NT, Luu Lam Thang T, Nam NH, Ng SJ, Abbas KS, et al. A consensus-based checklist for reporting of survey studies (CROSS). J Gen Intern Med. 2021;36(10):3179–87.
Pasina L, Casadei G, Nobili A. A survey among hospital specialists and pharmacists about biosimilars. Eur J Intern Med. 2016;35:e31–3.
Cassar K, Zammit Dimech D, Grech L, Balzan D, Cutajar A, Cassar PJ. SAT0637-HPR biosimilars: the perception amongst Maltese clinicians. Ann Rheum Dis. 2016;75(Suppl. 2):1294.
Cohen HP, Blauvelt A, Rifkin RM, Danese S, Gokhale SB, Woollett G. Switching reference medicines to biosimilars: a systematic literature review of clinical outcomes. Drugs. 2018;78(4):463–78.
US Food and Drug Administration. Guidance for industry: immunogenicity assessment for therapeutic protein products. https://www.fda.gov/media/85017/download.
Hoven Avd. Biosimilar medicines: practical EU experience and perspectives. Paper presented at the AAM Biosimilars Council Conference; 2017; Washington, DC.
Barsell A, Rengifo-Pardo M, Ehrlich A. A survey assessment of US dermatologists’ perception of biosimilars. J Drugs Dermatol. 2017;16(6):612–5.
Adé A, Bourdon O, Bussières JF. A survey of pharmacists’ knowledge and views of biosimilars in Quebec and France. Ann Pharm Fr. 2017;75(4):267–75.
Waller J, Sullivan E, Piercy J, Black CM, Kachroo S. Assessing physician and patient acceptance of infliximab biosimilars in rheumatoid arthritis, ankylosing spondyloarthritis and psoriatic arthritis across Germany. Patient Prefer Adherence. 2017;11:519–30.
Sullivan E, Piercy J, Waller J, Black CM, Kachroo S. Assessing gastroenterologist and patient acceptance of biosimilars in ulcerative colitis and Crohn’s disease across Germany. PLoS One. 2017;12(4):e0175826.
Narayanan S, Nag S. Likelihood of use and perception towards biosimilars in rheumatoid arthritis: a global survey of rheumatologists. Clin Exp Rheumatol. 2016;34(1 Suppl. 95):S9-11.
van Overbeeke E, De Beleyr B, de Hoon J, Westhovens R, Huys I. Perception of originator biologics and biosimilars: a survey among Belgian rheumatoid arthritis patients and rheumatologists. BioDrugs. 2017;31(5):447–59.
Colloca L, Panaccione R, Murphy TK. The clinical implications of nocebo effects for biosimilar therapy. Front Pharmacol. 2019;10:1372.
Pouillon L, Socha M, Demore B, Thilly N, Abitbol V, Danese S, et al. The nocebo effect: a clinical challenge in the era of biosimilars. Expert Rev Clin Immunol. 2018;14(9):739–49.
Brower CK, editor. Too long and too boring: the effects of survey length and interest on careless responding. 2018. Wright State University, Ohio.
Meade AW, Craig SB. Identifying careless responses in survey data. Psychol Methods. 2012;17(3):437–55.
Acknowledgements
We thank all the pediatric rheumatologists who participated in the study worldwide. We also thank Nicola Ruperto and PRINTO (Paediatric Rheumatology INternational Trials Organisation) and the PReS (European Society for Pediatric Rheumatology)-EMERGE (Emerging Rheumatologists and Researchers) group for disseminating the survey. All persons and members of the groups named in the Acknowledgments section have given us written permission to be named in the article.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
No funding was received for the preparation of this article.
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Istanbul University (no. 21.05.2021-204228).
Consent to participate
Each of the respondents filled out the questionnaire voluntarily.
Consent for publication
No personal or confidential data were collected.
Availability of data and material
The data underlying this article will be shared on reasonable request to the corresponding author.
Code availability
Not applicable.
Authors’ contributions
All authors whose names appear on the submission: (1) made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data; or the creation of new software used in the work; (2) drafted the work or revised it critically for important intellectual content; (3) approved the version to be published; and (4) agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed to the study conception and design. Methodology: Nuray Aktay Ayaz. Formal analysis and investigation: Fatma Gül Demirkan. Writing, original draft preparation: Fatma Gül Demirkan, Hafize Emine Sönmez, Özlem Akgün. Writing, review, and editing: Lovro Lamot, Betül Sözeri.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Demirkan, F.G., Sönmez, H.E., Lamot, L. et al. Embracing Change: An International Survey Study on the Beliefs and Attitudes of Pediatric Rheumatologists Towards Biosimilars. BioDrugs 36, 421–430 (2022). https://doi.org/10.1007/s40259-022-00526-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-022-00526-w