Abstract
Globally, patents on several well established biologic agents used to treat rheumatic diseases have already or will expire over the next few years, allowing for the availability of subsequent entry biologics (SEBs or biosimilars). The objective of this study was to identify gaps in knowledge and attitudes towards SEBs among Canadian rheumatologists. Eighty-one rheumatologists completed the survey and were included in the analysis (22 % of the 369 who were contacted). We found that one third of physicians (31 %) were familiar with SEBs and that physicians with greater than 20 years of practice were significantly more likely to be familiar or very familiar with SEBs compared to respondents with less than 10 years or 10–20 years of experience (OR 11.1, 95 % CI: 2.1–55.5, p = 0.004 and OR 4.5, 95 % CI: 1.2–16.2, p = 0.023, respectively). A third (32 %) of physicians agreed or strongly agreed that they would be comfortable with indication extrapolation. Most respondents (88 %) would feel concerned or very concerned if a pharmacist had the ability to substitute a biologic drug for an SEB without the physician’s approval. This survey was the first study that evaluated the position of rheumatologists on key areas surrounding SEBs from a nationwide Canadian perspective. Current physician attitudes and perceptions of SEBs can inform future educational initiatives and highlight important issues for payers, policy makers, and other stakeholders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past decade, biologic therapies, such as therapeutic monoclonal antibodies and fusion proteins, have grown to play an important role in the management of rheumatic diseases [1]. Throughout the world, patents on several of these biologic agents (originator or reference products) have already or will expire over the next few years, allowing for competition in the form of subsequent entry biologics (SEBs or biosimilars). While SEBs can be conceptually thought of as “generics” of biologics, unlike generics, they are similar but not identical to the originator molecule [2]. The complexity of the originator molecule makes it extremely difficult to create an identical copy, hence the term biosimilar [3–5]. SEBs are expected to be marketed at a lower price than their reference product, once the respective patents have expired [6]. The first SEB of infliximab has been approved in many jurisdictions.
Biologics, including SEBs, are derived from living organisms and are subject to inherent variation in post-translational modifications that are sensitive to changes in cell-lines and/or manufacturing processes [3]. This variation can even result in subtle differences among commercial batches of the originator biologic product [4]. This notion is especially true for monoclonal antibodies and fusion proteins because of their large size and structural complexity [7]. Due to the manufacturing challenges and complexity of originator biologics, there has been controversy over the amount and type of data required before approval of SEBs, and their placement in the therapeutic arena [5].
Concerns arise from difficulties in establishing how the potential variability between SEBs and their reference product may contribute to differences in the safety and efficacy of these drugs, especially due to the reduced submission requirements for SEBs stipulated by regulatory authorities. With limited efficacy and safety data available prior to approval, post-marketing pharmacovigilance of SEBs addressing the issues of immunogenicity, long-term safety, and sustained efficacy is essential [8].
Many countries have approved SEBs for all indications where approval exists for the original molecule, despite the absence of randomized clinical trials in other indications: a process referred to as extrapolation. This raises several issues, as some of the diseases are treated with doses and regimens different to that used in the limited development program.
Another issue is substitution, where the prescribed originator is replaced by an SEB. It is unknown if pharmacists or payers can implement or demand substitution without the physician’s approval. This also raises concerns over interchangeability in a patient already receiving the reference product, or at the time of initiation of the biologic [5]. This situation will grow more complex when more than one SEB exists for the same originator.
On the other hand, SEBs will reduce costs and improve accessibility to patients, and reduce some of the burden on healthcare budgets [9, 10]. Clinicians should have an understanding of specific issues surrounding SEBs in order to balance the cost with the clinical risk/benefit profiles. With the recent approval of an infliximab SEB, educational efforts to prepare clinicians for the arrival of SEBs have become increasingly important. The positions of various expert groups have been published [5, 11, 12]. However, there is no data on the collective opinion of the rheumatology community at large. Current physician attitudes and perceptions towards SEBs can inform future educational initiatives and highlight important issues for payers, policy makers, and other stakeholders.
The objective of this study, therefore, was to identify gaps in knowledge and attitudes towards SEBs among Canadian rheumatologists.
Materials and methods
Survey
The survey was pretested on a few rheumatologists and revised to eliminate redundancy and any perceived bias. An electronic survey written in both English and French (FluidSurveys; Ottawa, Ontario) was distributed by e-mail to 369 rheumatologist members of the Canadian Rheumatology Association (CRA) in February 2014. Two subsequent reminder e-mails followed the initial invitation to complete the survey, which was available for 30 days. The survey consisted of 29 questions: 3 evaluated basic respondent information, 4 evaluated biologic drug prescribing practices, 2 evaluated familiarity with SEBs, and 10 statements evaluated levels of agreement regarding attitudes towards prescribing new drugs. The final section consisted of 10 clinical scenario questions where respondents were also asked to provide comments. For most questions, responses were captured using a 5-point Likert scale. Respondents were given the opportunity to enter free text comments after most questions, and at the end of the survey (Appendix A shows the actual survey).
Respondents who completed the survey were provided with an additional link, where they could enter their contact information into a draw for a gift card valued at $500. Prior to commencing the survey, respondents were required to read and agree to the informed consent. This study was approved by the University of Toronto Social Sciences, Humanities & Education Research Ethics Board.
Analysis
Since a main focus of this study was to assess the degree of knowledge and awareness of SEBs, we analyzed whether responses to the following questions were correlated with SEB familiarity: geographic region of practice, years of practice, confidence in an SEB’s long-term safety and sustainability, concern over automatic substitution, and opinion on price reduction. In circumstances where response rates were low, responses were pooled. For example, respondents who indicated “very familiar” and “familiar” when asked about their degree of familiarity with SEBs were combined (as were those who responded very unfamiliar and unfamiliar). Odds ratios with 95 % confidence intervals were calculated using the proportional odds logit model, or using the cumulative odds logit model if the proportional odds assumption was violated. All statistical analyses were performed using SAS analytics software (SAS Institute Version 9.3; Cary, NC).
Results
Ninety rheumatologists responded, of whom 81 completed the survey and were included in the results and analysis (22 % response rate). Most were from Ontario (44 %), followed by Western Canada (36 %) and Eastern Canada (20 %). Thirty-seven (46 %) had over 20 years of practice, 21 (26 %) had between 10 and 20 years of practice, and 23 (28 %) had fewer than 10 years of practice. We did not distinguish between academic and community-based physicians.
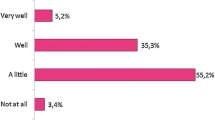
Overall, 31 % indicated that they were familiar or very familiar with SEBs (Fig. 1). Those with greater than 20 years of practice were significantly more likely to be familiar or very familiar with SEBs compared to respondents with less than 10 years or 10–20 years of experience (OR 11.1, 95 % CI: 2.1–55.5, p = 0.004 and OR 4.5, 95 % CI: 1.2–16.2, p = 0.023, respectively, Table 1). Comparisons of responses to select clinical scenarios, as well as to geographic region of practice, did not reveal any statistically significant association with the doctors’ familiarity with SEBs (Table 2).
When given the scenario where a biologic naïve patient is an ideal candidate for an anti-TNFα biologic, where cost is not an issue, 72 % of respondents were unlikely or very unlikely to offer an SEB as initial therapy. Only 11 % of respondents were likely or very likely to offer an SEB as initial therapy, and 16 % were neutral. Greater familiarity with established brand name drugs and uncertainty over the long-term safety of SEBs were often cited among those who were unlikely or very unlikely to offer an SEB in this scenario.
In a scenario where the provincial payer or insurance company mandated using an SEB of an anti-TNFα biologic not typically prescribed by the respondent, half of respondents (54 %) were likely or very likely to offer the SEB to the patient.
Thirty-two percent of respondents agreed, or strongly agreed, that they would be comfortable with using an SEB to treat patients with diseases such as ankylosing spondylitis (AS) and psoriatic arthritis (PsA) when the SEB had demonstrated equivalent safety and efficacy in a well-designed head-to-head trial with the reference biologic in only patients with rheumatoid arthritis (RA) (Fig. 1). Conversely, over half (54 %) disagreed or strongly disagreed with this extrapolated use of the SEB, and 14 % were neutral. A scenario where a Phase III head-to-head trial comparing an anti-TNFα brand name biologic to an SEB, and where the SEB demonstrated similar safety and efficacy at 30 weeks, found half (49 %) were not confident in the long-term sustainability profile of the SEB, whereas 19 % were confident or very confident, and one third were neutral. Most respondents (88 %) would feel concerned or very concerned if a pharmacist had the ability to substitute a biologic drug for an SEB without the physician’s approval (Fig. 1).
Nearly half (42 %) indicated a 30 % price reduction, and a third said a ≥50 % price reduction, would be reasonable before payers mandated the use of SEBs over brand name biologics.
Further opinions on issues of brand name biologics and SEBs can be found in Table 3. The majority of respondents either strongly agreed or agreed (94 %) that they were generally comfortable prescribing biologic drugs, yet only 31 % agreed or strongly agreed that they would be comfortable prescribing an SEB if they were currently available. When asked whether SEBs are essentially the same as generic drugs, two thirds agreed.
Discussion
With the recent approval of an SEB of infliximab in Canada and elsewhere, rheumatologists are among the first physician groups to be faced with decisions over adopting biosimilars of exceedingly complex therapeutic agents (e.g., monoclonal antibodies) in clinical practice. Overall familiarity with SEBs was relatively low (1/3 were familiar or very familiar with them). One possibility for this is the comparative lack of communication by SEB manufacturers up to the time of the survey. Over 45 % of respondents indicated that they have been practicing for more than 20 years, illustrating a mature rheumatology community. We found that these rheumatologists (with >20 years of experience) were significantly more likely to be familiar with SEBs compared to those with 10–20 years of experience, as well as those with fewer than 10 years of experience. Possible explanations include the recognition that physicians practising for longer have more experience with biologics and are more comfortable with their use.
No significant association was found between SEB familiarity and attitudes towards automatic substitution, pricing considerations, or long-term safety and efficacy profiles. Nevertheless, we observed reservations among rheumatologists on these key issues surrounding SEBs. When presented with a scenario about automatic substitution, the majority of rheumatologists expressed a high degree of concern, should this practice be enforced. When faced with a scenario about initiating a new biologic in a patient in the absence of financial constraints, the majority of rheumatologists would rather use an originator molecule, and would avoid using an SEB. Moreover, most rheumatologists would use an SEB only when mandated by the payer. In addition, most rheumatologists do not consider 30-week head-to-head trial data as adequate evidence to support the equivalence of long-term safety and efficacy to that of the originator molecule. These responses suggest that there is still uncertainty about the efficacy and safety of SEBs in rheumatic diseases. The accummulation of more data on SEBs will be required before rheumatologists will be comfortable using these therapies for the management of rheumatic diseases in Canada.
When presented with a hypothetical scenario where clinical data in RA was extrapolated to grant PsA and AS indications, the majority of respondents disagreed or strongly disagreed with this practice, suggesting that indication extrapolation is not supported by rheumatologists. The controversy over extrapolation has been discussed by Canadian experts in rheumatology [5, 12], and the stance against this practice has been documented by the Canadian Dermatology Association [13] and the Canadian Association of Gastroenterologists [14]. Our data is the first report of the position taken by Canadian rheumatologists over the extrapolation of indications for an SEB, indicating that a majority of rheumatologists do not agree with this practice. The rationale provided in their written comments by more than 25 % of the respondents who disagreed with indication extrapolation mentioned that these diseases are clinically or pathophysiologically different. These claims parallel arguments discussed in the literature that extrapolating indications to diseases that implicate the same molecular target, yet differing in the underlying pathophysiology, would be very challenging [5]. These opinions about extrapolation will play an important role in the adoption of the first approved infliximab SEB. Remsima/Inflectra (Celltrion/Hospira) was granted indications for RA, PsA, AS, and psoriasis (PsO), based on data from Phase I [15] and Phase III [16] trials in AS and RA, respectively.
Among the considerations a clinician could face when SEBs become increasingly available is the switching of those patients who are stable on biologic therapy onto an SEB, which could pose a risk of loss of disease control. Indeed, the notion that the risk of alternating, or switching, between the SEB and brand name biologic should not exceed the risk of using the brand name biologic in the absence of switching has been discussed [5, 7]. The question was posed in our survey, and a majority of respondents either disagreed or strongly disagreed with the idea of switching stable patients to an SEB (38 % and 30 %, respectively; Table 3). It has been suggested that a switch from a brand name biologic to an SEB (or vice versa) should be considered similar to switching between brand name biologics [5], which is supported by Health Canada’s guidelines declaring that SEBs are standalone products [17]. It is notable that a 48-week open-label extension study of a head-to-head trial comparing Remicade and Remsima, in which patients on infliximab were switched onto the SEB, found no significant difference in the safety and tolerability between the switch and maintenance groups [18]. However, the safety of switching to an SEB was not compared to a group who switched to another brand-name anti-TNFα biologic.
Further, the topic which suggested the most consensus was on interchangeability and substitution. When physicians were asked to gauge their level of concern over automatic substitution by pharmacists, 96 % had some degree of concern, of which 72 % indicated “very concerned” should this practice be extended to SEBs. Regardless of possible future circumstances, the question of automatic substitution by pharmacists remains an issue that resonates with many of our survey’s respondents. Health Canada has stated that the granting of market authorization of an SEB does not deem the SEB interchangeable [19], leaving this decision to the provincial health authorities. Our findings are in agreement with the positions discussed by Canadian experts in rheumatology, gastroenterology, and dermatology that do not support automatically switching a patient to an SEB [11, 13, 14].
Much like small-molecule drug generics, SEBs present an opportunity for significant cost-savings, especially in chronic disease pharmacotherapy [20]. We analyzed the importance of the cost of biologic therapy to physicians. Over 75 % of respondents indicated that patient assistance programs have an impact when considering biologic therapy, indicating that the high cost of biologic drugs is taken into account by physicians. Several respondents indicated that the reduction in price must be substantial in order to offset the risk of switching to an SEB, with over one in three rheumatologists demanding the SEB be discounted by ≥50 %. We asked whether physicians would prescribe an SEB to patients who are ideal for anti-TNFα therapy if cost was not an issue; almost three quarters of respondents (73 %) were unlikely or highly unlikely to prescribe an SEB to those patients. However, if payers mandated prescribing an SEB of an anti-TNFα biologic that physicians would normally not prescribe (e.g., adalimumab is the preferred product, but an SEB of infliximab is mandated), roughly half of respondents (54 %) would indeed prescribe the SEB. Interestingly, 46 % of survey respondents indicated that they would use a non-anti-TNFα biologic to avoid using the SEB. These trends suggest that physicians recognize the cost of biologic therapeutics to their patients. However a preference for incumbent therapies exists, and doctors may not entirely be prepared to concede to payers if forced to use the SEB. This would have implications for SEB and innovator manufacturers, as the payers’ decisions will have the most significant impact on whether SEBs will be widely used in Canada.
There are several limitations to the survey that must be considered when interpreting these results. Firstly, our findings are limited by the survey response rate (22 %). However, previous surveys among Canadian physicians have obtained comparable response rates using similar methodology [21–23]. Per our protocol, a survey was considered complete when the participant proceeded to the final question and acknowledged their completion of the survey. As a result, respondent data were excluded from the final analysis set if the participant terminated the survey prematurely. Because some categories had low responses, it was necessary to pool responses in order to perform sensible statistical analysis (e.g., combining “Agree” and “Strongly agree” into one category). Secondly, a selection bias could be present as rheumatologists who are not familiar with SEBs may be less willing to participate. It is also important to recognize that community physicians may have obtained their concepts from expert opinion publications [5, 11, 12] on this topic and therefore can have a bias in favour of experts. Finally, as with other surveys, there exists a risk of social desirability bias [24].
This survey was the first study that evaluated the position of rheumatologists on key areas surrounding SEBs from a nationwide Canadian perspective. We report moderate familiarity with SEBs among these physicians, with significantly greater familiarity among those who have been longer in practice. The survey highlighted several issues of debate that may be addressed by tailored educational efforts as the presence and demand for SEBs continues to grow.
References
Upchurch KS, Kay J (2012) Evolution of treatment of rheumatoid arthritis. Rheumatology 51:vi28–vi36
Revers L, Furczon E (2010) An introduction to biologics and biosimilars. Part II: Subsequent Entry Biologics: Biosame or Biodifferent? Can Pharm J 143:184-191
Scheinberg MA, Kay J (2012) The advent of biosimilar therapies in rheumatology — “O brave new world.” Nat Rev Rheumatol 8:430–436
Schiestl M, Stangler T, Torella C, Čepeljnik T, Toll H, Grau R (2011) Acceptable changes in quality attributes of glycosylated biopharmaceuticals. Nature Biotechnol 29:310–312
Kay J, Feagan BG, Guirguis MS, Keystone EC, Klein AV, Lubiniecki AS, Mould DR, Nyarko KA, Ridgway AAG, Trudeau ME, Wang J (2012) Health Canada/BIOTECanada Summit on Regulatory and Clinical Topics Related to Subsequent Entry Biologics (Biosimilars), Ottawa, Canada, 14 May 2012. Biologicals 40:517–527
Farfan-Portet MI, Gerkens S, Lepage-Nefkens I, Vinck I, Hulstaert F (2014) Are biosimilars the next tool to guarantee cost-containment for pharmaceutical expenditures? Eur J Health Econ 15:223
Dörner TSV, Castañeda-Hernández G, Ferraccioli G, Isaacs JD, Kvien TK, Martin-Mola E, Mittendorf T, Smolen JS, Burmester GR (2013) The role of biosimilars in the treatment of rheumatic diseases. Ann Rheum Dis 72:322–328
Papp K, Bourcier M, Ho V, Burke V, Haraoui B (2013) Preparing for subsequent entry biologics in dermatology and rheumatology in Canada. J Cutan Med Surg 17:340–346
Ndegwa S, Quansah K. Subsequent entry biologics – Emerging trends in regulatory and health technology assessment frameworks [Environmental Scan, Issue 43, ES0284]. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2014
Haustein R, de Millas C, Höer A, Häussler H (2012) Saving money in the European healthcare systems with biosimilars. Generics Biosimilars Initiat J, 1(3-4):120–126
Ontario Rheumatology Association (2012) Position paper on subsequent entry biologics/biosimilars
Russell AS, Vandana A, Barnabe C, Jamal S, Offer RC, Olszynski WP, Shojania K, Haraoui B (2012) Subsequent entry biologics/biosimilars: A viewpoint from Canada. Clin Rheumatol 31:1289–1292
Canadian Dermatology Association (2013) Canadian Dermatology Association Position Statement: Biosimilars
Devlin SM, Bessler B, Bernstein CN, Fedorak RN, Bitton A, Singh H, Feagan BG (2013) Overview of subsequent entry biologics for management of inflammatory bowel disease and Canadian Association of Gastroenterology statement on subsequent entry biologics. Can J Gastroenterol 27:567–571
Park W, Hrycaj P, Jeka S, Kovalenko V, Lysenko G, Miranda P, Mikazane H, Gutierrez-Ureña S, Lim M, Lee YA, Kim H, Yoo DH, Braun J (2013) A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: The PLANETAS study. Ann Rheum Dis 72:1605–1612
Yoo DH, Hrycaj P, Miranda P, Ramiterre E, Piotrowski M, Shevchuk S, Kovalenko V, Prodanovic N, Abello-Banfi M, Gutierrez-Ureña S, Morales-Olazabal L, Tee M, Jimenez R, Zamani O, Lee SJ, Kim H, Park W, Müller-Ladner U (2013) A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: The PLANETRA study. Ann Rheum Dis 72:1613–1620
Health Canada Food and Drugs Branch (2010) Guidance for Sponsors: Information and Submission Requirements for Subsequent Entry Biologics (SEBs). Health Canada, Ottawa, Canada
Yoo DH, Prodanovic N, Jaworski J, Miranda P, Ramiterre EB, Baranauskaite A, Wiland P, Abud-Mendoza C, Oparanoc B, Son YK, Park W, Muller-Ladner U. Efficacy and safety of CT-P13 (infliximab biosimilar) over two years in patients with rheumatoid arthritis: Comparison between continued CT-P13 and switching from infliximab to CT-P13. Abstract presented at: Annual Meeting of the American College of Rheumatology; 26-29 October 2013; San Diego, CA
Health Canada Food and Drugs Branch (2010) Questions & Answers to Accompany the Final Guidance for Sponsors: Information and Submission Requirements for Subsequent Entry Biologics (SEBs). Health Canada, Ottawa, Canada
Colbert RA, Cronstein BN (2011). Biosimilars: the debate continues. Arthritis Rheum 63:2848–2850
Haraoui B, Bensen W, Bessette L, Le Clercq S, Thorne C, Wade J (2012) Treating rheumatoid arthritis to target: A Canadian physician survey. J Rheumatol 39:949–953
National Physician Survey (2013) 2013 National Physician Survey (NPS): Demographics for internal medicine specialists. National Physician Survey, Mississauga
National Physician Survey (2010) 2010 National Physician Survey (NPS): Demographics for internal medicine specialists. National Physician Survey, Mississauga
Nederhof AJ (1985) Methods of coping with social desirability bias: A review. Eur J Soc Psychol 15:263–280
Acknowledgments
We would like to thank all the physicians who took time to complete our survey and share their opinions, as well as the Canadian Rheumatology Association (CRA) for making this study possible. We would also like to thank Christine Charnock and Virginia Hopkins of the CRA for their continuous support in coordinating the survey during the launch, maintenance, and closure phases. This study was funded by MBiotech Innovation Bursaries awarded to Messrs. Grabowski, Henderson and Lam. The lottery incentive for the survey was provided by the CRA.
Conflict of interest
Drs. Keystone, Thorne, Jamal, Pope, Haraoui, and Revers have received honoraria from the pharmaceutical industry in the past, but declare no conflict of interest in relation to the present study. Messrs. Grabowski, Henderson and Lam have been undertaking this research as part of their graduate training at the Institute of Management of Innovation, University of Toronto Mississauga. Messrs. Grabowski and Lam are currently employees of Boehringer Ingelheim and Janssen, respectively. Mr. Henderson was an employee of Amgen and Janssen during the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
David Grabowski, Bradley Henderson, and Dennis Lam contributed to this work equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 67 kb)
Rights and permissions
About this article
Cite this article
Grabowski, D., Henderson, B., Lam, D. et al. Attitudes towards subsequent entry biologics/biosimilars: A survey of Canadian rheumatologists. Clin Rheumatol 34, 1427–1433 (2015). https://doi.org/10.1007/s10067-014-2835-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-014-2835-4